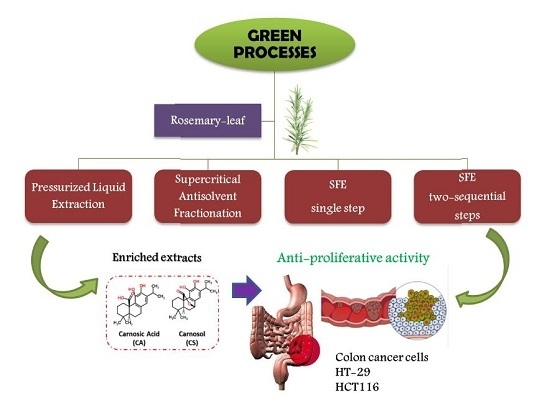
Comparative Study of Green Sub- and Supercritical Processes to Obtain Carnosic Acid and Carnosol-Enriched Rosemary Extracts with in Vitro Anti-Proliferative Activity on Colon Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
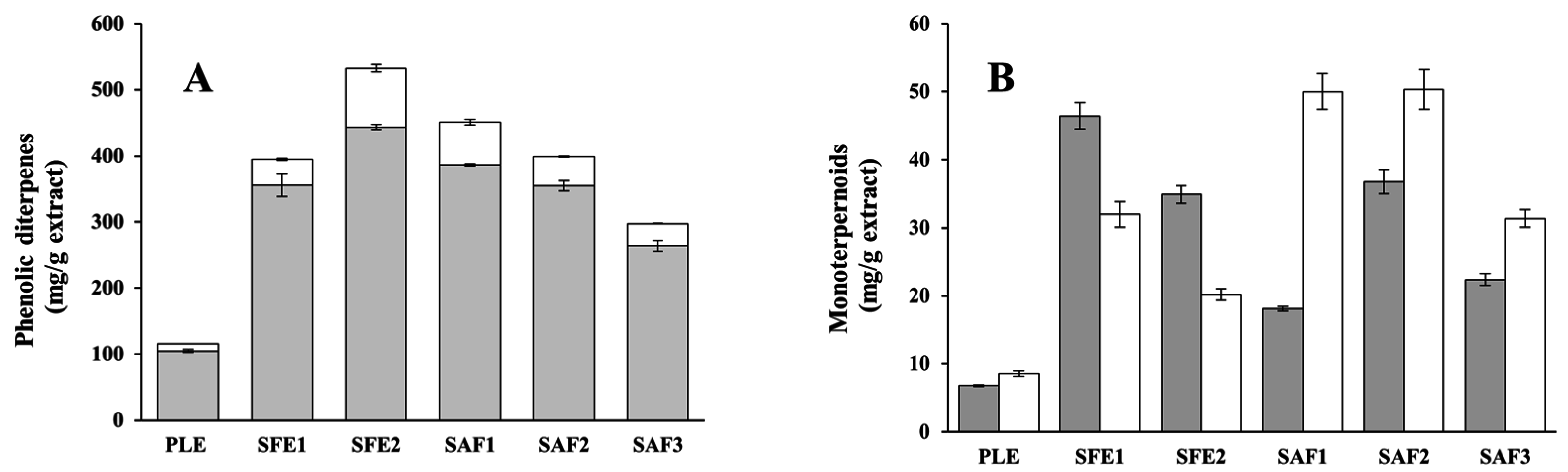
2.1. Yield of Phenolic Compounds and Antioxidant Properties of the Extracts
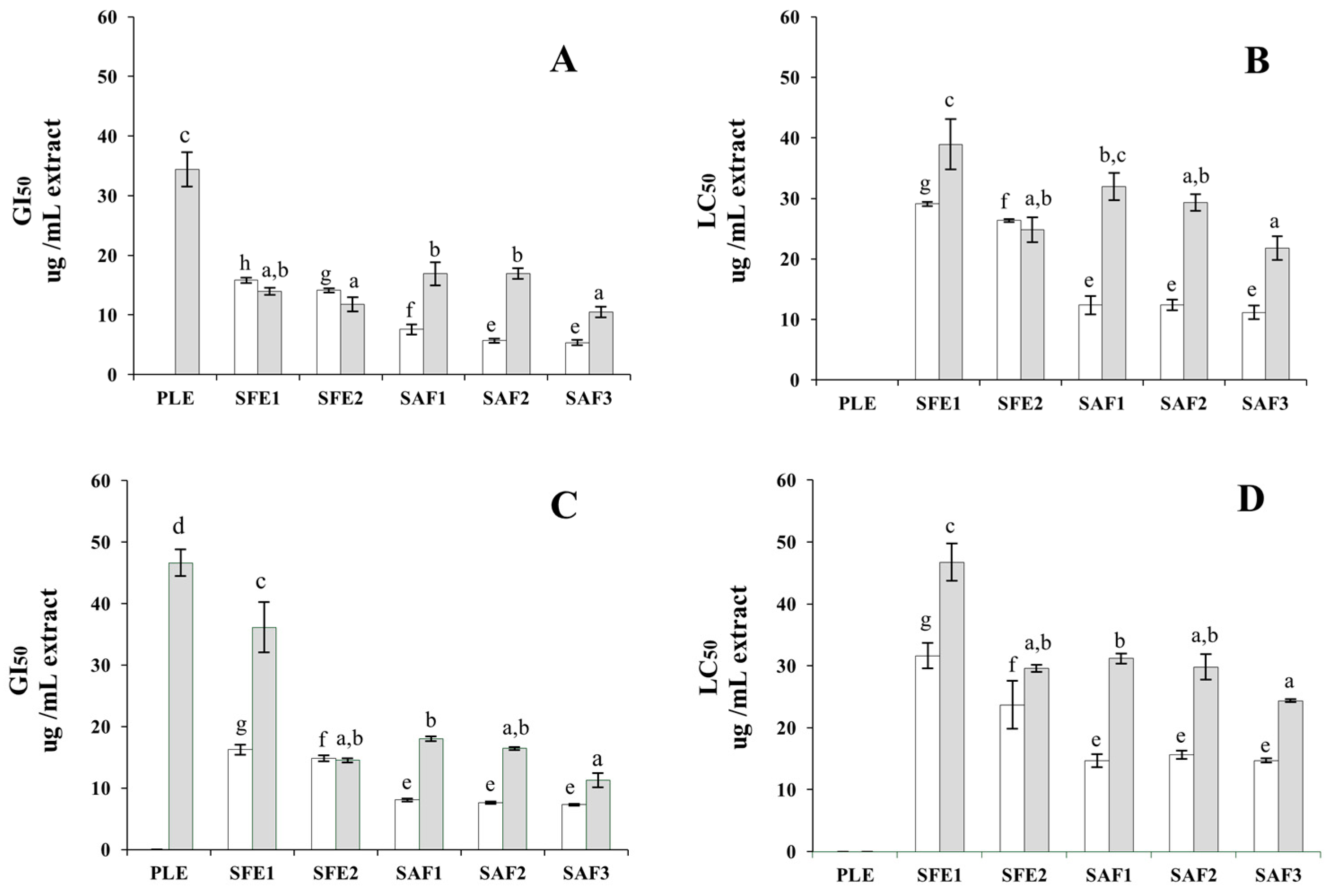
2.2. Anti-Proliferative Activity of the Extracts
2.3. Chemical Characterization of the Extracts by LC-DAD-MS, LC-Q/TOF-MS, and GC-MS
3. Materials and Methods
3.1. Samples and Reagents
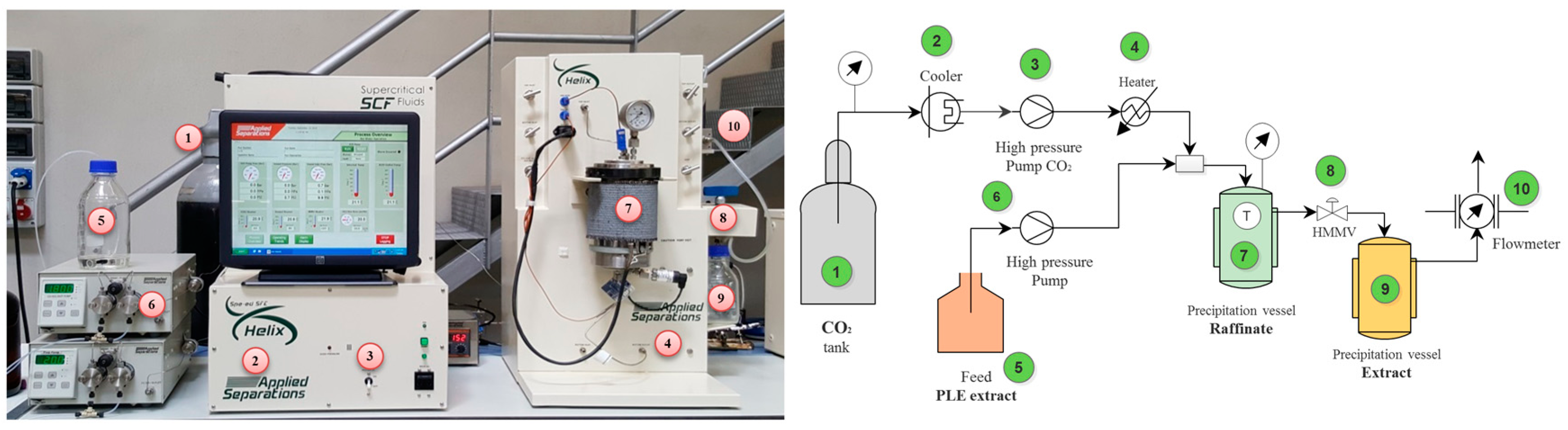
3.2. Rosemary-Leaf Extraction Procedures
3.2.1. Pressurized Liquid Extraction (PLE)
3.2.2. Single-Step SFE Process
3.2.3. Two-Step Sequential SFE
3.2.4. Supercritical Antisolvent Fractionation (SAF) at Pilot Scale
3.3. Chemical Characterization of Rosemary Extracts
3.3.1. Liquid Chromatography-Diode Array Detection-Mass Spectrometry
3.3.2. Liquid Chromatography-Quadrupole Time-Of-Flight Mass Spectrometry (LC-Q/TOF-MS)
3.3.3. Gas Chromatography-Mass Spectrometry (GC-MS)
3.4. Total Phenols Content (Folin-Ciocateu Method)
3.5. Antioxidant Activity In Vitro Assays
3.5.1. DPPH Radical Scavenging Assay
3.5.2. TEAC Assay
3.6. Cell Culture
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- King, J.W. Modern supercritical fluid technology for food applications. Annu. Rev. Food Sci. Technol. 2014, 5, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC-Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Pereira, C.G.; Nunes, S.B.; Padilha, F.F.; Umsza-Guez, M.A. Supercritical fluid extraction using CO2: Main applications and future perspectives. Sep. Sci. Technol. 2013, 48, 2741–2760. [Google Scholar] [CrossRef]
- Yen, H.W.; Yang, S.C.; Chen, C.H.; Jesisca; Chang, J.S. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2015, 184, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Castro-Puyana, M.; Mendiola, J.A.; Ibañez, E. Compressed fluids for the extraction of bioactive compounds. TrAC-Trends Anal. Chem. 2013, 43, 67–83. [Google Scholar] [CrossRef]
- Herrero, M.; Sánchez-Camargo, A.d.P.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC-Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Zinnai, A.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Venturi, F. Supercritical fluid extraction from microalgae with high content of LC-PUFAs. A case of study: SC-CO2 oil extraction from Schizochytrium sp. J. Supercrit. Fluids 2016, 116, 126–131. [Google Scholar] [CrossRef]
- Zinnai, A.; Venturi, F.; Sanmartin, C.; Andrich, G. A Simplified method to estimate the time evolution of oil extraction from different substrates by supercritical CO2. Am. J. Anal. Chem. 2012, 2012, 939–945. [Google Scholar] [CrossRef]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae—A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Vicente, G.; Martín, D.; García-Risco, M.R.; Fornari, T.; Reglero, G. Supercritical carbon dioxide extraction of antioxidants from rosemary (Rosmarinus officinalis) leaves for use in edible vegetable oils. J. Oleo Sci. 2012, 61, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Barreto, H.M.; Silva Filho, E.C.; Lima, E.d.O.; Coutinho, H.D.M.; Morais-Braga, M.F.B.; Tavares, C.C.A.; Tintino, S.R.; Rego, J.V.; de Abreu, A.P.L.; Lustosa, M.; et al. Chemical composition and possible use as adjuvant of the antibiotic therapy of the essential oil of Rosmarinus officinalis L. Ind. Crops Prod. 2014, 59, 290–294. [Google Scholar] [CrossRef]
- Bassolé, I.H. N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Bor, T. Antimicrobials from herbs, spices, and plants. Fruits Veg. Herbs 2016, 551–578. [Google Scholar]
- Valdés, A.; García-Cañas, V.; Rocamora-Reverte, L.; Gómez-Martínez, Á.; Ferragut, J.A.; Cifuentes, A. Effect of rosemary polyphenols on human colon cancer cells: Transcriptomic profiling and functional enrichment analysis. Genes Nutr. 2013, 8, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; García-Cañas, V.; Simó, C.; Ibáñez, C.; Micol, V.; Ferragut, J.A.; Cifuentes, A. Comprehensive foodomics study on the mechanisms operating at various molecular levels in cancer cells in response to individual rosemary polyphenols. Anal. Chem. 2014, 86, 9807–9815. [Google Scholar] [CrossRef] [PubMed]
- González-Vallinas, M.; Molina, S.; Vicente, G.; Zarza, V.; Martín-Hernández, R.; García-Risco, M.R.; Fornari, T.; Reglero, G.; de Molina, A.R. Expression of MicroRNA-15b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of rosemary diterpenes in colon and pancreatic cancer. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Jungbauer, A.; Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 2012, 71, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Identification and quantification of a major anti-oxidant and anti-inflammatory phenolic compound found in basil, lemon thyme, mint, oregano, rosemary, sage, and thyme. Int. J. Food Sci. Nutr. 2011, 62, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Satyajit Patra, S.N. Review of medicinal plants for anti-obesity activity. Transl. Biomed. 2015, 6, 1–23. [Google Scholar]
- Gamboa-Gómez, C.I.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Vázquez-Cabral, B.D.; González-Laredo, R.F. Plants with potential use on obesity and its complications. EXCLI J. 2015, 14, 809–831. [Google Scholar] [PubMed]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, F.; Autrup, H.; Barlow, S.; Castle, L.; Crebelli, R.; Dekant, W.; Engel, K.-H.; Gontard, N.; Gott, D.; Grilli, S.; et al. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to. EFSA J. 2008, 628–633, 1–19. [Google Scholar]
- Johnson, J.J. Carnosol: A promising anti-cancer and anti-inflammatory agent. Cancer Lett. 2011, 305, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Ho, C.T.; Lin-Shiau, S.Y.; Lin, J.K. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-κB and c-Jun. Biochem. Pharmacol. 2005, 69, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Kyung-Soo Chun, R.; Kundu, J.; Gyeong Chae, I.; Kumar Kundu, J. Carnosol: A phenolic diterpene with cancer chemopreventive potential. J. Cancer Prev 2014, 1919, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Al Dhaheri, Y.; Attoub, S.; Ramadan, G.; Arafat, K.; Bajbouj, K.; Karuvantevida, N.; AbuQamar, S.; Eid, A.; Iratni, R. Carnosol induces ROS-mediated beclin1-independent autophagy and apoptosis in triple negative breast cancer. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; VasanthaSelvan, M.; Silvan, S.; Baskaran, N.; Kumar Singh, A.; Vinoth Kumar, V. Carnosic acid: A potent chemopreventive agent against oral carcinogenesis. Chem. Biol. Interact. 2010, 188, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Lin, C.Y.; Lin, H.H.; Chen, J.H. Carnosic acid, a rosemary phenolic compound, induces apoptosis through reactive oxygen species-mediated p38 activation in human neuroblastoma IMR-32 cells. Neurochem. Res. 2011, 36, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Oleanolic acid and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Kanjoormana, M.; Kuttan, G. Antiangiogenic activity of ursolic acid. Integr. Cancer Ther. 2010, 9, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Novotný, L.; Vachálková, A.; Biggs, D. Ursolic acid: An anti-tumorigenic and chemopreventive activity. Neoplasma 2001, 48, 241–246. [Google Scholar] [PubMed]
- Peng, C.-H.; Su, J.-D.; Chyau, C.-C.; Sung, T.-Y.; Ho, S.-S.; Peng, C.-C.; Peng, R.Y. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Biosci. Biotechnol. Biochem. 2007, 71, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine 2012, 19, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.P.; Mendiola, J.A.; Valdés, A.; Castro-Puyana, M.; García-Cañas, V.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Supercritical antisolvent fractionation of rosemary extracts obtained by pressurized liquid extraction to enhance their antiproliferative activity. J. Supercrit. Fluids 2016, 107, 581–589. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Valdés, A.; Sullini, G.; García-Cañas, V.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Two-step sequential supercritical fluid extracts from rosemary with enhanced anti-proliferative activity. J. Funct. Foods 2014, 11, 293–303. [Google Scholar] [CrossRef]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Green processes for the extraction of bioactives from Rosemary: Chemical and functional characterization via ultra-performance liquid chromatography-tandem mass spectrometry and in-vitro assays. J. Chromatogr. A 2010, 1217, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Zabot, G.L.; Moraes, M.N.; Carvalho, P.I.N.; Meireles, M.A.A. New proposal for extracting rosemary compounds: Process intensification and economic evaluation. Ind. Crops Prod. 2015, 77, 758–771. [Google Scholar] [CrossRef]
- Catchpole, O.; Tallon, S.; Dyer, P.; Montanes, F.; Moreno, T.; Vagi, E.; Eltringham, W.; Billakanti, J. Integrated supercritical fluid extraction and bioprocessing. Am. J. Biochem. Biotechnol. 2012, 8, 263–287. [Google Scholar]
- Sanders, J.P.M.; Clark, J.H.; Harmsen, G.J.; Heeres, H.J.; Heijnen, J.J.; Kersten, S.R.A.; van Swaaij, W.P.M.; Moulijn, J.A. Process intensification in the future production of base chemicals from biomass. Chem. Eng. Process. Process Intensif. 2012, 51, 117–136. [Google Scholar] [CrossRef]
- Lee, K.T.; Lim, S.; Pang, Y.L.; Ong, H.C.; Chong, W.T. Integration of reactive extraction with supercritical fluids for process intensification of biodiesel production: Prospects and recent advances. Prog. Energy Combust. Sci. 2014, 45, 54–78. [Google Scholar] [CrossRef]
- Martinez-Correa, H.A.; Cabral, F.A.; Magalhães, P.M.; Queiroga, C.L.; Godoy, A.T.; Sánchez-Camargo, A.P.; Paviani, L.C. Extracts from the leaves of Baccharis dracunculifolia obtained by a combination of extraction processes with supercritical CO2, ethanol and water. J. Supercrit. Fluids 2012, 63, 31–39. [Google Scholar] [CrossRef]
- Garcia-Mendoza, M.P.; Paula, J.T.; Paviani, L.C.; Cabral, F.A.; Martinez-Correa, H.A. Extracts from mango peel by-product obtained by supercritical CO2 and pressurized solvent processes. LWT-Food Sci. Technol. 2015, 62, 131–137. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F.; Carvalho, P.I.N.; Rostagno, M.A.; Meireles, M.A.A. Process integration for turmeric products extraction using supercritical fluids and pressurized liquids: Economic evaluation. Food Bioprod. Process. 2016, 98, 227–235. [Google Scholar] [CrossRef]
- Gonzalez-Coloma, A.; Martín, L.; Mainar, A.M.; Urieta, J.S.; Fraga, B.M.; Rodríguez-Vallejo, V.; Díaz, C.E. Supercritical extraction and supercritical antisolvent fractionation of natural products from plant material: Comparative results on Persea indica. Phytochem. Rev. 2012, 11, 433–446. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Bedir, E.; Vardar Sukan, F. In vitro antioxidant activities of Rosmarinus officinalis extracts treated with supercritical carbon dioxide. Food Chem. 2007, 101, 1457–1464. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standarized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Simó, C.; Ibáñez, C.; Rocamora-Reverte, L.; Ferragut, J.A.; García-Cañas, V.; Cifuentes, A. Effect of dietary polyphenols on K562 leukemia cells: A Foodomics approach. Electrophoresis 2012, 33, 2314–2327. [Google Scholar] [CrossRef] [PubMed]
- Vicente, G.; Molina, S.; González-Vallinas, M.; García-Risco, M.R.; Fornari, T.; Reglero, G.; de Molina, A.R. Supercritical rosemary extracts, their antioxidant activity and effect on hepatic tumor progression. J. Supercrit. Fluids 2013, 79, 101–108. [Google Scholar] [CrossRef]
- González-Vallinas, M.; Reglero, G.; Ramírez de Molina, A. Rosemary (Rosmarinus officinalis L.) extract as a potential complementary agent in anticancer therapy. Nutr. Cancer 2015, 5581, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Pérez-Sánchez, A.; Lozano-Sánchez, J.; Barrajón-Catalán, E.; Arráez-Román, D.; Cifuentes, A.; Micol, V.; Carretero, A.S. A bioguided identification of the active compounds that contribute to the antiproliferative/cytotoxic effects of rosemary extract on colon cancer cells. Food Chem. Toxicol. 2015, 80, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Almela, L.; Sánchez-Muñoz, B.; Fernández-López, J.A.; Roca, M.J.; Rabe, V. Liquid chromatograpic-mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J. Chromatogr. A 2006, 1120, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Sifringer, M.; Kaczor, J.; Wejksza, K.; Zdzisińska, B.; Kandefer-Szerszeń, M. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn. Schmiedebergs. Arch. Pharmacol. 2006, 374, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kommera, H.; Kaluđerović, G.N.; Kalbitz, J.; Paschke, R. Lupane Triterpenoids—Betulin and Betulinic acid derivatives induce apoptosis in tumor cells. Investig. New Drugs 2011, 29, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Sun, M.; Luo, S.; Xu, T.; Cao, Y.; Yan, X.; Wang, Y. A 3D QSAR study of betulinic acid derivatives as anti-tumor agents using topomer CoMFA: model building studies and experimental verification. Molecules 2013, 18, 10228–10241. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Jaggi, M.; Singh, M.K.; Mukherjee, R.; Burman, A.C. Pharmacological evaluation of C-3 modified Betulinic acid derivatives with potent anticancer activity. Investig. New Drugs 2008, 26, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Dutta, D.; Mukherjee, S.; Das, S.; Maiti, N.C.; Das, P.; Chowdhury, C. Synthesis and biological evaluation of a novel betulinic acid derivative as an inducer of apoptosis in human colon carcinoma cells (HT-29). Eur. J. Med. Chem. 2015, 102, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Tschiggerl, C.; Bucar, F. Investigation of the volatile fraction of rosemary infusion extracts. Sci. Pharm. 2010, 78, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Yoshimura, Y.; Suzuki, M.; Shuto, K.; Ohkohchi, N.; et al. Antitumor effect of 1,8-cineole against colon cancer. Oncol. Rep. 2013, 30, 2647–2652. [Google Scholar] [PubMed]
- Nikolić, B.; Vasilijević, B.; Mitić-Ćulafić, D.; Vuković-Gačić, B.; Knežević-Vukćević, J. Comparative study of genotoxic, antigenotoxic and cytotoxic activities of monoterpenes camphor, eucalyptol and thujone in bacteria and mammalian cells. Chem. Biol. Interact. 2015, 242, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Koşar, M.; Dorman, H.J.D.; Hiltunen, R. Effect of an acid treatment on the phytochemical and antioxidant characteristics of extracts from selected Lamiaceae species. Food Chem. 2005, 91, 525–533. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. JNCI J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
| Process | Sample Name | Pressure (Bar) | Temperature (°C) | Feed/SC-CO2 Ratio | %H2O (w/w) | %Ethanol (w/w) | Process Time (min) |
|---|---|---|---|---|---|---|---|
| PLE | PLE | 100 | 150 | - | 24.0 | 76.0 ** | 20 |
| Single-step SFE | SFE1 | 150 | 40 | - | - | 7.0 * | 300 |
| Two-step SFE | SFE2 | 300 | 40 | - | - | 0 | 60 |
| 150 | 40 | - | - | 7.0 * | 120 | ||
| SAF | SAF1 | 100 | 40 | 0.025 | 55.8 | 44.2 ** | 180 |
| SAF | SAF2 | 100 | 40 | 0.100 | 55.8 | 44.2 ** | 60 |
| SAF | SAF3 | 100 | 40 | 0.025 | 24.0 | 76.0 ** | 180 |
| Sample | Yield (g/100 g Sample) | TPC 1 | TEAC 2 | EC50 3 |
|---|---|---|---|---|
| PLE | 38.46 d ± 1.99 | 233.88 f ± 4.43 | 2.75 b ± 0.04 | 7.70 d ± 0.33 |
| SFE1 | 6.74 a ± 0.33 | 134.42 a ± 4.51 | 1.87 a ± 0.05 | 7.03 e ± 0.15 |
| SFE2 | 4.68 a ± 0.01 | 169.01 b ± 8.61 | 2.64 b ± 0.03 | 5.61 c ± 0.11 |
| SAF1 | 20.65 c ± 1.74 | 220.05 e ± 5.82 | 4.09 d ± 0.15 | 3.39 a ± 0.08 |
| SAF2 | 5.74 a ± 0.45 | 203.04 d ± 7.81 | 3.67 c ± 0.09 | 4.12 b ± 0.12 |
| SAF3 | 15.36 b ± 1.41 | 188.55 c ± 1.06 | 3.80 c ± 0.20 | 7.83 d ± 0.22 |
| Peak | Rt (min) | [H-M]− | Molecular Formula | Identification | Peak Area (Mean ± SD) (× 106) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLE | SFE1 | SFE2 | SAF1 | SAF2 | SAF3 | |||||
| 1 | 0.479 | 387.1171 | C13 H24 O13 | NI1 | 3.91 ± 0.24 | - | - | - | - | - |
| 2 | 0.994 | 198.0528 | C9 H10 O5 | Siringic acid | 0.36 ± 0.02 | - | - | - | - | - |
| 3 | 1.737 | 306.0798 | C15 H14 O7 | Gallocathechin | 0.67 ± 0.04 | - | - | - | - | - |
| 4 | 1.797 | 387.1678 | C18 H28 O9 | NI2 | 0.99 ± 0.03 | - | - | - | - | - |
| 5 | 2.274 | 360.0845 | C18 H16 O8 | Rosmarinic acid | 4.10 ± 0.12 | - | - | - | - | - |
| 6 | 2.730 | 207.0636 | C11 H12 O4 | Tryhydroxycinnamic acid derivate | - | - | - | 1.024 c ± 0.006 | 0.71 b ± 0.01 | 0.61 a ± 0.05 |
| 7 | 3.178 | 345.1737 | C20 H26 O5 | Rosmanol | 0.930 a ± 0.009 | 6.16 f ± 0.42 | 4.80 e ± 0.15 | 3.90 d ± 0.02 | 2.37 b ± 0.01 | 2.87 c ± 0.11 |
| 8 | 3.275 | 345.1706 | C20 H26 O5 | Epirosmanol/Isorosmanol | - | 1.72 b ± 0.06 | 1.53 a ± 0.01 | 2.68 c ± 0.15 | 1.80 b ± 0.007 | 1.69 a,b ± 0.07 |
| 9 | 3.392 | 283.0617 | C16 H12 O5 | Genkwanin | 0.78 a ± 0.05 | 2.29 d ± 0.02 | 1.79 b ± 0.01 | 2.02 c ± 0.01 | 1.88 b ± 0.02 | 1.90 b,c ± 0.06 |
| 10 | 3.843 | 343.1563 | C20 H24 O5 | Safficinolide | - | - | - | 0.57 ± 0.01 | - | - |
| 11 | 3.883 | 331.1562 | C19 H24 O5 | NI3 | - | - | - | 2.06 c ± 0.09 | 1.26 b ± 0.02 | 0.99 a ± 0.05 |
| 12 | 3.960 | 329.1754 | C20 H26 O4 | Carnosol | 3.76 a ± 0.12 | 15.49 b ± 0.17 | 30.90 e ± 0.90 | 29.76 e ± 0.88 | 22.63 d ± 0.07 | 18.84 c ± 0.16 |
| 13 | 4.111 | 343.1630 | C20 H24 O5 | Rosmadial | - | 0.92 b ± 0.06 | - | 0.99 c ± 0.01 | 0.91 b ± 0.01 | 0.708 a ± 0.002 |
| 14 | 4.158 | 373.2037 | C22 H30 O5 | 11,12-Dimethylrosmanol | - | - | 2.17 a ± 0.14 | 4.53 c ± 0.23 | 3.22 b ± 0.03 | 3.26 b ± 0.02 |
| 15 | 4.258 | 325.1865 | C21 H26 O3 | NI4 | 1.39 a ± 0.06 | 2.29 c,d ± 0.25 | 1.86 b ± 0.14 | 1.96 b,c ± 0.24 | 2.51 d ± 0.03 | 1.37 c ± 0.08 |
| 16 | 4.338 | 331.1952 | C20 H28 O4 | Carnosic Acid | 21.80 a ± 1.04 | 42.86 b ± 0.36 | 50.78 d ± 2.44 | 49.28 d ± 1.09 | 50.01 d ± 0.25 | 45.95 c ± 0.53 |
| 17 | 4.575 | 345.2133 | C21 H30 O4 | Methyl carnosate/12-methoxy-carnosic acid | 1.50 a ± 0.04 | 8.04 b ± 0.25 | 12.63 d ± 0.65 | 12.42 d ± 0.89 | 12.33 d ± 0.14 | 9.95 c ± 0.43 |
| 18 | 4.753 | 315.1965 | C20 H28 O3 | Rosmaridiphenol | - | - | - | 0.50 b ± 0.02 | 0.509 b ± 0.008 | 0.21 a ±0.02 |
| 19 | 4.799 | 317.2107 | C20 H30 O3 | NI5 | - | 2.27 a,b ± 0.07 | 2.47 b ± 0.14 | 2.15 a ± 0.08 | 2.138 a ± 0.006 | 2.40 b ± 0.11 |
| 20 | 4.818 | 455.3422 | C26 H48 O6 | Betulinic Acid * | 2.304 ± 0.003 | - | - | - | - | - |
| 21 | 4.976 | 455.3650 | C30 H48 O3 | Oleanolic acid * | 7.99 c ± 0.52 | 5.56 b ± 0.22 | 5.15 b ± 0.08 | 4.08 a ± 0.14 | 5.25 b ± 0.03 | 5.14 b ± 0.32 |
| 22 | 5.110 | 455.3515 | C30 H48 O3 | Ursolic acid * | - | 1.84 c ± 0.19 | 1.51 b ± 0.05 | 1.368 a ± 0.009 | 1.542 b ± 0.007 | 2.24 d ± 0.02 |
| 23 | 5.441 | 479.2785 | C30 H40 O5 | NI6 | - | 1.07 d ± 0.02 | 0.60 a ± 0.04 | 0.94 c ± 0.07 | 0.759 b ± 0.007 | 1.15 d ± 0.08 |
| 24 | 5.621 | 331.1921 | C20 H28 O4 | NI7 | - | 0.12 a ± 0.02 | 1.61 d ± 0.10 | 1.89 e ± 0.01 | 0.521 c ± 0.004 | 0.33 b ± 0.03 |
| 25 | 6.414 | 467.3168 | C30 H44 O4 | NI8 | 1.23 a ± 0.01 | 4.42 d ± 0.16 | 4.99 e ± 0.31 | 1.63 b,c ± 0.04 | 1.79 c ± 0.03 | 5.48 f ± 0.02 |
| 26 | 6.658 | 467.3184 | C30 H44 O4 | NI9 | 0.83 a ± 0.06 | 3.32 c ± 0.11 | 2.38 b ± 0.08 | 1.06 d ± 0.03 | 1.03 a ± 0.02 | 4.48 d ± 0.17 |
| 27 | 6.840 | 615.4061 | C33 H60 O10 | NI10 | 0.075 a ± 0.018 | 0.50 c ± 0.04 | 0.33 b ± 0.02 | - | - | 0.57 d ± 0.03 |
| 28 | 6.997 | 551.3749 | C35 H52 O5 | NI11 | - | 0.305 b ± 0.005 | 0.20 a ± 0.02 | - | - | 0.46 c ± 0.03 |
| 29 | 7.290 | 535.3794 | C35 H52 O4 | NI12 | - | - | 0.34 ± 0.02 | - | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Camargo, A.D.P.; García-Cañas, V.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Comparative Study of Green Sub- and Supercritical Processes to Obtain Carnosic Acid and Carnosol-Enriched Rosemary Extracts with in Vitro Anti-Proliferative Activity on Colon Cancer Cells. Int. J. Mol. Sci. 2016, 17, 2046. https://doi.org/10.3390/ijms17122046
Sánchez-Camargo ADP, García-Cañas V, Herrero M, Cifuentes A, Ibáñez E. Comparative Study of Green Sub- and Supercritical Processes to Obtain Carnosic Acid and Carnosol-Enriched Rosemary Extracts with in Vitro Anti-Proliferative Activity on Colon Cancer Cells. International Journal of Molecular Sciences. 2016; 17(12):2046. https://doi.org/10.3390/ijms17122046
Chicago/Turabian StyleSánchez-Camargo, Andrea Del Pilar, Virginia García-Cañas, Miguel Herrero, Alejandro Cifuentes, and Elena Ibáñez. 2016. "Comparative Study of Green Sub- and Supercritical Processes to Obtain Carnosic Acid and Carnosol-Enriched Rosemary Extracts with in Vitro Anti-Proliferative Activity on Colon Cancer Cells" International Journal of Molecular Sciences 17, no. 12: 2046. https://doi.org/10.3390/ijms17122046