Preparation of a Bis-GMA-Free Dental Resin System with Synthesized Fluorinated Dimethacrylate Monomers
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of FDMA
4.3. Preparation of Resin Formulation
4.4. Measurement of Viscosity of Dental Resin
4.5. Measurement of Double Bond Conversion
4.6. Measurement of Volumetric Shrinkage
4.7. Measurement of Water Sorption and Solubility
4.8. Measurement of Flexural Strength and Modulus
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Lassila, L.V.J.; Vallittu, P.K. Synthesis of antibacterial and radio-opaque dimethacrylate monomers and their potential application in dental resin. Dent. Mater. 2014, 30, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U. New developments of polymeric dental composites. Prog. Polym. Sci. 2001, 26, 535–576. [Google Scholar] [CrossRef]
- Liu, D.; Liu, F.; He, J.; Lassila, L.V.J.; Vallittu, P.K. Synthesis of a novel tertiary amine containing urethane dimethacrylate monomer (UDTMA) and its application in dental resin. J. Mater. Sci. Mater. Med. 2013, 24, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Guo, S.; Liu, F.; He, J. Synthesis of fluorinated dimethacrylate monomer and its application in preparing Bis-GMA free dental resin. J. Mater. Sci. Mater. Med. 2015, 51, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I.M. Structure-Property relationships in dimethacrylate networks based on Bis-GMA, UDMA and TEGDMA. Dent. Mater. 2009, 25, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Ellakwa, A.; Cho, N.; Lee, I.B. The effect of resin matrix composition on the polymerization shrinkage and rheological properties of experimental dental composites. Dent. Mater. 2007, 23, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, C.S.; Shelton, Z.R.; Braga, R.R.; Windmoller, D.; Machado, J.C.; Stansbury, J.W. Characterization of dimethacrylate polymeric networks: A study of the crosslinked structure formed by monomers used in dental composites. Eur. Polym. J. 2011, 130, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, K.J.; Mariotti, A. BIS-GMA-based resins in dentistry: are they safe. J. Am. Dent. Assoc. 1999, 130, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; Zhou, Z.; Miao, M.; He, Y.; Wang, J.; Ferber, J.; Gao, E.; Yuan, W. Urine bisphenol-A (BPA) level in relation to semen quality. Fertil. Steril. 2011, 95, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Ehrlich, S.; Toth, T.L.; Wright, D.L.; Calafat, A.M.; Trisini, A.T.; Ye, X.; Hauser, R. Semen quality and sperm DNA damage in relation to urinary Bisphenol A among men from an infertility clinic. Reprod. Toxicol. 2010, 30, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Song, T.B.; Cai, Y.Y.; Zhou, J.S.; Song, X.; Zhao, X.; Wu, X.L. Bisphenol A exposure induces apoptosis and upregulation of Fas/FasL and caspase-3 expression in the testes of mice. Toxicol. Sci. 2009, 108, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Yamaguchi, K.; Ishikawa, T.; Ando, M.; Chiba, K.; Hashimoto, H.; Shiotani, M.; Fujisawa, M. Molecular changes induced by bisphenol-A in rat Sertoli cell culture. Syst. Biol. Reprod. Med. 2011, 57, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Lubick, N. Cardiovascular health: Exploring a potential link between BPA and heart disease. Environ. Health Perspect. 2010, 118, A116. [Google Scholar] [CrossRef] [PubMed]
- Silver, M.K.; O’Neill, M.S.; Sowers, M.R.; Park, S.K. Urinary bisphenol A and type-2 diabetes in U.S. adults: Data from NHANES 2003–2008. PLoS ONE 2011, 6, e26868. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Preiss, A.; Arenholt-Bindslev, D. Bisphenol-A content of reins monomers and related degradation products. Clin. Oral Investig. 1999, 3, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.C.; Diamond, F.; Eichmiller, F.; Selwitz, R.; Jones, G. Stability of bisphenol A, triethylene-glycol dimethacrylate, and bisphenol A dimethacrylate in whole saliva. Dent. Mater. 2002, 18, 128–135. [Google Scholar] [CrossRef]
- Kadoma, Y.; Tanaka, M. Acid and base-catalyzed hydrolysis of biphenol A-related compounds. Dent. Mater. J. 2000, 19, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Puglar, R.; Olea-Serrano, M.F.; Novillo-Fertrell, A.; Rivas, A.; Pazos, P.; Pedraza, V.; Navajas, J.M.; Olea, N. Determination of Bisphenol A and related aromatic compounds released from Bis-GMA-based composites and sealants by high performance liquid chromatography. Environ. Health Perspect. 2000, 108, 21–27. [Google Scholar]
- Papakonstantinou, A.E.; Eliades, T.; Cellesi, F.; Watts, D.C.; Silikas, N. Evaluation of UDMA’s potential as a substitute for Bis-GMA in orthodontic adhesives. Dent. Mater. 2013, 29, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Floyd, C.J.E.; Dickens, S.H. Network structure of Bis-GMA- and UDMA-based resin systems. Dent. Mater. 2006, 22, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Magali, D.; Delphine, T.B.; Jacques, D.; Gaёtane, L. Volume contraction in photocured dental resin: The shrinkage-conversion relationship revisited. Dent. Mater. 2006, 22, 359–365. [Google Scholar]
- Liang, X.; Liu, F.; He, J. Synthesis of none Bisphenol A structure dimethacrylate monomer and characterization for dental composite applications. Dent. Mater. 2014, 30, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, F.; He, J. Preparation and characterization of low polymerization shrinkage and Bis-GMA free dental resin system. Adv. Polym. Technol. 2015, 34, 12503. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Fukushima, T.; Horibe, T. Effect of monomer structure on the mechanical properties of light-cured composite resins. Dent. Mater. J. 1989, 8, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, S.; Hirasawa, T. Improvement to light transmittance in light-cured composite resins by the utilisation of low refractive index dimethacrylates. Dent. Mater. J. 1990, 9, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Huang, Q.; Liu, F.; Lin, Z.; He, J. Synthesis of antibacterial methacrylate monomer derived from thiazole and its application in dental resin. J. Mech. Behav. Biomed. Mater. 2015, 49, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, R.B.; Wikant, A.; Mj Øsund, H.; Kopperud, H.M.; Haasum, J.; Gedde, U.W.; Örtengren, U.T. The influence of bis-EMA versus bis GMA on the degree of conversion and water susceptibility of experimental composite materials. Acta Odontol. Scand. 2014, 72, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Podgórski, M. Synthesis and characterization of novel dimethacrylates of different chain lengths as possible dental resins. Dent. Mater. 2010, 26, e188–e194. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Liu, F.; He, J. Preparation and characterization of Bis-GMA free dental resin system with synthesized dimethacrylate monomer TDDMMA derived from tricyclo[5.2.1.0(2,6)]-decanedimethanol. J. Mech. Behav. Biomed. Mater. 2016, 57, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.P.; Braden, M.; Davy, K.W.M. Polymerization shrinkage of methacrylate esters. Biomaterials 1987, 8, 53–56. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Trujilo-Lemon, M.; Lu, H.; Ding, C.; Lin, Y.; Ge, J. Conversion-dependent shrinkage stress and strain in dental resin and composites. Dent. Mater. 2005, 21, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Luo, Y.; Liu, F.; Lin, Z.; Ling, J.; Jia, D. Photopolymerization and properties of fluorene-based dimethacrylate monomer used as root canal sealer. Adv. Polym. Technol. 2008, 27, 108–116. [Google Scholar] [CrossRef]
- He, J.; Liu, F.; Luo, Y.; Jia, D. Synthesis and characterization of a dimethacrylates monomer with low shrinkage and water sorption for dental application. J. Appl. Polym. Sci. 2012, 125, 114–120. [Google Scholar] [CrossRef]
- He, J.; Liu, F.; Vallittu, P.K.; Lassila, L.V.J. Synthesis and characterization of new dimethacrylate monomer and its application in dental resin. J. Biomater. Sci. Polym. Ed. 2013, 24, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, F.; He, J. Preparation of low shrinkage methacrylate-based resin system without Bisphenol A structure by using a synthesized dendritic macromer (G-IEMA). J. Mech. Behav. Biomed. Mater. 2014, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, F.; He, J.; He, Y.; Lin, Z. Preparation of Bis-GMA-free dental restorative composites with dendritic macromer (G-IEMA). Adv. Polym. Technol. 2015, 34, 21519. [Google Scholar] [CrossRef]
- Soderholm, K.J.; Zigan, M.; Ragan, M.; Fischlshweiger, W.; Bergman, M. Hydrolytic degradation of dental composites. J. Dent. Res. 1984, 63, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Costella, A.M.; Trochmann, J.L.; Oliveria, W.S. Water sorption and diffusion coefficient through an experimental dental resin. J. Mater. Sci. Mater. Med. 2010, 21, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Karabela, M.M.; Vouvoudi, E.C. Dynamic thermo-mechanical properties and sorption characteristics of two commercial light cured dental resin composites. Dent. Mater. 2008, 24, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Condon, J.R. Rate of elution of leachable components from composite. Dent. Mater. 1990, 6, 282–287. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Antonucci, J.M. Dimethacrylate monomers with varied fluorine contents and distributions. Dent. Mater. 1999, 15, 166–173. [Google Scholar] [CrossRef]
- Chang, M.C.; Lin, L.D.; Chuang, F.H.; Chan, C.P.; Wang, T.M.; Lee, J.J.; Jeng, P.Y.; Tseng, W.Y.; Lin, H.J.; Jeng, J.H. Carboxylesterase expression in human dental pulp cells: Role in regulation of Bis-GMA-induced prostanoid production and cytotoxicity. Acta Biomater. 2012, 8, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, N.; Kurata, S. Synthesis of dimethacryloxy ethyl-1,1,6,6-tetrahydro-perfluoro-hexamethylene-1,6-dicarbamate as dental base monomers and the mechanical properties of the copolymers of the monomer and methyl methacrylate. Dent. Mater. J. 2011, 30, 103–108. [Google Scholar]


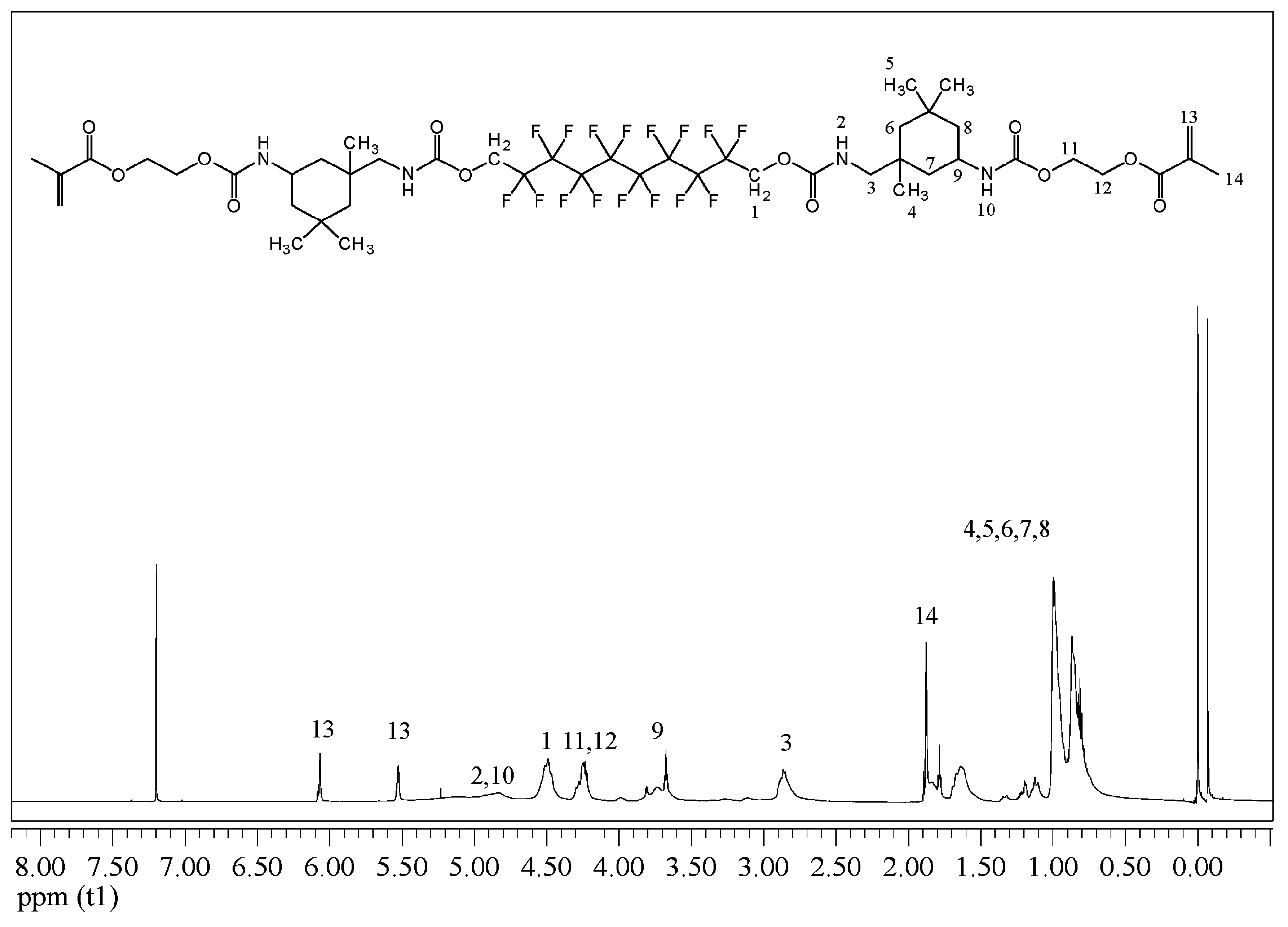
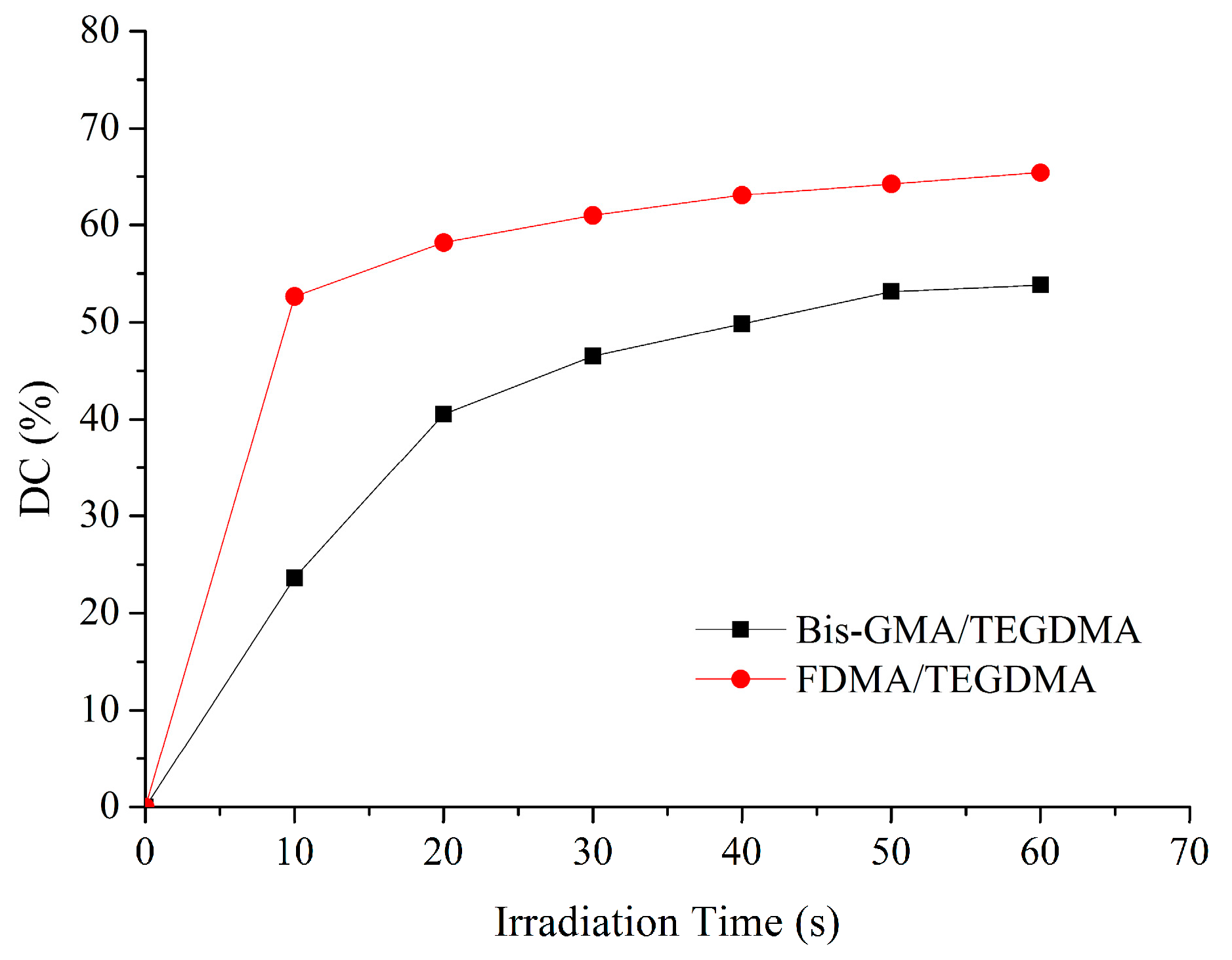
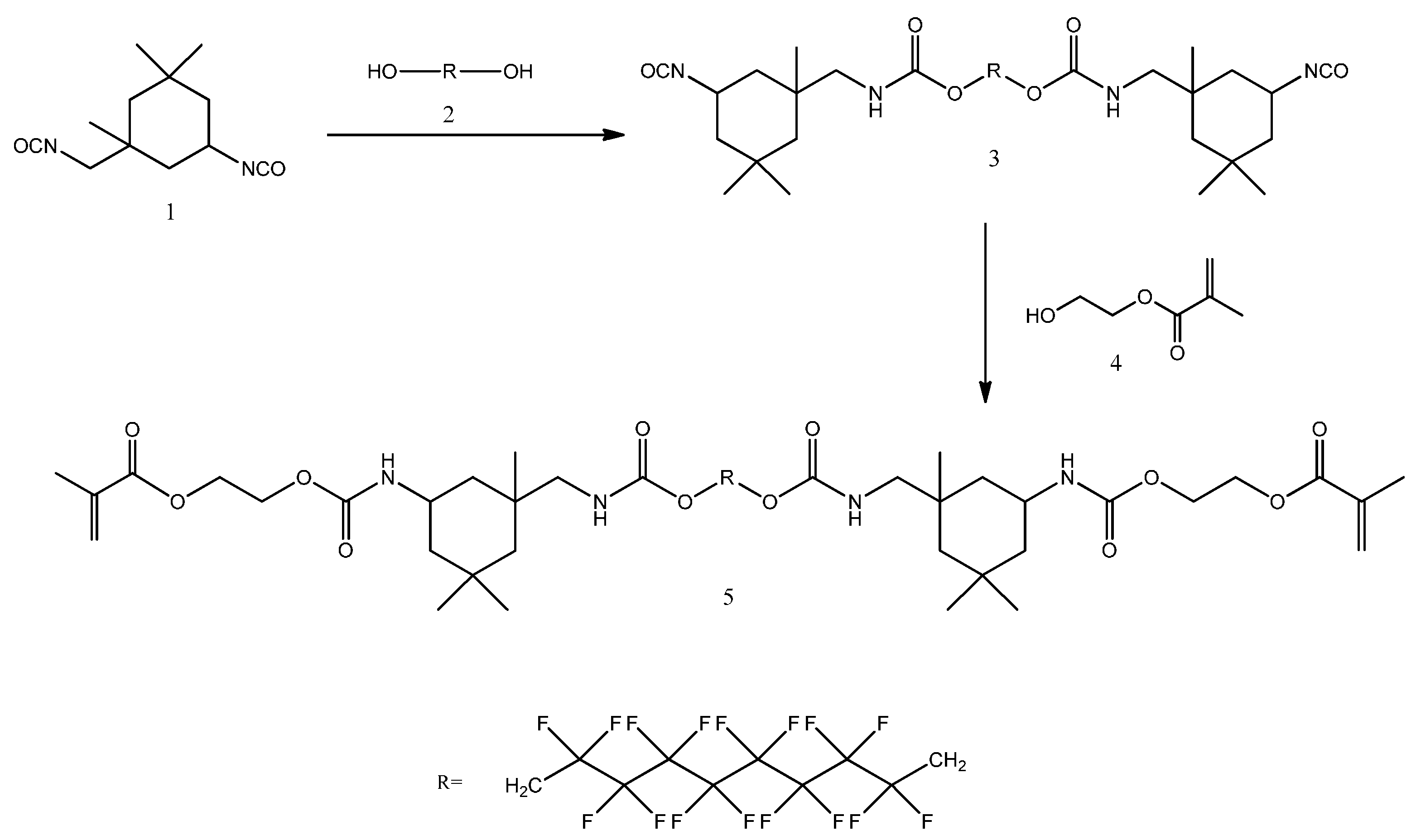
| Resin Formulation | Viscosity (mPa·s) | DC60s (%) | VS (%) |
|---|---|---|---|
| Bis-GMA/TEGDMA | 164.7 ± 1.5 A | 53.8 ± 2.1 a | 9.2 ± 0.8 a |
| FDMA/TEGDMA | 201.7 ± 1.5 B | 65.4 ± 3.7 b | 7.8 ± 0.9 b |
| Resin Formulation | FS (MPa) | FM (GPa) | WS (%) | WSL (%) | ||
|---|---|---|---|---|---|---|
| Before Water Immersion | After Water Immersion | Before Water Immersion | After Water Immersion | |||
| Bis-GMA/TEGDMA | 100.3 ± 6.0 a,A | 77.6 ± 5.3 a,B | 2.38 ± 0.05 a,C | 1.83 ± 0.10 a,D | 4.08 ± 0.05 a | 0.83 ± 0.08 a |
| FDMA/TEGDMA | 96.5 ± 4.6 a,A | 88.6 ± 4.5 b,B | 2.33 ± 0.08 a,C | 1.99 ± 0.13 a,D | 3.85 ± 0.04 b | 1.14 ± 0.09 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Zhu, W.; Liu, F.; He, J. Preparation of a Bis-GMA-Free Dental Resin System with Synthesized Fluorinated Dimethacrylate Monomers. Int. J. Mol. Sci. 2016, 17, 2014. https://doi.org/10.3390/ijms17122014
Luo S, Zhu W, Liu F, He J. Preparation of a Bis-GMA-Free Dental Resin System with Synthesized Fluorinated Dimethacrylate Monomers. International Journal of Molecular Sciences. 2016; 17(12):2014. https://doi.org/10.3390/ijms17122014
Chicago/Turabian StyleLuo, Shuzhen, Wenbin Zhu, Fang Liu, and Jingwei He. 2016. "Preparation of a Bis-GMA-Free Dental Resin System with Synthesized Fluorinated Dimethacrylate Monomers" International Journal of Molecular Sciences 17, no. 12: 2014. https://doi.org/10.3390/ijms17122014





