Perinatal Arterial Ischemic Stroke Is Associated to Materno-Fetal Immune Activation and Intracranial Arteritis
Abstract
:1. Introduction
2. Results
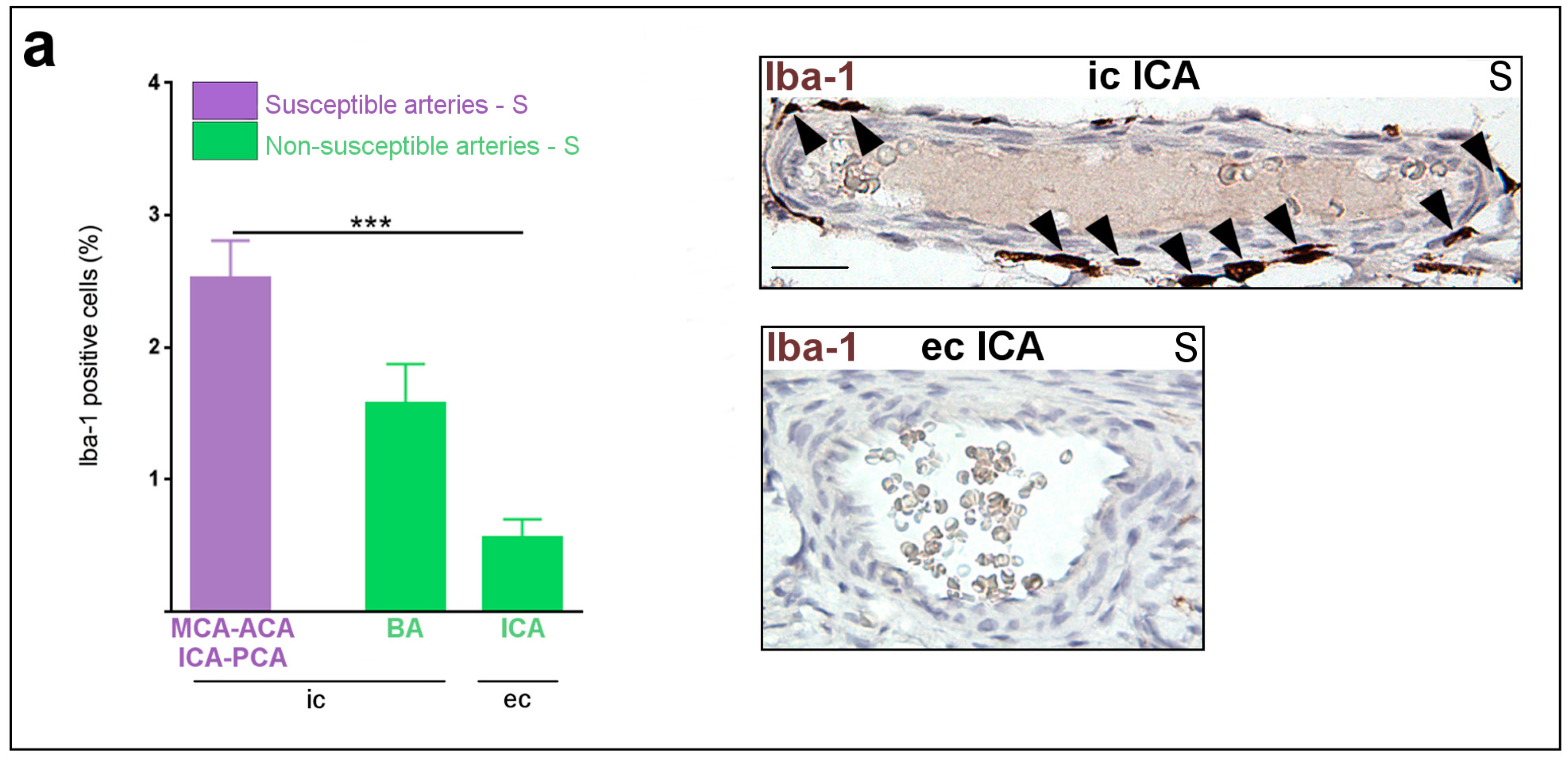
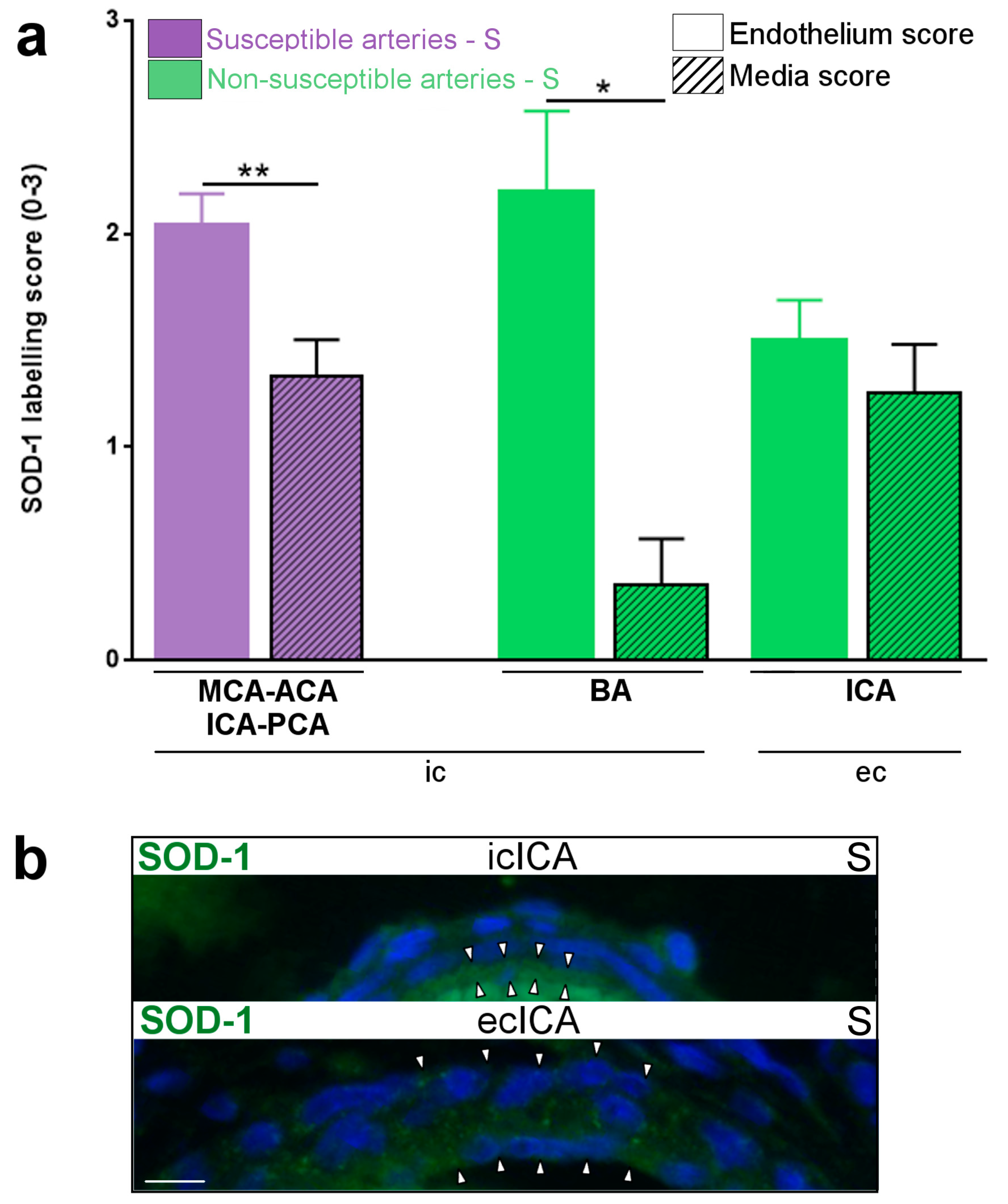
2.1. Constitutive Expression of Inflammatory Markers of Interest within the Medium-Sized Arterial Wall of Arteries Susceptible vs. Non-Susceptible to PAIS
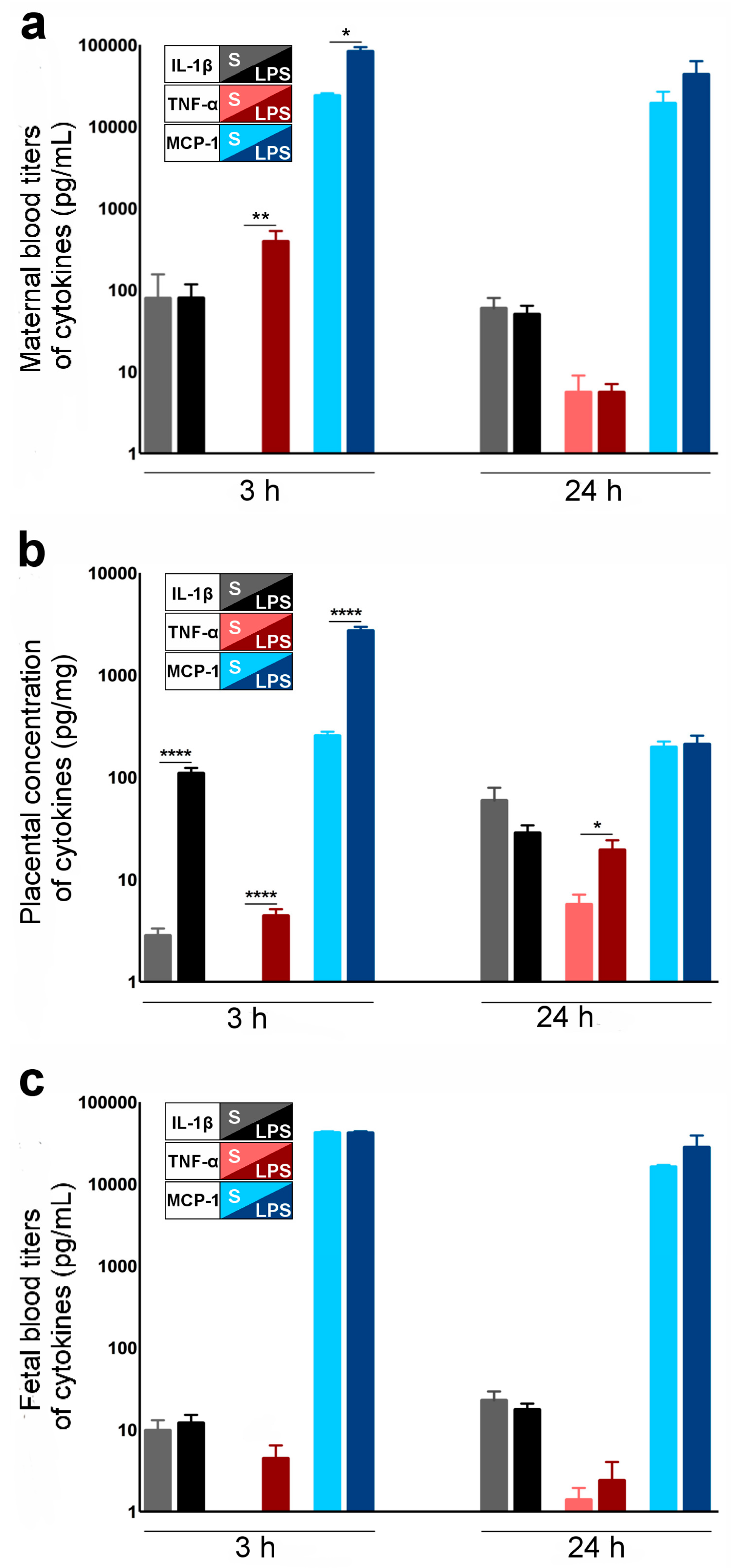
2.2. Cytokines and Chemokines Involved in LPS-Induced Materno-Fetal Inflammatory Responses
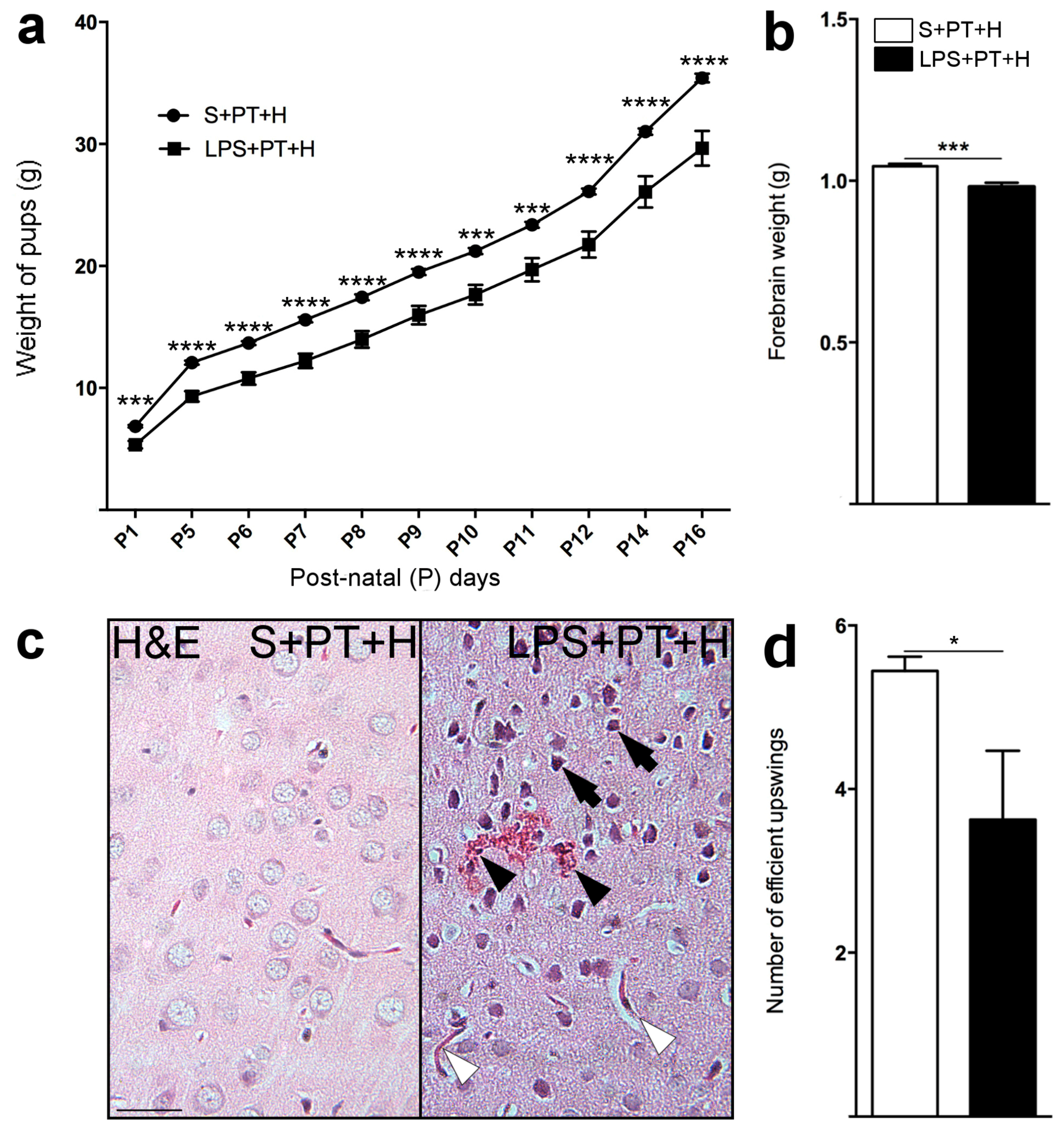
2.3. Susceptibility to Infarcts in the Pups from LPS-Exposed vs. S-Exposed Dams
2.4. Motor Behavior of Pups Exposed to S + PT + H vs. LPS + PT + H
2.5. Inflammatory Responses within Cervicocephalic Arterial Walls of Pups in Utero-Exposed to LPS vs. S
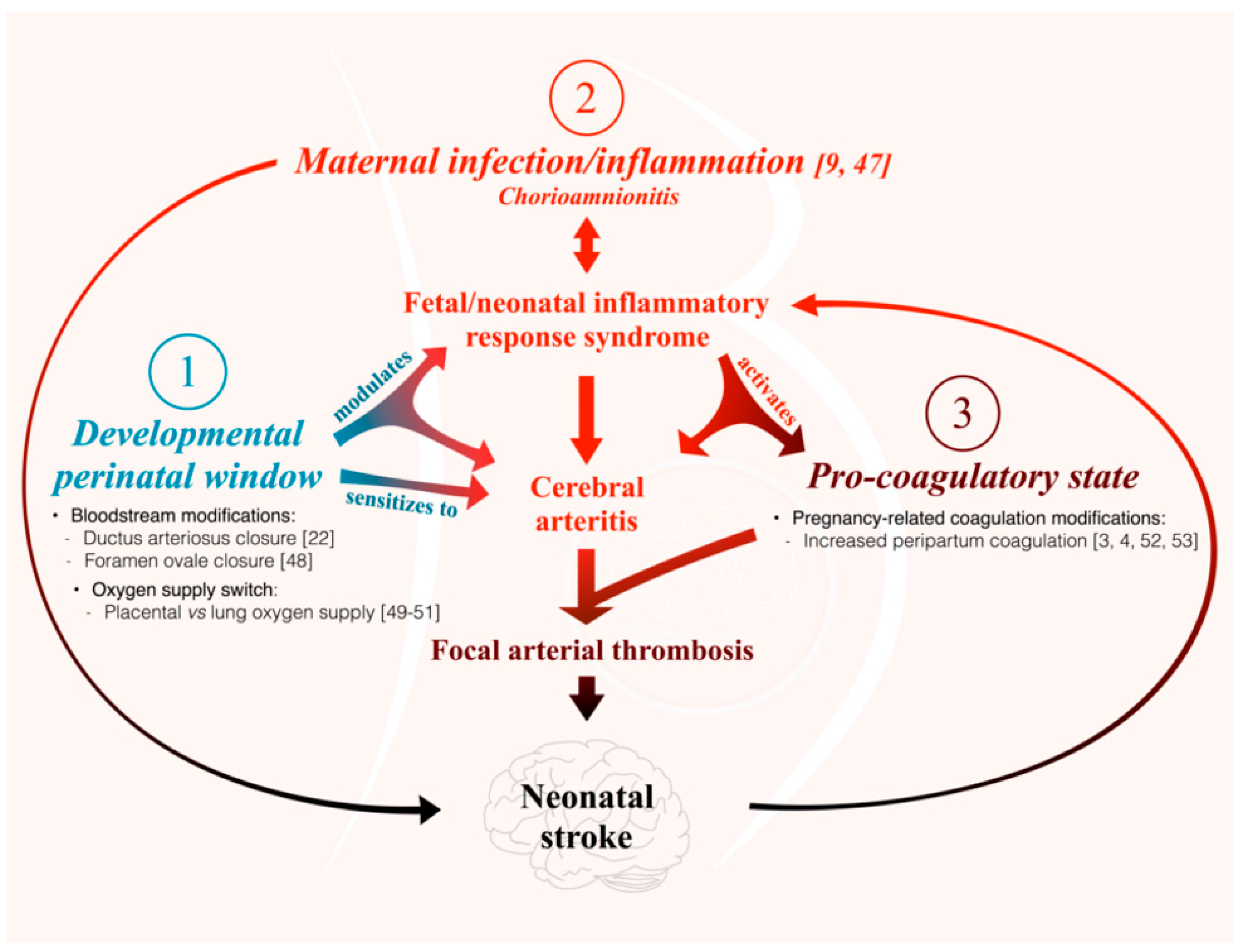
3. Discussion
- (i)
- The present study is the first to our knowledge to address the question of the differential susceptibility to stroke of the main cerebral arteries using an animal model. We are not aware of any previous works documenting the distribution of PAIS in rodents. However, previous experiments performed in our laboratory on this rat model of LPS-induced materno-fetal inflammatory response showed that fetuses from LPS-exposed dams developed macrophagic arteritis within their placenta and umbilical cord [13]. This observation led us to hypothesize that such macrophagic arteritis affecting the umbilical cord might propagate to other arteries, such as brain arteries. Based on these previous findings, we assumed that rat could be a pertinent animal model to deal with this research question and to study the biological mechanisms underpinning the various susceptibilities to stroke.
- (ii)
- Our study is based on semi-quantitative IHC due to the difficulty in extracting a sufficient amount of proteins from tiny cerebral arteries of rodents to make possible the use of some classic quantitative analyses. Selective dissections of such fragile arteries would have necessitated strenuous and lengthy dissections of the skull to access the small basal arteries. Such arterial isolation without contamination by other tissues (e.g., meningeal tissue) and without alterations due to too long delay and trauma remained an unresolved challenge in our hands. Further experiments on bigger animals might overcome this limitation. Due to the small amount of cerebrospinal fluid (CSF) and its difficult access in sufficient amounts in small animals, it was not possible to monitor CSF inflammation. It is possible that the adventitial macrophagic activation we observed in segments of arteries located within or in the vicinity of subarachnoidal spaces would result from LPS-induced CSF inflammation.
- (iii)
- An important question in relation to our model is whether LPS crosses the placental barrier. According to the literature, the effects of maternal LPS exposure on the developing fetus are not mediated directly by the LPS [25,26] but via an indirect effect that might implicate the fetal immune response [13,27,28,29].
- (iv)
- This study used newborn rat pups whose brain development corresponds to a stage of development equivalent to preterm human newborns whereas PAIS occurs in term human newborns. However, there is no data to our knowledge showing that the cerebral arteries of newborn rats are less mature than those of term human newborns. Our aim was to study the short-term interaction between end-gestational/placental inflammation and perinatal cervicocephalic arteries. This would not be feasible in rodents at P12, i.e., at a stage of brain development equivalent to term human newborns. Given that placental inflammation (chorioamnionitis) is a major risk factor of PAIS, and that PAIS most often occurs just a few hours after birth, designing our model in P1 newborn rats appeared to be relevant to the reality of this pathology.
- (v)
- Additional pre-clinical, as well as clinical studies, for instance using non-invasive neonatal arterial wall magnetic resonance imaging, are mandatory to confirm our preclinical findings, their translation to human, and thus, to further uncover the pathophysiological mechanisms underlying human PAIS.
4. Materials and Methods
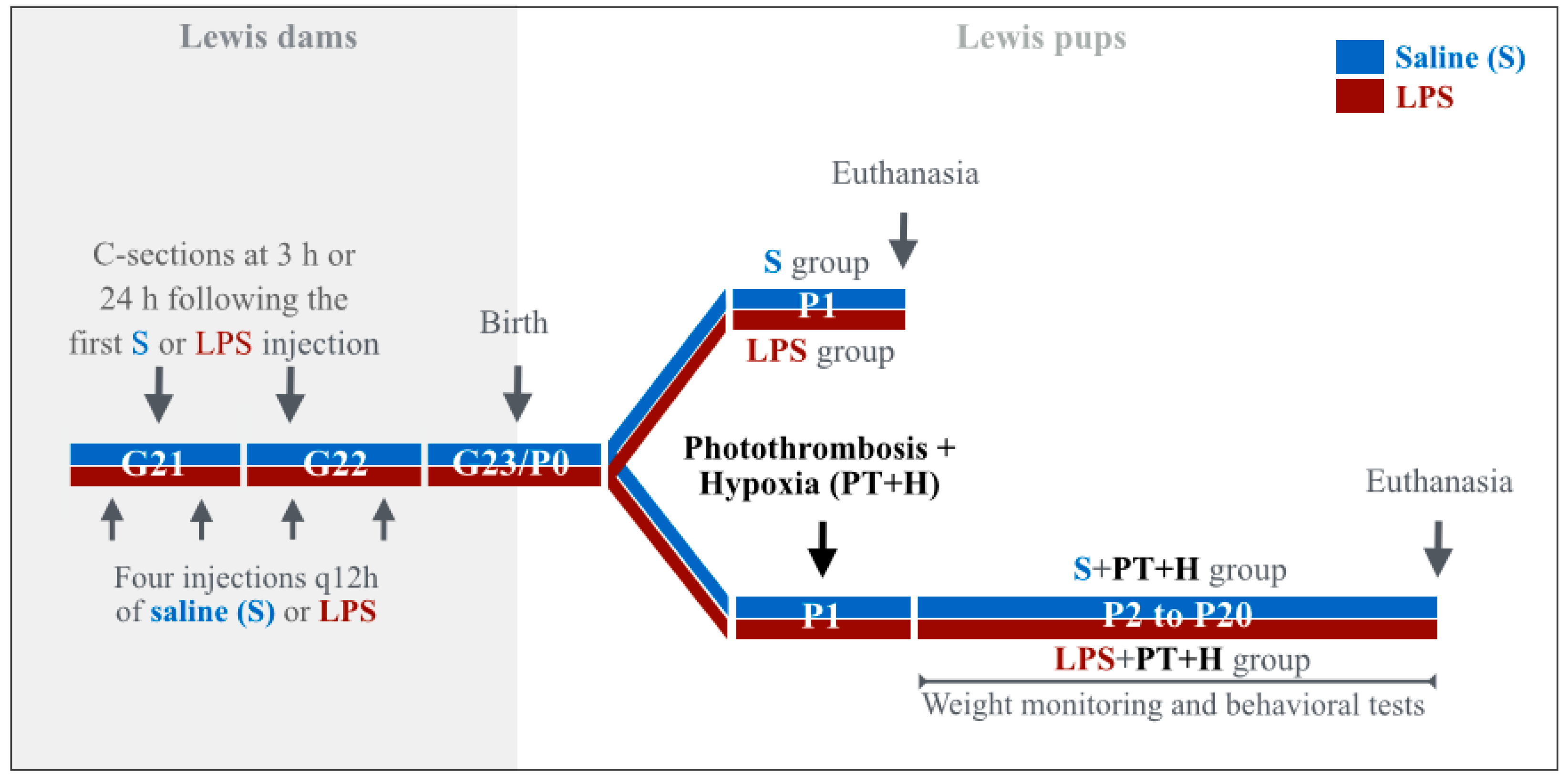
4.1. Animals
4.2. Immunohistochemistry (IHC), Immunofluorescence (IF)
4.3. ELISA
4.4. Behavioral Tests
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACA | Anterior cerebral artery |
| AlPcS4 | Aluminium phthalocyanine tetrasulfonate |
| Arg-1 | Arginase-1 |
| BA | Basilar artery |
| CD40 | Cluster of differentiation 40 |
| CD40L | Cluster of differentiation 40 ligand |
| CP | Cerebral palsy |
| CSF | Cerebrospinal fluid |
| DAB | 3,3-diaminobenzidine |
| EBST | Elevated Body Swing Test |
| ec | Extra-cranial |
| ELISA | Enzyme-linked immunosorbent assay |
| G | Gestational day |
| GIMP | GNU Image Manipulation Program |
| H | Hypoxia |
| Iba-1 | Ionized calcium binding adapter molecule-1 |
| ICAM-1 | Intercellular adhesion molecule-1 |
| ic | Intra-cranial |
| ICA | Internal carotid artery |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| IL-1β | Interleukin-1β |
| IL-1Ra | IL-1 receptor antagonist |
| iNOS | Inducible nitric oxide synthase |
| ip | Intra-peritoneally |
| LPS | Lipopolysaccharide |
| MCA | Middle cerebral artery |
| MCP-1 | Monocyte chemotactic protein 1 |
| mW | milliWatt |
| NO | Nitric oxide |
| P | Post-natal day |
| PAIS | Perinatal arterial ischemic stroke |
| PCA | Posterior cerebral artery |
| PCNA | Proliferating cell nuclear antigen |
| PMN | Polymorphonuclear neutrophils |
| PT | Photothrombosis |
| S | Saline |
| SEM | Standard error of the mean |
| SOD-1 | Superoxide dismutase-1 |
| TNF-α | Tumor necrosis factor-α |
| vWF | vonWillebrand factor |
References
- Chabrier, S.; Saliba, E.; Nguyen The Tich, S.; Charollais, A.; Varlet, M.N.; Tardy, B.; Presles, E.; Renaud, C.; Allard, D.; Husson, B.; et al. Obstetrical and neonatal characteristics vary with birthweight in a cohort of 100 term newborns with symptomatic arterial ischemic stroke. Eur. J. Paediatr. Neurol. 2010, 14, 206–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Aa, N.E.; Benders, M.J.; Groenendaal, F.; de Vries, L.S. Neonatal stroke: A review of the current evidence on epidemiology, pathogenesis, diagnostics and therapeutic options. Acta Paediatr. 2014, 103, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.B.; Lynch, J.K. Stroke in newborn infants. Lancet Neurol. 2004, 3, 150–158. [Google Scholar] [CrossRef]
- Chabrier, S.; Husson, B.; Dinomais, M.; Landrieu, P.; Nguyen The Tich, S. New insights (and new interrogations) in perinatal arterial ischemic stroke. Thromb. Res. 2011, 127, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.W.; Croen, L.A.; Shah, S.J.; Newman, T.B.; Najjar, D.V. Cerebral palsy in a term population: Risk factors and neuroimaging findings. Pediatrics 2006, 118, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Kirton, A.; de Veber, G. Paediatric stroke: Pressing issues and promising directions. Lancet Neurol. 2015, 14, 92–102. [Google Scholar] [CrossRef]
- Osborn, A.G. Diagnostic Cerebral Angiography, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1998; p. 480. [Google Scholar]
- Van der Aa, N.E.; Dudink, J.; Benders, M.J.; Govaert, P.; van Straaten, H.L.; Porro, G.L.; Groenendaal, F.; de Vries, L.S. Neonatal posterior cerebral artery stroke: Clinical presentation, MRI findings, and outcome. Dev. Med. Child Neurol. 2013, 55, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kirton, A.; Armstrong-Wells, J.; Chang, T.; Deveber, G.; Rivkin, M.J.; Hernandez, M.; Carpenter, J.; Yager, J.Y.; Lynch, J.K.; Ferriero, D.M. International Pediatric Stroke Study Investigators Symptomatic neonatal arterial ischemic stroke: The International Pediatric Stroke Study. Pediatrics 2011, 128, e1402–e1410. [Google Scholar] [CrossRef] [PubMed]
- Koelfen, W.; Freund, M.; Varnholt, V. Neonatal stroke involving the middle cerebral artery in term infants: Clinical presentation, EEG and imaging studies, and outcome. Dev. Med. Child Neurol. 1995, 37, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Barmada, M.A.; Moossy, J.; Shuman, R.M. Cerebral infarcts with arterial occlusion in neonates. Ann. Neurol. 1979, 6, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Husson, B.; Hertz-Pannier, L.; Adamsbaum, C.; Renaud, C.; Presles, E.; Dinomais, M.; Kossorotoff, M.; Landrieu, P.; Chabrier, S. MR angiography findings in infants with neonatal arterial ischemic stroke in the middle cerebral artery territory: A prospective study using circle of Willis MR angiography. Eur. J. Radiol. 2016, 85, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.; Tremblay, L.; Lepage, M.; Sébire, G. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J. Immunol. 2010, 184, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Larouche, A.; Roy, M.; Kadhim, H.; Tsanaclis, A.M.; Fortin, D.; Sébire, G. Neuronal injuries induced by perinatal hypoxic-ischemic insults are potentiated by prenatal exposure to lipopolysaccharide: Animal model for perinatally acquired encephalopathy. Dev. Neurosci. 2005, 27, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, Y.; Takahashi, H.; Takahashi, K. Cerebrovascular Moyamoya disease. Eur. J. Pediatr. 1984, 142, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.P.; Bulder, M.M.; Chabrier, S.; Kirkham, F.J.; Uiterwaal, C.S.; Tardieu, M.; Sébire, G. The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain 2009, 132, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Amlie-Lefond, C.; Sébire, G.; Fullerton, H.J. Recent developments in childhood arterial ischaemic stroke. Lancet Neurol. 2008, 7, 425–435. [Google Scholar] [CrossRef]
- Bergwerff, M.; Verberne, M.E.; DeRuiter, M.C.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Neural crest cell contribution to the developing circulatory system: Implications for vascular morphology? Circ. Res. 1998, 82, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Renesto, P.; Chignard, M. Tumor necrosis factor-alpha enhances platelet activation via cathepsin G released from neutrophils. J. Immunol. 1991, 146, 2305–2309. [Google Scholar] [PubMed]
- Jang, J.Y.; Min, J.H.; Chae, Y.H.; Baek, J.Y.; Wang, S.B.; Park, S.J.; Oh, G.T.; Lee, S.H.; Ho, Y.S.; Chang, T.S. Reactive oxygen species play a critical role in collagen-induced platelet activation via SHP-2 oxidation. Antioxid. Redox Signal. 2014, 20, 2528–2540. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, S.E.; Hansmann, G. Patent ductus arteriosus of the preterm infant. Pediatrics 2010, 125, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.N.; Girard, S.; Holmes, W.M.; Parkes, L.M.; Williams, S.R.; Parry-Jones, A.R.; Allan, S.M. Systemic inflammation impairs tissue reperfusion through endothelin-dependent mechanisms in cerebral ischemia. Stroke 2014, 45, 3412–3419. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, B.; Tann, C.J.; Degos, V.; Sigaut, S.; van Steenwinckel, J.; Schang, A.L.; Kichev, A.; Robertson, N.J.; Mallard, C.; Hagberg, H.; et al. Inflammation-induced sensitization of the brain in term infants. Dev. Med. Child Neurol. 2015, 57, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Ashdown, H.; Dumont, Y.; Ng, M.; Poole, S.; Boksa, P.; Luheshi, G.N. The role of cytokines in mediating effects of prenatal infection on the fetus: Implications for schizophrenia. Mol. Psychiatry 2006, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Robinson, S.M. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav. Immun. 2010, 24, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, R.; Heikkinen, T.; Hakala, K.; Laine, K.; Alanen, A. Transfer of proinflammatory cytokines across term placenta. Obstet. Gynecol. 2005, 106, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, J.; Samuelsson, A.M.; Jansson, T.; Holmäng, A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr. Res. 2006, 60, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, M.V.; Alexander, J.M.; Byrd, W.; Bawdon, R.E. Transfer of inflammatory cytokines across the placenta. Obstet. Gynecol. 2004, 103, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.; Kadhim, H.; Larouche, A.; Roy, M.; Gobeil, F.; Sébire, G. Pro-inflammatory disequilibrium of the IL-1β/IL-1Ra ratio in an experimental model of perinatal brain damages induced by lipopolysaccharide and hypoxia-ischemia. Cytokine 2008, 43, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Wang-Fischer, Y. Manual of Stroke Models in Rats; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Ali, H.; Cauchon, N.; van Lier, J.E. Pd-catalyzed Heck reaction for the synthesis of isomeric metallo tetravinylsulfo phthalocyanines and their photosensitizing properties. Photochem. Photobiol. Sci. 2009, 8, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.V.; West, C.M.; Whitehurst, C. The biology of photodynamic therapy. Phys. Med. Biol. 1997, 42, 913–935. [Google Scholar] [CrossRef] [PubMed]
- Levine, S. Anoxic-ischemic encephalopathy in rats. Am. J. Pathol. 1960, 36, 1–17. [Google Scholar] [PubMed]
- Brochu, M.E.; Girard, S.; Lavoie, K.; Sébire, G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study. J. Neuroinflamm. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Iqbal, A.J. Epigenetic Control of Macrophage Polarisation and Soluble Mediator Gene Expression during Inflammation. Mediat. Inflamm. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Manfredi, G.; Germain, D. SOD1, an unexpected novel target for cancer therapy. Genes Cancer 2014, 5, 15–21. [Google Scholar] [PubMed]
- Paxinos, G.; Ashwell, K.W.S.; Tork, I. Atlas of the Developing Rat Nervous System, 3rd ed.; Elsevier: Boston, MA, USA; Amsterdam, The Netherlands, 2008. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: Sydney, Australia; Orlando, FL, USA, 1986; p. xxvi, 237p. of plates. [Google Scholar]
- Girard, S.; Sébire, G.; Kadhim, H. Proinflammatory orientation of the interleukin 1 system and downstream induction of matrix metalloproteinase 9 in the pathophysiology of human perinatal white matter damage. J. Neuropathol. Exp. Neurol. 2010, 69, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Savard, A.; Brochu, M.E.; Chevin, M.; Guiraut, C.; Grbic, D.; Sébire, G. Neuronal self-injury mediated by IL-1β and MMP-9 in a cerebral palsy model of severe neonatal encephalopathy induced by immune activation plus hypoxia-ischemia. J. Neuroinflamm. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, H.; Tabarki, B.; Verellen, G.; de Prez, C.; Rona, A.M.; Sébire, G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology 2001, 56, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health ImageJ. Available online: http://rsb.info.nih.gov/ij/ (accessed on 7 September 2016).
- GIMP GNU Image Manipulation Program. Available online: http://www.gimp.org/ (accessed on 7 September 2016).
- Savard, A.; Lavoie, K.; Brochu, M.E.; Grbic, D.; Lepage, M.; Gris, D.; Sebire, G. Involvement of neuronal IL-1β in acquired brain lesions in a rat model of neonatal encephalopathy. J. Neuroinflamm. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Kirton, A.; de Veber, G. Advances in perinatal ischemic stroke. Pediatr. Neurol. 2009, 40, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Teitel, D. Recognition of Undiagnosed Neonatal Heart Disease. Clin. Perinatol. 2016, 43, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Sola, A.; Baquero, H.; Neira, F.; Alvis, R.; Deulofeut, R.; Critz, A. Pulse oxygen saturation levels and arterial oxygen tension values in newborns receiving oxygen therapy in the neonatal intensive care unit: Is 85% to 93% an acceptable range? Pediatrics 2008, 121, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D.; Sejersted, Y.; Solberg, R.; Wollen, E.J.; Bjørås, M. Oxygenation of the Newborn: A Molecular Approach. Neonatology 2012, 101, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Uslu, S.; Bulbul, A.; Can, E.; Zubarioglu, U.; Salihoglu, O.; Nuhoglu, A. Relationship between oxygen saturation and umbilical cord pH immediately after birth. Pediatr. Neonatol. 2012, 53, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, S.J. Innate immunity, coagulation and placenta-related adverse pregnancy outcomes. Thromb. Res. 2009, 124, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Günther, G.; Junker, R.; Sträter, R.; Schobess, R.; Kurnik, K.; Heller, C.; Kosch, A.; Nowak-Göttl, U. Childhood Stroke Study Group Symptomatic ischemic stroke in full-term neonates: Role of acquired and genetic prothrombotic risk factors. Stroke 2000, 31, 2437–2441. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guiraut, C.; Cauchon, N.; Lepage, M.; Sébire, G. Perinatal Arterial Ischemic Stroke Is Associated to Materno-Fetal Immune Activation and Intracranial Arteritis. Int. J. Mol. Sci. 2016, 17, 1980. https://doi.org/10.3390/ijms17121980
Guiraut C, Cauchon N, Lepage M, Sébire G. Perinatal Arterial Ischemic Stroke Is Associated to Materno-Fetal Immune Activation and Intracranial Arteritis. International Journal of Molecular Sciences. 2016; 17(12):1980. https://doi.org/10.3390/ijms17121980
Chicago/Turabian StyleGuiraut, Clémence, Nicole Cauchon, Martin Lepage, and Guillaume Sébire. 2016. "Perinatal Arterial Ischemic Stroke Is Associated to Materno-Fetal Immune Activation and Intracranial Arteritis" International Journal of Molecular Sciences 17, no. 12: 1980. https://doi.org/10.3390/ijms17121980





