Towards Clinical Application of Neurotrophic Factors to the Auditory Nerve; Assessment of Safety and Efficacy by a Systematic Review of Neurotrophic Treatments in Humans
Abstract
:1. Introduction
2. Results
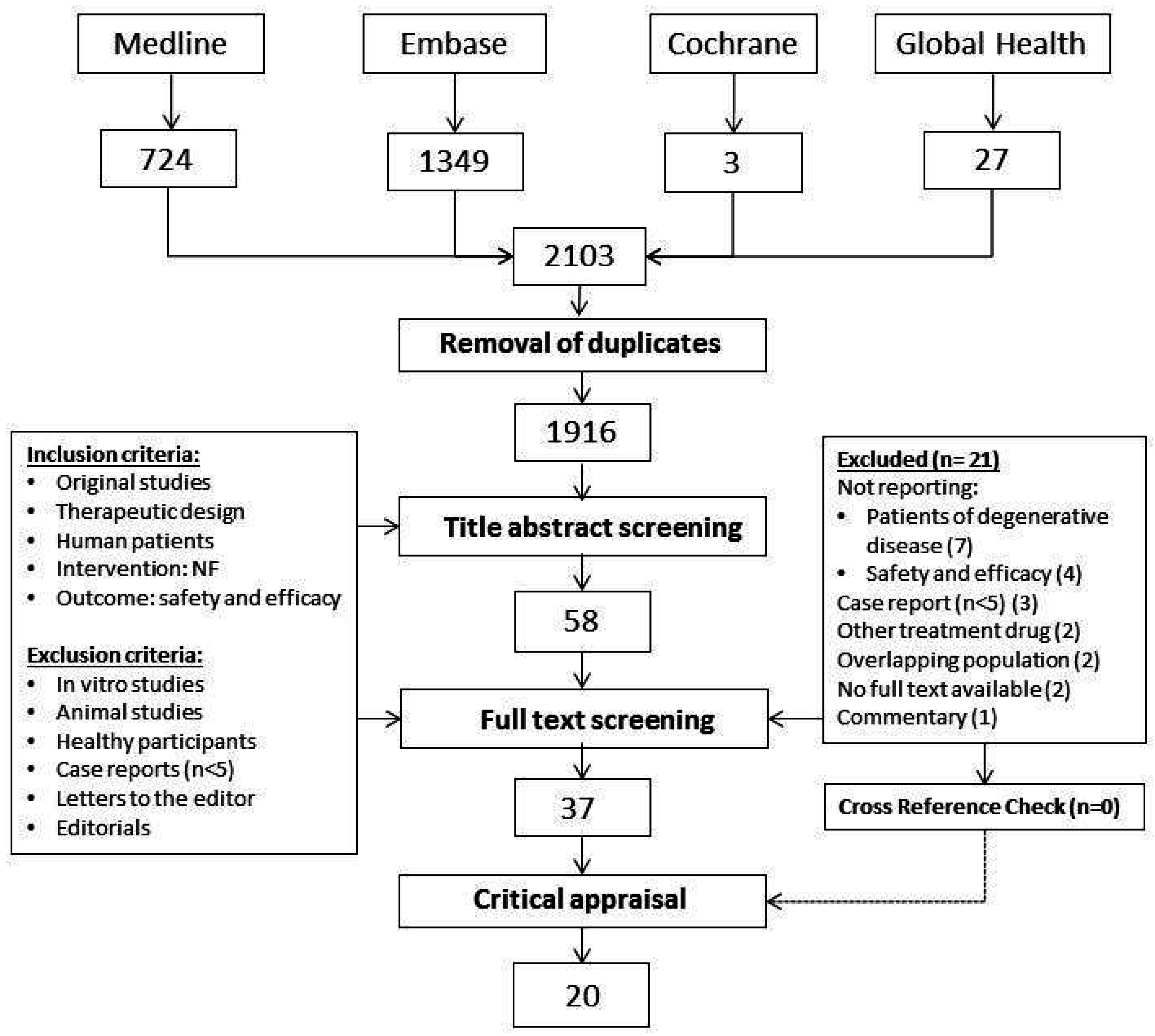
2.1. Search and Selection
2.2. Critical Appraisal Assessment
2.3. Patient Characteristics and Treatment Modalities in Selected Studies
2.4. Safety Assessment and Adverse Events Reported in Selected Studies
2.5. Relatedness of Adverse Events to the Study Drug in Selected Studies
2.6. Efficacy Assessment from Selected Studies
2.7. Influence of the Administration Route and Size of the Effect
3. Discussion
3.1. Safety and Efficacy of Neurotrophic Therapies of Degenerative Disorders
3.2. Translation from Animal Studies to Human Trials
3.3. Applicability of NF Therapies to the Auditory Nerve
4. Methods
4.1. Search Strategy and Study Selection
4.2. Quality Assessment of Selected Study
4.3. Data Extraction
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Database | Syntax | |
|---|---|---|
| Medline | 1 | Exp Nerve Growth Factors/ |
| 2 | (BDNF or (brain adj3 (neurotrophic* or neuro trophic* or neurotropic* or neuro tropic*))).tw. | |
| 3 | (NGF or ((nerve or neurotropic* or neuro tropic* or neurotrophi* or neuro trophi* or neuronotrophi* or neurono trophi* or neurite*) adj3 (outgrowth or growth or factor*)).tw. | |
| 4 | 1 or 2 or 3 | |
| 5 | Animals/ not (Animals/ and Humans/) | |
| 6 | 4 not 5 | |
| 7 | Exp In Vitro Techniques/ | |
| 8 | “in vitro”.ti. | |
| 9 | 7 or 8 | |
| 10 | 6 not 9 | |
| 11 | Limit 10 to yr=”1995-Current” | |
| 12 | Limit 11 to clinical trial, all | |
| Embase | Modeled search strategy for Medline, in title/abstract | |
| Cochrane | Modeled search strategy for Cochrane, in title/abstract | |
| Global health | Modeled search strategy for Global Health, in title/abstract | |
References
- Seyyedi, M.; Viana, L.M.; Nadol, J.B. Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otol. Neurotol. 2014, 35, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Sly, D.J.; Hampson, A.J.; Minter, R.L.; Heffer, L.F.; Li, J.; Millard, R.E.; Winata, L.; Niasari, A.; O’Leary, S.J. Brain-derived neurotrophic factor modulates auditory function in the hearing cochlea. J. Assoc. Res. Otolaryngol. 2012, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pinyon, J.L.; Tadros, G.D.; Froud, K.E.; Wong, A.C.Y.; Tompson, I.T.; Crawford, E.N.; Ko, M.; Morris, R.; Klugmann, M.; Housley, G.D. Close-field electroporation gene delivery using the cochlear implant electrode array enhances the bionic ear. Sci. Transl. Med. 2014, 6, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Ramekers, D.; Versnel, H.; Strahl, S.B.; Klis, S.F.L.; Grolman, W. Temporary neurotrophin treatment prevents deafness-induced auditory nerve degeneration and preserves function. J. Neurosci. 2015, 35, 12331–12345. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Liberman, L.D.; Maison, S.F. Efferent feedback slows cochlear aging. J. Neurosci. 2014, 34, 4599–4607. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015, 300, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Ramekers, D.; Versnel, H.; Grolman, W.; Klis, S.F.L. Neurotrophins and their role in the cochlea. Hear Res 2012, 288, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, P.J.; Wise, A.K.; Flynn, B.O.; Nayagam, B.A.; Hume, C.R.; O’Leary, S.J.; Shepherd, R.K.; Richardson, R.T. Neurotrophin gene therapy for sustained neural preservation after deafness. PLoS ONE 2012, 7, e52338. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H. Gene therapy for neurological disorders. Expert Opin. Biol. Ther. 2003, 8, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. The neurotrophin family of neurotrophic factors: An overview. Methods Mol. Biol. 2012, 846, 1–12. [Google Scholar] [PubMed]
- Levi-Montalcini, R.; Hamburger, V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J. Exp. Zool. 1951, 116, 321–361. [Google Scholar] [CrossRef] [PubMed]
- Weissmiller, A.M.; Chengbiao, W. Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl. Neurodegener. 2012, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Chi, D.H.; P’Keeffe, L.J.; Kruszka, P.; Raphael, Y.; Altschuler, R.A. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int. J. Dev. Neurosci. 1997, 15, 631–643. [Google Scholar] [CrossRef]
- Miller, J.M.; Miller, A.L.; Yamagata, T.; Bredberg, G.; Altschuler, R.A. Protection and regrowth of the auditory nerve after deafness: Neurotrophins, antioxidants and depolarization are effective in vivo. Audiol. Neurotol. 2002, 7, 175–179. [Google Scholar] [CrossRef]
- Agterberg, M.J.H.; Versnel, H.; de Groot, J.C.M.J.; Smoorenburg, G.F.; Albers, F.W.J.; Klis, S.F.L. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res. 2008, 244, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Agterberg, M.J.H.; Versnel, H.; van Dijk, L.M.; de Groot, J.C.M.J.; Klis, S.F.L. Enhanced survival of spiral ganglion cells after cessation of treatment with brain-derived neurotrophic factor in deafened guinea pigs. J. Assoc. Res. Otolaryngol. 2009, 10, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Havenith, S.; Versnel, H.; Agterberg, M.J.H.; de Groot, J.C.M.J.; Sedee, R.J.; Grolman, W.; Klis, S.F.L. Spiral ganglion cell survival after round window membrane application of brain-derived neurotrophic factor using gelfoam as carrier. Hear Res. 2011, 272, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Havenith, S.; Versnel, H.; Klis, S.F.L.; Grolman, W. Local delivery of brain-derived neurotrophic factor on the perforated round window membrane in guinea pigs: A possible clinical application. Otol. Neurotol. 2015, 36, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Corfas, G.; Liberman, M.C. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci. Rep. 2016, 6, 24907. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, L.N.; Shepherd, R.K. Clinical application of neurotrophic factors: The potential for primary auditory neuron protection. Eur. J. Neurosci. 2005, 22, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Sendter, M.; Schmalbruch, H.; Stockli, K.A.; Carroll, P.; Kreutzberg, G.W.; Thoenen, H. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature 1992, 358, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; Ikeda, K.; Klinkosz, B.; Cedarbaum, J.M.; Wong, V.; Lindsay, R.M. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science 1994, 65, 1107–1110. [Google Scholar] [CrossRef]
- Tomac, A.; Lindqvist, E.; Lin, L.F.N.; Ogren, S.O.; Young, D.; Hoffer, B.J.; Olson, L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 1995, 373, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Gash, D.M.; Zhang, Z.; Ovadia, W.A.G.; Cass, W.A.; Yi, A.; Simmerman, L.; Russell, D.; Martin, D.; Lapchak, P.A.; Collins, F.; et al. Functional recovery in Parkinsonian monkeys treated with GDNF. Nature 1996, 380, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Ekestern, E. Neurotrophic factors and amyotrophic lateral sclerosis. Neurodegener. Dis. 2004, 1, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.B.; Soderstrom, K.; Bakay, R.A.; Korfower, J.H. Neurotrophic factor therapy for Parkinson’s disease. Prog. Brain Res. 2010, 184, 237–264. [Google Scholar] [PubMed]
- Cedarbaum, J.M.; ALS CNTF Treatment Study Group. A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. Neurology 1996, 46, 1244–1249. [Google Scholar]
- Miller, R.G.; Petajan, J.H.; Bryan, W.W.; Armon, C.; Barohn, R.J.; Goodpasture, J.C.; Hoagland, R.J.; Parry, G.J.; Ross, M.A.; Stromatt, S.C. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. Ann. Neurol. 1996, 39, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendsen, C.N.; Heywood, P. Direct brain infusion of glial cell line–derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.K.; Bunnage, M.; Plaha, P.; Svendsen, C.N.; Heywood, P.; Gill, S.S. Intraputamenal infusion of glial cell line–derived neurotrophic factor in PD: A two-year outcome study. Ann. Neurol. 2005, 57, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Burchiel, K.J.; Comella, C.L.; Jankovic, J.; Lang, A.E.; Laws, E.R., Jr.; Lozano, A.M.; Penn, R.D.; Simpson, R.K., Jr.; Stacy, M.; et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 2003, 60, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Slevin, J.T.; Gerhardt, G.A.; Smith, C.D.; Gash, D.M.; Kryscio, R.; Young, B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line–derived neurotrophic factor. J. Neurosurg. 2005, 102, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Slevin, J.T.; Gash, D.M.; Smith, C.D.; Gash, D.M.; Kryscio, R.; Young, B. Unilateral intraputaminal glial cell line–derived neurotrophic factor in patients with Parkinson disease: Response to 1 year each of treatment and withdrawal. J. Neurosurg. 2006, 20, E1. [Google Scholar] [CrossRef] [PubMed]
- Slevin, J.T.; Gash, D.M.; Smith, C.D.; Gerhardt, G.A.; Kryscio, R.; Chebrolu, H.; Walton, A.; Wagner, R.; Young, A.B. Unilateral intraputamenal glial cell line–derived neurotrophic factor in patients with Parkinson disease: Response to 1 year of treatment and 1 year of withdrawal. J. Neurosurg. 2007, 106, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Borasio, G.D.; Robberecht, W.; Leigh, P.N.; Emile, J.; Guiloff, R.J.; Jerusalem, F.; Silani, V.; Vos, P.E.; Wokke, J.H.; Dobbins, T. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. Neurology 1998, 51, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Aloe, L.; Russo, A.; Cesari, M.; Onder, G.; Bonini, S.; Carbonin, P.U.; Bernabei, R. Topical treatment of pressure ulcers with nerve growth factor. A randomized clinical trial. Ann. Intern. Med. 2003, 139, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Bensa, S.; Hadden, R.D.M.; Hahn, A.; Hughes, R.A.C.; Willison, H.J. Randomized controlled trial of brain-derived neurotrophic factor in Guillain-Barre syndrome: A pilot study. Eur. J. Neurol. 2000, 7, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.C.; Felice, K.J.; Festoff, B.W.; Gawel, M.J.; Gelinas, D.F.; Kratz, R.; Murphy, M.F.; Natter, H.M.; Norris, F.H.; Rudnicki, S.A. Effect of recombinant human insulin-like growth factor on progression of ALS. A placebo-controlled study. Neurology 1997, 49, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- The ALS CNTF Treatment Study (ACTS) Phase I-II Study Group. A phase I study of recombinant human ciliary neurotrophic factor (rHCNTF) in patients with amyotrophic lateral sclerosis. Clin. Neuropharmacol. 1995, 18, 515–532. [Google Scholar]
- Miller, R.G.; Bryan, W.W.; Dietz, M.A.; Munsat, T.L.; Petajan, J.H.; Smith, S.A.; Goodpasture, J.C. Toxicity and tolerability of recombinant human ciliary neurotrophic factor in patients with amyotrophic lateral sclerosis. Neurology 1996a, 47, 1329–1331. [Google Scholar] [CrossRef]
- Ochs, G.; Penn, R.D.; York, M.; Giess, R.; Beck, M.; Tonn, J.; Haigh, J.; Malta, E.; Traub, M.; Sendtner, M.; et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 201–206. [Google Scholar] [CrossRef]
- Lang, A.E.; Gill, S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P.; et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, E.J.; Windbank, A.J.; Mandrekar, J.N.; Bamlet, W.R.; Appel, S.H.; Armon, C.; Barkhaus, P.E.; Bosch, P.; Boylan, K.; David, W.S.; et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology 2008, 71, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Apfel, S.C.; Schwarts, S.; Adornato, B.T.; Freeman, R.; Biton, V.; Rendell, M.; Vinik, A.; Giuliani, M.; Stevens, J.C.; Barbano, R.; et al. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: A randomized controlled trial. rhNGF Clinical Investigator Group. JAMA 2000, 284, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Wellmer, A.; Misra, V.P.; Sharief, M.; Kopelman, P.G.; Anand, P. A double-blind placebo-controlled clinical trial of recombinant human brain-derived neurotrophic factor (rhBDNF) in diabetic polyneuropathy. J. Peripher. Nerv. Syst. 2001, 6, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Rolan, P.E.; O’Neil, G.; Versage, E.; Rana, J.; Tang, Y.; Galluppi, G.; Aycardi, E. First-in-human, double-blind, placebo-controlled, randomized, dose-escalation study of BG00010, a glial cell line-derived neurotrophic factor family member, in subjects with unilateral sciatica. PLoS ONE 2015, 10, e0125034. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.G.; Weleber, R.G.; Duncan, J.L.; Jaffe, G.J.; Tao, W. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am. J. Ophthalmol. 2013, 156, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Apfel, S.C.; Kessler, J.A.; Adornato, B.T.; Litchy, W.J.; Sanders, C.; Rask, C.A. Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. Neurology 1998, 51, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, M.P.; Littlejohn, T.W.; Schwartz, S.L.; Weiss, S.R.; McIlwain, H.H.; Heymsfield, S.B.; Bray, G.A.; Roberts, W.G.; Heyman, E.R.; Stambler, N.; et al. Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults: A randomized, dose-ranging study. JAMA 2003, 289, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hopkins, J.J.; Heier, J.S.; Birch, D.G.; Halperin, L.S.; Albini, T.A.; Brown, D.M.; Jaffe, G.J.; Tao, W.; Williams, G.A. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6241–6245. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Kumakawa, K.; Usami, S.I.; Hato, N.; Tabuchi, K.; Takahashi, M.; Fujiwara, K.; Sasaki, A.; Komune, S.; Sakamoto, T.; et al. A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med. 2014, 12, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arakawa, Y.; Sendtner, M.; Thoenen, H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: Comparison with other neurotrophic factors and cytokines. J. Neurosci. 1990, 10, 3507–3515. [Google Scholar] [PubMed]
- Sendtner, M.; Kreutzberg, G.W.; Thoenen, H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature 1990, 345, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Yuen, E.C.; Mobley, W.C. Therapeutic potential of neurotrophic factors for neurological disorders. Ann. Neurol. 1996, 40, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Perel, P.; Roberts, I.; Sena, E.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.; Mignini, L.E.; Jayaram, P.; Khan, K.S. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. BMC Med. 2007, 334, 197. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Ramirez, G.A.; Kuhn, H.G.; Peterson, D.A.; Day-Lollini, P.A.; Stewart, G.R.; Tuszynski, M.H.; Gage, F.H.; Thal, L.J. Reversible Schwann cell hyperplasia and sprouting of sensory and sympathetic neurites after intraventricular administration of nerve growth factor. Ann. Neurol. 1997, 41, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Thoenen, H.; Sendtner, M. Neurotrophins: From enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat. Neurosci. 2002, 5, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.; Pitzer, C.; Schneider, A. Neurotrophic Growth Factors for the Treatment of Amyotrophic Lateral Sclerosis: Where Do We Stand? Front. Neurosci. 2010, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Sakane, T.; Pardridge, W.M. Carboxyl-directed pegylation of brain-derived neurotrophic factor markedly reduces systemic clearance with minimal loss of biologic activity. Pharm. Res. 1997, 14, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N. Neurotrophic factors: Promising candidates in tissue regeneration. Neural Regen. Res. 2016, 11, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting Items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
| Study | Directness of Evidence (DoE) | DoE Score | Risk of Bias (RoB) | RoB Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication Year | Study Design | Indication for Treatment | Demographic Data | Treatment Approach | Efficacy Outcome Measures | Safety Assessment | Follow-Up | Randomization | Blinding | Standardization (T) | Standardization (O) | Standardization (FU) | Missing Data | |||
| Cedarbaum et al. [39] | 1995 | RCT | ● | ◑ | ● | ○ | ● | ◑ | M | ● | ● | ◑ | ● | ● | ● | L |
| Cedarbaum [27] | 1996 | RCT | ● | ◑ | ● | ● | ◑ | ● | H | ● | ● | ○ | ● | ● | ● | L |
| Miller et al. [40] | 1996a | RCT | ● | ◑ | ● | ○ | ● | ◑ | M | ● | ◑ | ◑ | ● | ● | ● | L |
| Miller et al. [28] | 1996b | RCT | ● | ◑ | ● | ● | ● | ◑ | H | ● | ◑ | ◑ | ● | ● | ● | L |
| Lai et al. [38] | 1997 | RCT | ● | ◑ | ● | ● | ● | ○ | M | ● | ● | ● | ● | ● | ● | L |
| Apfel et al. [48] | 1998 | RCT | ● | ◑ | ● | ● | ● | ◑ | H | ● | ● | ◑ | ● | ● | ◑ | L |
| Borasia et al. [35] | 1998 | RCT | ● | ○ | ● | ● | ◑ | ◑ | M | ● | ● | ● | ● | ● | ● | L |
| Apfel et al. [44] | 2000 | RCT | ● | ◑ | ● | ● | ● | ○ | M | ● | ● | ● | ● | ● | ● | L |
| Bensa et al. [37] | 2000 | RCT | ● | ● | ● | ○ | ● | ○ | M | ● | ● | ● | ● | ● | ◑ | L |
| Ochs et al. [41] | 2000 | RCT | ● | ◑ | ● | ○ | ● | ◑ | M | ● | ● | ○ | ● | ● | ● | L |
| Wellmer et al. [45] | 2001 | RCT | ● | ◑ | ● | ● | ● | ◑ | H | ● | ● | ◑ | ● | ● | ◑ | L |
| Ettinger et al. [49] | 2003 | RCT | ● | ◑ | ● | ● | ● | ● | H | ● | ● | ◑ | ● | ● | ● | L |
| Nutt et al. [31] | 2003 | RCT | ● | ◑ | ● | ● | ● | ◑ | H | ● | ● | ◑ | ● | ● | ◑ | L |
| Landi et al. [36] | 2003 | RCT | ● | ● | ● | ● | ○ | ● | H | ● | ● | ● | ● | ● | ● | L |
| Lang et al. [42] | 2006 | RCT | ● | ◑ | ● | ● | ● | ◑ | H | ● | ● | ● | ● | ● | ● | L |
| Sorenson et al. [43] | 2008 | RCT | ● | ◑ | ● | ● | ● | ● | H | ● | ● | ● | ● | ● | ● | L |
| Zhang et al. [50] | 2011 | RCT | ● | ◑ | ● | ● | ● | ○ | M | ● | ● | ◑ | ● | ● | ● | L |
| Birch et al. [47] | 2013 | RCT | ● | ◑ | ● | ● | ● | ● | H | ● | ◑ | ◑ | ● | ● | ● | L |
| Nakagawa et al. [51] | 2014 | RCT | ● | ◑ | ● | ● | ● | ● | H | ● | ● | ● | ● | ● | ◑ | L |
| Rolan et al. [46] | 2015 | RCT | ● | ◑ | ● | ● | ● | ● | H | ● | ● | ◑ | ● | ● | ● | L |
| Valk et al. | 1996 | RCT | ● | ◑ | ● | ● | ○ | ● | M | ● | ● | ◑ | ● | ● | ○ | M |
| Lambiase et al. | 1998 | CT | ● | ● | ● | ● | ○ | ● | H | ○ | ○ | ◑ | ● | ● | ● | H |
| Bonini et al. | 2000 | CT | ● | ◑ | ● | ● | ○ | ● | M | ○ | ○ | ● | ● | ● | ● | M |
| Parkman et al. | 2003 | RCT | ● | ◑ | ● | ● | ● | ○ | M | ● | ● | ○ | ● | ● | ◑ | M |
| Gill et al. [29] | 2003 | PCS | ● | ● | ● | ● | ● | ● | H | ○ | ○ | ○ | ● | ● | ● | H |
| Patel et al. [30] | 2005 | |||||||||||||||
| Beck et al. | 2005 | RCT | ● | ◑ | ● | ● | ○ | ○ | L | ● | ● | ◑ | ● | ● | ○ | M |
| Tuszynski et al. | 2005 | CT | ● | ◑ | ● | ● | ○ | ● | M | ○ | ○ | ● | ● | ● | ◑ | H |
| Slevin et al. | 2005 | PCS | ● | ● | ● | ● | ● | ● | H | ○ | ○ | ◑ | ● | ● | ● | H |
| Slevin et al. | 2006 | |||||||||||||||
| Slevin et al. | 2007 | |||||||||||||||
| Nguyen et al. | 2006 | RCT | ● | ◑ | ● | ○ | ● | ◑ | M | ● | ○ | ◑ | ● | ● | ● | M |
| Sieving et al. | 2006 | PCS | ● | ◑ | ● | ● | ● | ● | H | ○ | ○ | ● | ● | ● | ○ | H |
| Marks et al. | 2008 | CT | ● | ● | ● | ● | ● | ● | H | ○ | ○ | ◑ | ● | ● | ● | H |
| Nguyen et al. | 2009 | RCT | ● | ◑ | ● | ○ | ● | ● | M | ● | ○ | ◑ | ● | ● | ● | M |
| Zhou et al. | 2009 | RCT | ● | ○ | ● | ● | ○ | ◑ | L | ● | ○ | ○ | ● | ● | ● | M |
| Sacca et al. | 2011 | RCT | ● | ◑ | ● | ● | ● | ○ | M | ● | ● | ○ | ● | ● | ◑ | M |
| Zein et al. | 2014 | CT | ● | ● | ● | ● | ● | ● | H | ○ | ○ | ● | ● | ● | ◑ | H |
| Chew et al. | 2015 | CT | ● | ◑ | ● | ● | ● | ● | H | ○ | ○ | ◑ | ● | ● | ● | H |
| Tan et al. | 2015 | CT | ● | ● | ● | ● | ○ | ○ | M | ○ | ○ | ◑ | ● | ● | ● | H |
| Directness of Evidence (DoE) | |
|---|---|
| Study design | CT, clinical trial PCS, prospective case series RCS, retrospective case series RCT, randomized control trial |
| Indication for treatment Diagnosis | Clearly reported, ● Not clearly reported, ○ |
| Demographic data Age at treatment | Individually reported, ● Means reported, ◑ Not reported, ○ |
| Treatment approach NF used, dosage, route of administration | Reported, ● Not reported, ○ |
| Efficacy outcome measures Pre and post treatment assessment | Reported, ● Not reported, ○ |
| Safety assessment Quantifiable adverse events per patient If drug was attributed to reported adverse events | Reported per patient or per event, ● Events reported but not quantified, ◑ Not reported, ○ |
| Follow-up Duration of follow-up at the end of treatment for all tested individuals | ˃2 months, ● <2 months, ◑ not reported, ○ |
| Overall DoE score | High, ≥5 points Moderate, between 4–5 points Low, <4 |
| Risk of Bias (RoB) | |
| Randomization | Randomized or concealed, ● Not randomized or concealed, ○ |
| Blinding | Blinding of patient, researcher, observer, ● Single blind, ◑ No blinding, ○ |
| Standardization of treatment | All patients received the same therapy, ● Different types of NFs or dosage used, ◑ Dosage modified throughout trial, ○ |
| Standardization of outcome measures | Identical outcome reports, ● Reported however not standardized, ◑ Not reported, ○ |
| Standardization of follow up | Identical follow up for all patients, ● Reported however not standardized, ◑ Not reported, ○ |
| Missing data | No missing data; missing data mentioned/quantified and Method of handling described, ● Missing data mentioned in study but method of handling Not described, ◑ Missing data not reported, ○ |
| Overall RoB score | Low, ≥5 points Moderate, between 4–5 points High, <4 |
| Study | Diagnosis | Total pts (NF Group) | NF | Dose | Administration | Safety Conclusion | Efficacy Conclusion |
|---|---|---|---|---|---|---|---|
| Cedarbaum et al. (1995) [39] | ALS | 57 (43) | CNTF | 0.5, 1, 3, 7, 10, 30 µg/kg | Thrice weekly s/c injection per week for 2 weeks | Safe | N/A |
| Cedarbaum et al. (1996) [27] | ALS | 730 (485) | CNTF | 15, 30 µg/kg | Thrice weekly s/c injection per week for 9 months | Not safe | Not effective |
| Miller et al. (1996b) [28] | ALS | 44 (33) | CNTF | 0.5, 2, 5, 10, 20 µg/kg | Thrice weekly s/c injection for 1 month | Safe (0.5, 2, and 5 µg/kg) | N/A |
| Miller et al. (1996a) [40] | ALS | 483 (360) | CNTF | 0.5, 2, 5 µg/kg | Daily s/c injection for 6 months | Safe | Not effective |
| Lai et al. (1997) [38] | ALS | 266 (176) | IGF-I | 0.05, 0.1 mg/kg | Twice daily s/c injection for 9 months | Safe | Effective |
| Apfel et al. (1998) [48] | Diabetic polyneuropathy | 250 (168) | NGF | 0.1, 0.3 μg/kg | Thrice weekly s/c injection per week for 6 months | Safe | Effective |
| Borasio et al. (1998) [35] | ALS | 183 (124) | IGF-I | 0.10 mg/kg | Daily s/c injection for 9 months | Safe | Not effective |
| Apfel et al. (2000) [44] | Diabetic polyneuropathy | 836 (394) | NGF | 0.1 μg/kg | Thrice weekly s/c injection per week for 12 months | Safe | Not effective |
| Bensa et al. (2000) [37] | Guillain–Barre syndrome | 10 (6) | BDNF | 25 µg/kg | Daily s/c injection for a maximum of 6 months | Safe | Not effective |
| Ochs et al. (2000) [41] | ALS | 25 (20) | BDNF | 25, 60, 150, 400, 1000 µg/kg | Daily intrathecal delivery for 3 months | Safe (25, 60, 150 µg/day) | N/A |
| Wellmer et al. (2001) [45] | Diabetic polyneuropathy | 27 (19) | BDNF | 25, 100 µg/kg | Daily s/c injection for 3 months | Safe | Not effective |
| Nutt et al. (2003) [31] | PD | 50 (38) | GDNF | 150, 361, 559, 1588, 3311 µg | ICV for 8 months | Not safe | Not effective |
| Ettinger et al. (2003) [49] | Obesity | 173 (141) | CNTF | 0.3,1, 2 µg/kg | Daily s/c injection for 2–3 months | Safe (0.3, 1 µg/kg) | Effective |
| Landi et al. (2003) [36] | Pressure ulcer of the foot | 36 (18) | NGF | 50 µg | Daily topical drop for a maximum of 6 weeks | Safe | Effective |
| Lang et al. (2006) [42] | PD | 34 (17) | GDNF | 14 µg | Daily intraputamenal continuous infusion for 6 months | Safe | Not effective |
| Sorenson et al. (2008) [43] | ALS | 302 (150) | IGF-I | 0.05 mg/kg | Twice daily s/c injections for 2 years | Safe | Not effective |
| Zhang et al. (2011) [50] | Macular degeneration | 36 (24) | CNTF | 5 or 10 ng daily release | Intraocular encapsulated cell implant for 12 months | Safe | Effective |
| Birch et al. (2013) [47] | Retinitis pigmentosa | 266 (133) | CNTF3 CNTF4 | 5 or 20 ng daily release | Intraocular encapsulated cell implant for 12 months | Safe | Not effective |
| Nakagawa et al. (2014) [51] | Sudden deafness | 118 (60) | IGF-I | 10 mg/mL | Intratympanic Gelfoam | Safe | Effective |
| Rolan et al. (2015) [46] | Unilateral sciatica | 48 (36) | GDNF | 0.3, 1, 3, 10, 25, 50, 100, 200, 400, 800 μg/kg | i/v or s/c injection of a single dose | Safe | Not effective |
| Characteristics | Included, n (%) |
|---|---|
| n, total patients in trials | 3974 |
| n, patients in NF group | 2445 (61.5%) |
| n, patients in placebo group | 1529 (38.5%) |
| Age at treatment, in years (NF group) | |
| Mean ± SD | 55.2 ± 10.4 |
| Diagnosis, n, patients | |
| ALS | 2090 (52.6%) |
| Diabetic polyneuropathy | 1113 (28%) |
| Retinitis pigmentosa | 266 (6.7%) |
| Obesity | 173 (4.4%) |
| Sudden deafness | 118 (3%) |
| Parkinson’s disease | 84 (2.1%) |
| Sciatica | 48 (1.2%) |
| Macular degeneration | 36 (0.9%) |
| Pressure ulcer of foot | 36 (0.9%) |
| Guillain–Barre syndrome | 10 (0.2%) |
| NF used, n, patients (NF group) | |
| CNTF | 1219 (49.9%) |
| NGF | 580 (23.7%) |
| IGF-I | 510 (20.9%) |
| GDNF | 91 (3.7%) |
| BDNF | 45 (1.8%) |
| Administration route, n, patients | |
| s/c | 3385 (85.2%) |
| Intraocular encapsulated implant | 302 (7.6%) |
| Intratympanic gelfoam infiltrated | 118 (3%) |
| ICV | 50 (1.3%) |
| Topical | 36 (0.9%) |
| Intraputamenal | 34 (0.9%) |
| Intrathecal | 25 (0.6%) |
| i/v | 24 (0.6%) |
| Administration type, n, patients | |
| Systemic | 3409 (85.8%) |
| Local | 565 (14.2%) |
| Duration of treatment | |
| >6 months | 2669 (67.2%) |
| 1–6 months | 1082 (27.2%) |
| <1 month | 105 (2.6%) |
| Unknown | 118 (3%) |
| Adverse Events in Patients Receiving NF, n, Events | Included, n (%) |
|---|---|
| n of patients receiving NF via injection | 1144 |
| injection site pain or reaction | 699 (61.1%) |
| n of patients receiving NF | 1836 |
| Asthenia, fatigue, weakness | 436 (23.7%) |
| Gastrointestinal disturbances a | 372 (20.3%) |
| Cough | 193 (10.5%) |
| Headache | 173 (9.4%) |
| Mood changes b | 141 (7.7%) |
| Dizziness, vertigo, incoordination | 135 (7.4%) |
| Fever/chills/sweating | 134 (7.3%) |
| Dyspnea/respiratory failure | 127 (6.9%) |
| Weight loss/anorexia | 112 (6.1%) |
| Sensation of warmth/shock/paresthesias | 98 (5.3%) |
| Rhinitis | 69 (3.8%) |
| Ophthalmological symptoms c | 68 (2.7%) |
| Tinnitus | 51 (2.8%) |
| Infection | 40 (2.2%) |
| Pain other than injection site | 40 (2.2%) |
| Dyskinesia | 34 (1.9%) |
| Rash/pruritus | 32 (1.7%) |
| Aural fullness | 32 (1.7%) |
| Hypoglycemia | 21 (1.1%) |
| Peripheral edema/joint swelling/hypertension/IOP | 20 (1.1%) |
| Relatedness of adverse event to NF drug, n, study | |
| n of studies reporting adverse events | 19 |
| Yes | 8 (42.1%) |
| No | 6 (31.6%) |
| Some | 4 (21.1%) |
| N/A | 1 (5.3%) |
| Overall safety assessment, n, study | |
| n of studies reporting on safety | 20 |
| Safe | 15 (75%) |
| Not safe | 2 (10%) |
| Safe for lower doses | 3 (15%) |
| Overall Efficacy Assessment, n, Study | n (%) |
|---|---|
| n of studies reporting on efficacy | 17 |
| Effective | 6 (35.3%) |
| Not effective | 11 (64.7%) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezdjian, A.; Kraaijenga, V.J.C.; Ramekers, D.; Versnel, H.; Thomeer, H.G.X.M.; Klis, S.F.L.; Grolman, W. Towards Clinical Application of Neurotrophic Factors to the Auditory Nerve; Assessment of Safety and Efficacy by a Systematic Review of Neurotrophic Treatments in Humans. Int. J. Mol. Sci. 2016, 17, 1981. https://doi.org/10.3390/ijms17121981
Bezdjian A, Kraaijenga VJC, Ramekers D, Versnel H, Thomeer HGXM, Klis SFL, Grolman W. Towards Clinical Application of Neurotrophic Factors to the Auditory Nerve; Assessment of Safety and Efficacy by a Systematic Review of Neurotrophic Treatments in Humans. International Journal of Molecular Sciences. 2016; 17(12):1981. https://doi.org/10.3390/ijms17121981
Chicago/Turabian StyleBezdjian, Aren, Véronique J. C. Kraaijenga, Dyan Ramekers, Huib Versnel, Hans G. X. M. Thomeer, Sjaak F. L. Klis, and Wilko Grolman. 2016. "Towards Clinical Application of Neurotrophic Factors to the Auditory Nerve; Assessment of Safety and Efficacy by a Systematic Review of Neurotrophic Treatments in Humans" International Journal of Molecular Sciences 17, no. 12: 1981. https://doi.org/10.3390/ijms17121981






