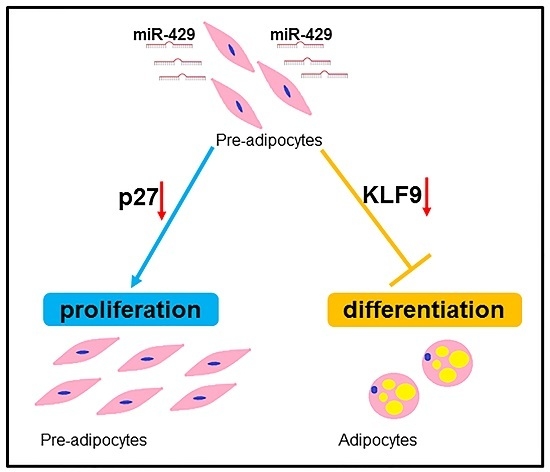
miR-429 Inhibits Differentiation and Promotes Proliferation in Porcine Preadipocytes
Abstract
:1. Introduction
2. Results
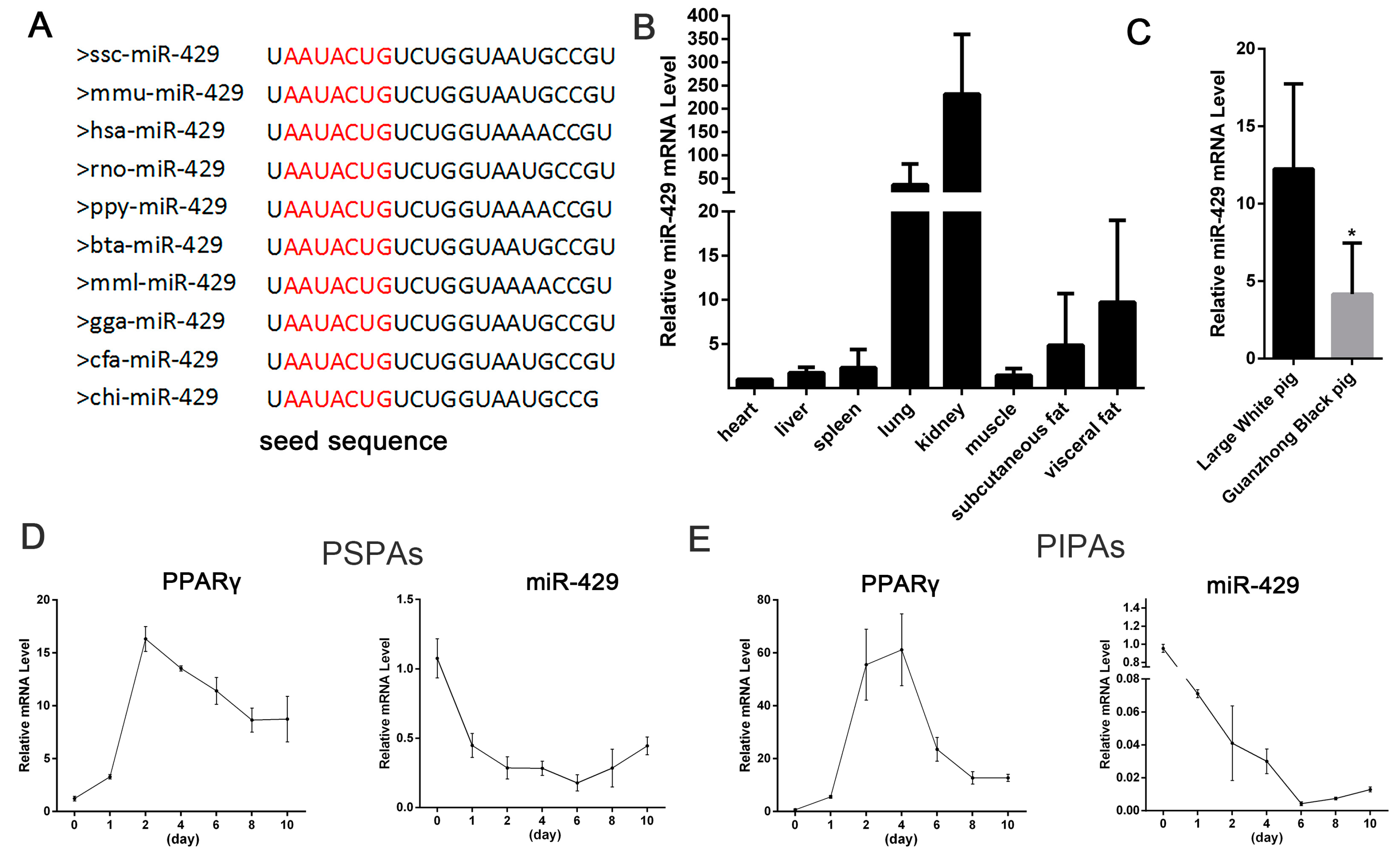
2.1. miR-429 Is Ubiquitously Expressed in Various Porcine Tissue Types and Downregulated in Early Differentiation of Porcine Preadipocytes
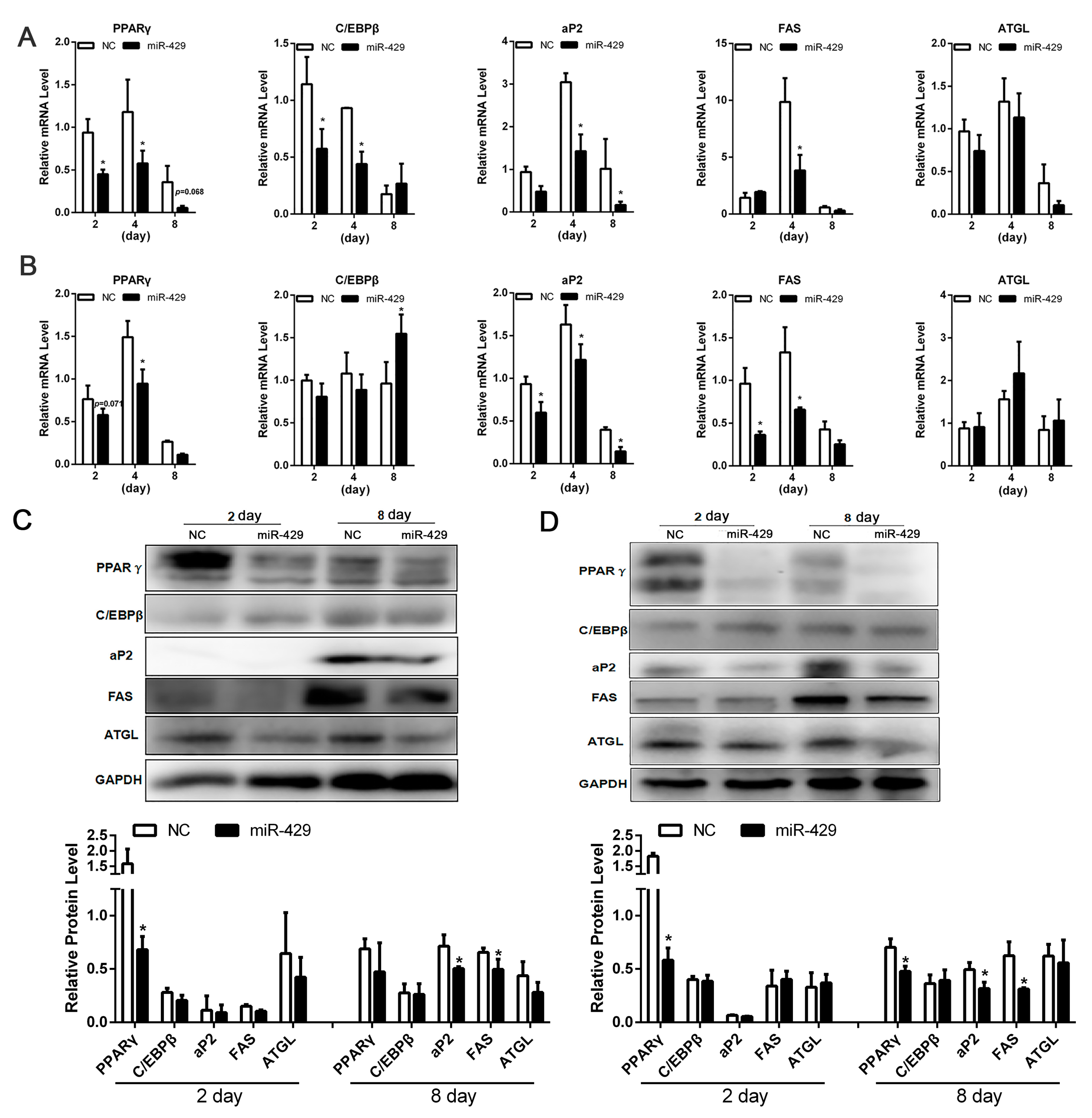
2.2. miR-429 Inhibits Differentiation of Porcine Subcutaneous Pre-Adipocytes (PSPAs) and Porcine Intramuscular Pre-Adipocytes (PIPAs)
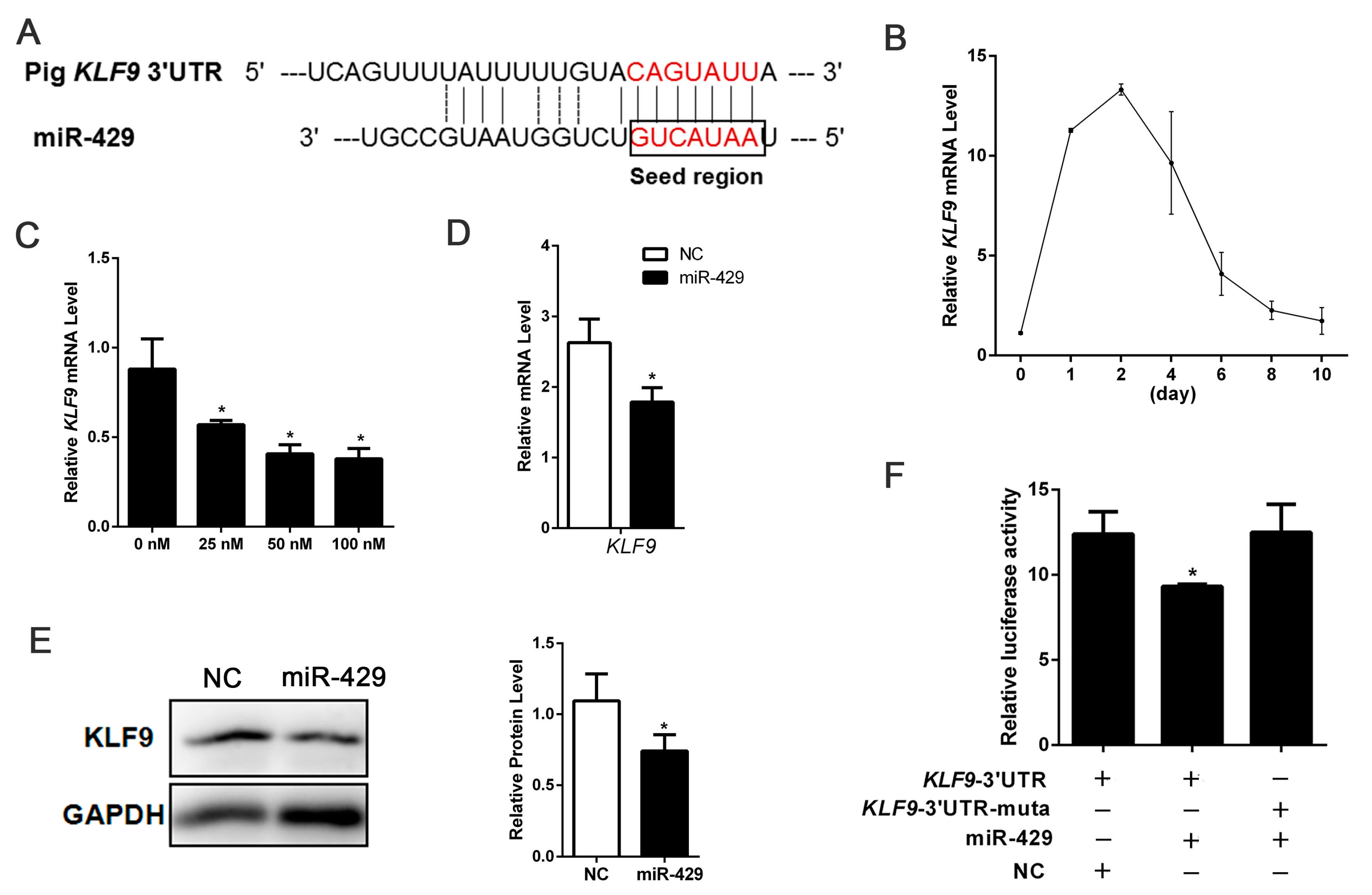
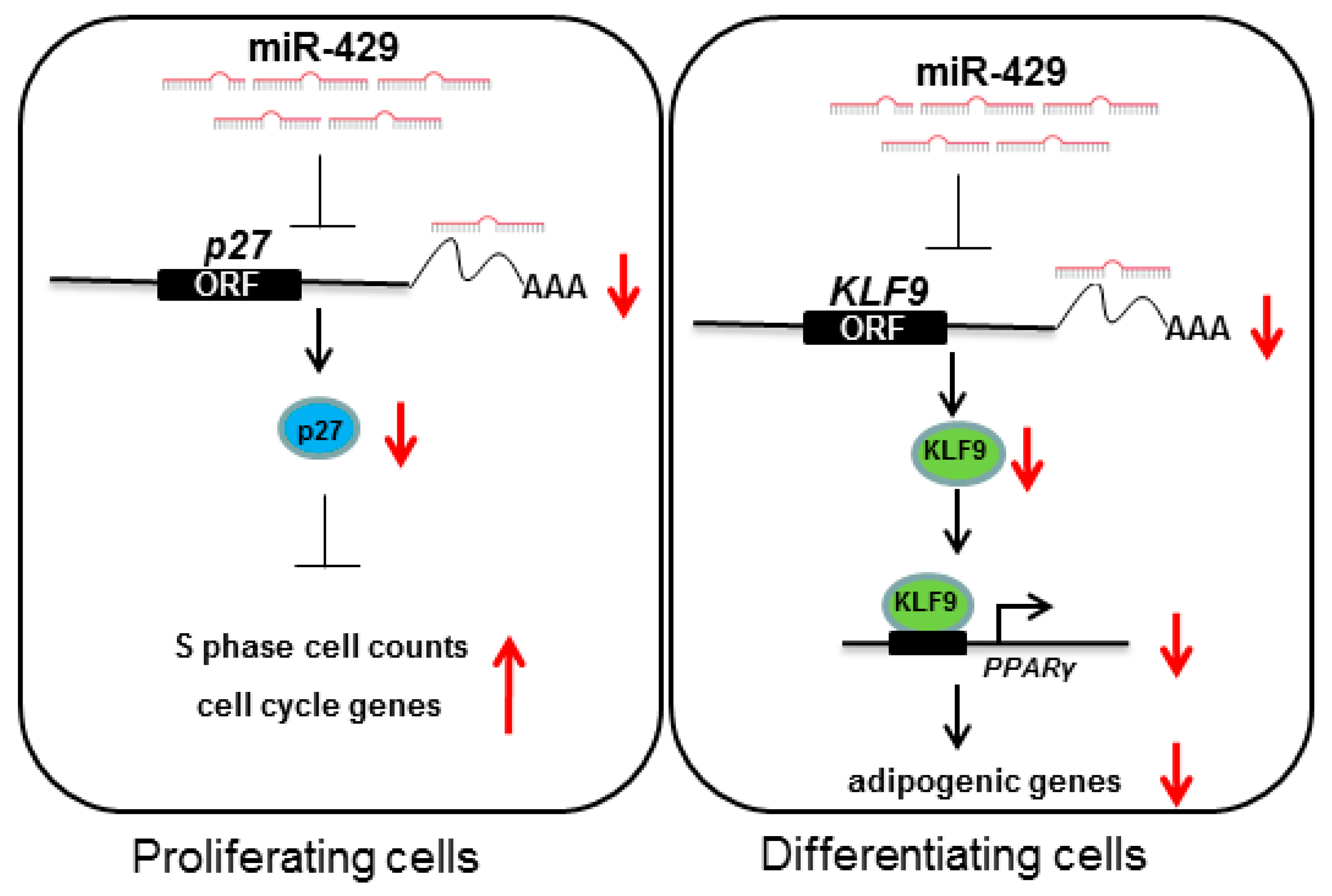
2.3. miR-429 Can Target on KLF9 in PSPAs
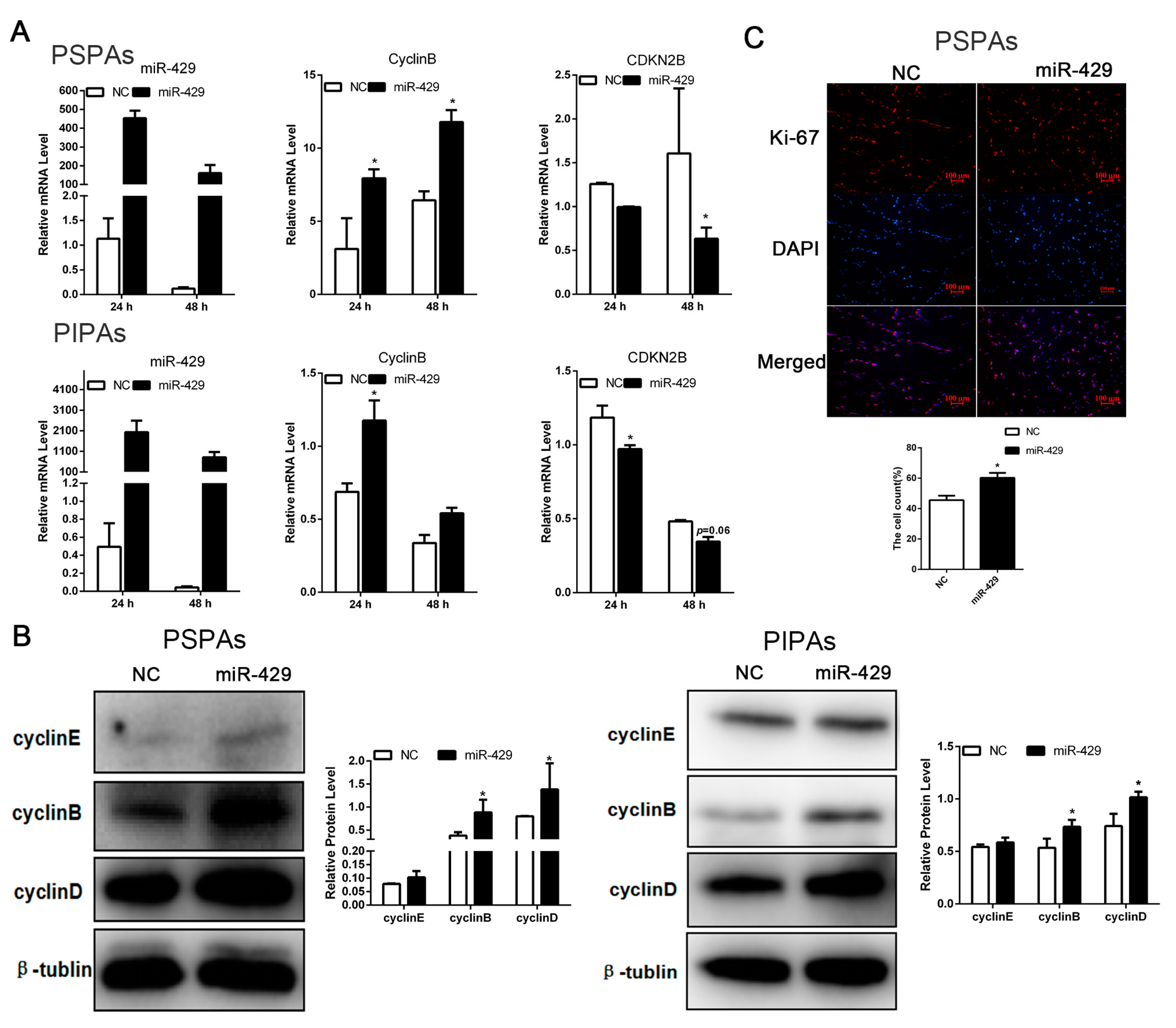
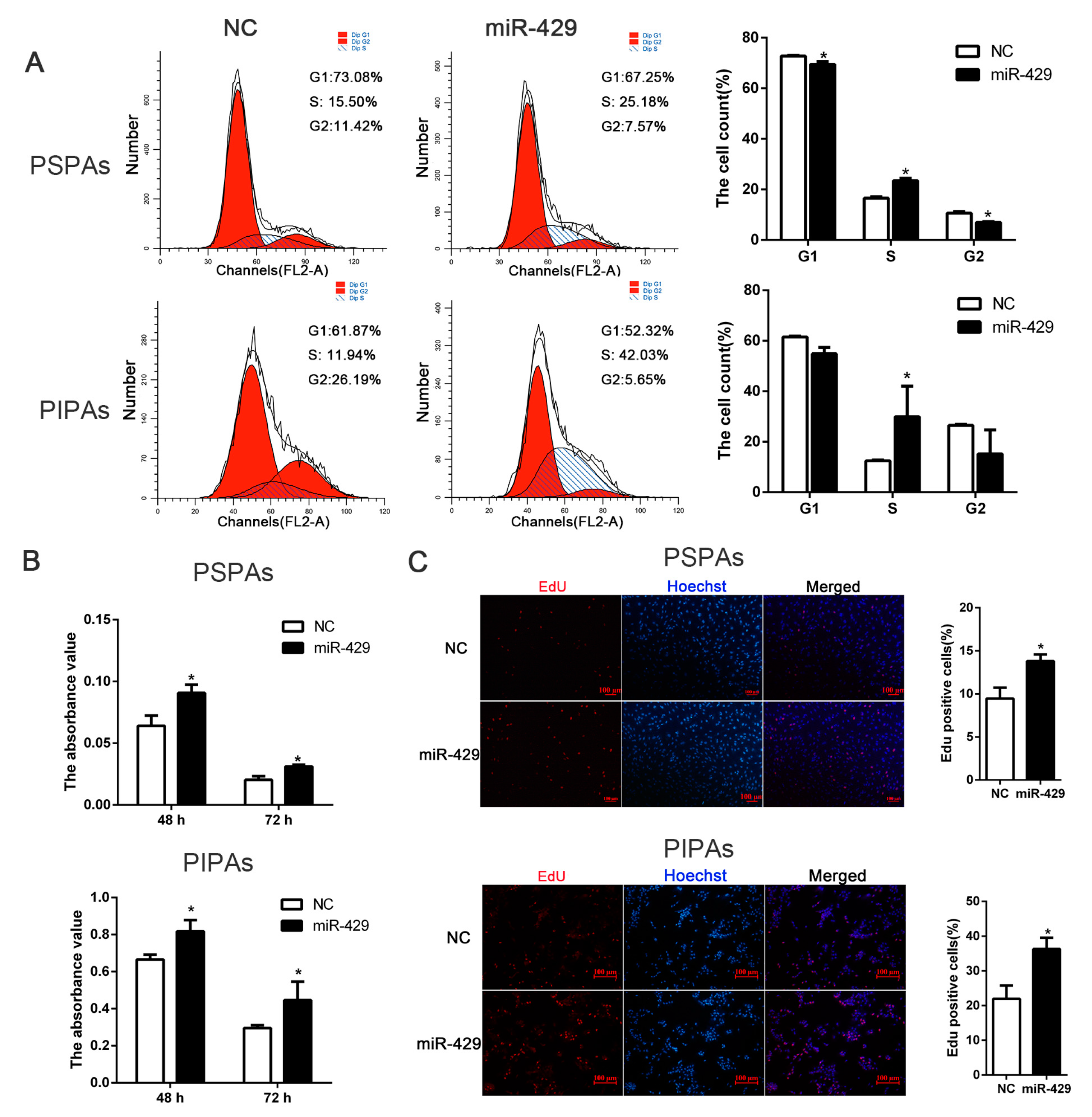
2.4. miR-429 Promotes Cell Cycle Progression in PSPAs and PIPAs
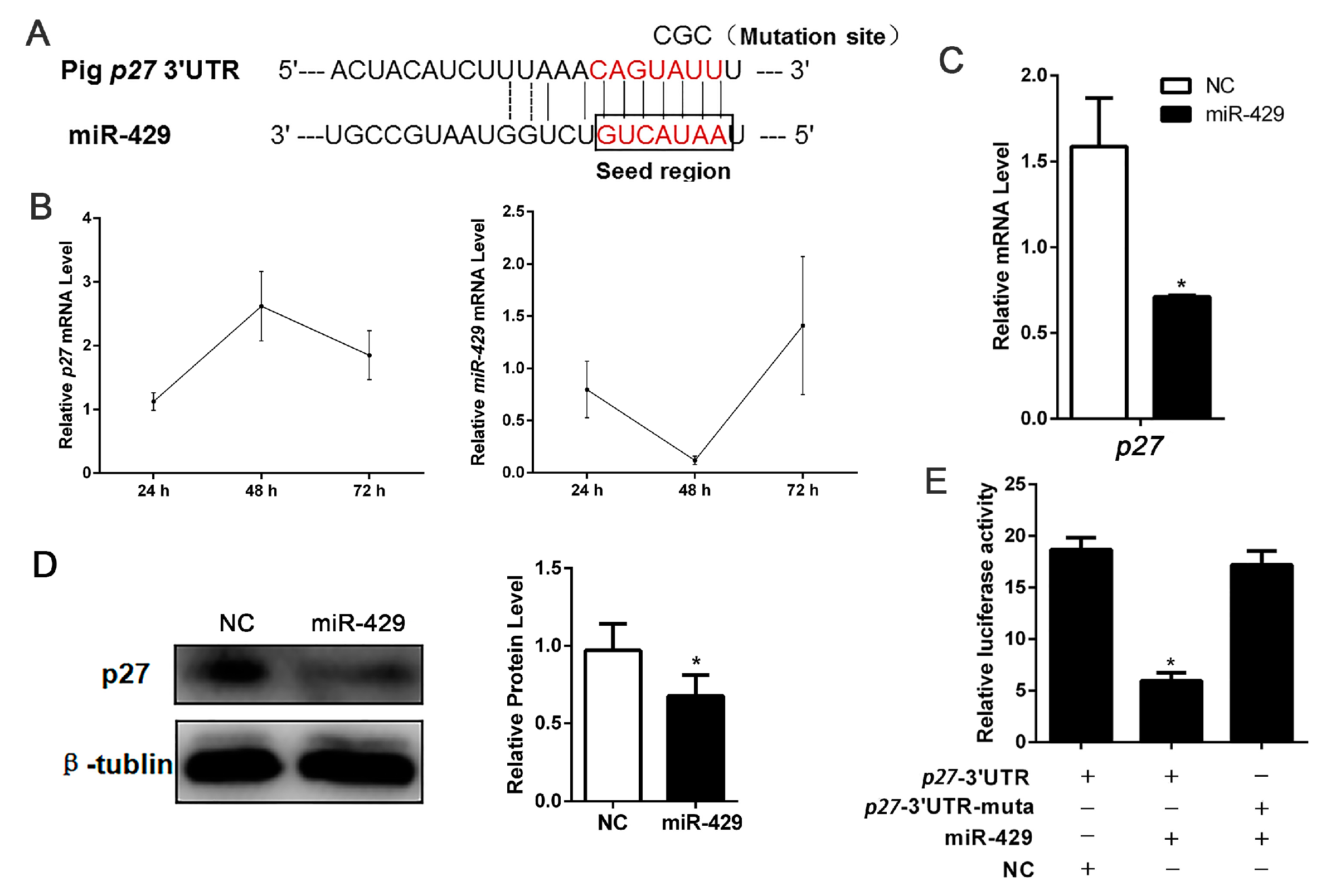
2.5. miR-429 Is Capable of Targeting p27 in the Proliferation Stage of Porcine Preadipocytes
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of PSPAs and PIPAs
4.2. Transfection of miRNA Agomir
- Ssc-miR-429 sense: 5′-UAAUACUGUCUGGUAAUGCCGU-3′;
- Antisense: 5′-GGCAUUACCAGACAGUAUUAUU-3′;
- NC sense: 5′-UUCUCCGAACGUGUCACGUTT-3′;
- Antisense: 5′-ACGUGACACGUUCGGAGAATT-3′.
4.3. RNA Extractions and RT-qPCR
4.4. Western Blot Analysis
4.5. Luciferase Reporter Assay
4.6. Bodipy Staining of Lipid Droplets
4.7. Flow Cytometry
4.8. Cell Counting Kit-8
4.9. EdU Staining
4.10. Immunofluorescent Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PSPAs | Porcine subcutaneous pre-adipocytes |
| PIPAs | Porcine intramuscular pre-adipocytes |
| PPARγ | Peroxisome proliferator activated receptor γ |
| aP2 | Adipocyte fatty acid-binding protein 4 |
| FAS | Fatty acid sythetase |
| ATGL | Adipose triglyceride lipase |
| Ki-67 | Marker of proliferation Ki-67 |
| Cyclin E | Cell cycle protein E |
| Cyclin B | Cell cycle protein B |
| Cyclin D | Cell cycle protein D |
| KLF9 | Krüppel-like transcription factor 9 |
| p27 | Cyclin-dependent kinase inhibitor 1B |
| ssc | Sus scrofa |
| mmu | Mus musculus |
| hsa | Homo sapiens |
| rno | Rattus norvegicus |
| ppy | Pongo pygmaeus |
| bta | Bos taurus |
| mml | Macaca mulatta |
| gga | Gallus gallus |
| cfa | Canis familiaris |
References
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, X.; Monin, G.; Talmant, A.; Mourot, J.; Lebret, B. Influence of intramuscular fat content on the quality of pig meat—1. Composition of the lipid fraction and sensory characteristics of m. Longissimus lumborum. Meat Sci. 1999, 53, 59–65. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Gardan, D.; Gondret, F.; Louveau, I. Lipid metabolism and secretory function of porcine intramuscular adipocytes compared with subcutaneous and perirenal adipocytes. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E372–E380. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Peng, Y.D.; Yu, S.L.; Li, H.A.; Xiang, H.; Peng, J.; Jiang, S.W. MicroRNAs: Emerging roles in adipogenesis and obesity. Cell Signal. 2014, 26, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Song, J.; Cui, J.; Hou, J.; Zheng, X.; Li, C.; Liu, L. MicroRNAs regulate adipocyte differentiation. Cell Biol. Int. 2013, 37, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Mo, D.; Zhang, Y.; Zhang, Y.; Cong, P.; Xiao, S.; He, Z.; Liu, X.; Chen, Y. MicroRNAome comparison between intramuscular and subcutaneous vascular stem cell adipogenesis. PLoS ONE 2012, 7, e45410. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Chen, X.; Guan, L.Z.; Qi, Q.; Shu, G.; Jiang, Q.Y.; Yuan, L.; Xi, Q.Y.; Zhang, Y.L. miRNA-181a regulates adipogenesis by targeting tumor necrosis factor-α (TNF-α) in the porcine model. PLoS ONE 2013, 8, e71568. [Google Scholar]
- Taniguchi, M.; Nakajima, I.; Chikuni, K.; Kojima, M.; Awata, T.; Mikawa, S. MicroRNA-33b downregulates the differentiation and development of porcine preadipocytes. Mol. Biol. Rep. 2014, 41, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xi, Q.Y.; Cheng, X.; Dong, T.; Zhu, X.T.; Shu, G.; Wang, L.N.; Jiang, Q.Y.; Zhang, Y.L. miR-146a-5p inhibits TNF-α-induced adipogenesis via targeting insulin receptor in primary porcine adipocytes. J. Lipid Res. 2016, 57, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.E.; Li, Y.F.; Jia, L.; Ji, H.L.; Song, Z.Y.; Cheng, J.; Wu, G.F.; Song, C.C.; Zhang, Q.L.; Zhu, J.Y.; et al. MicroRNA-199a-5p affects porcine preadipocyte proliferation and differentiation. Int. J. Mol. Sci. 2014, 15, 8526–8538. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.F.; Yang, X.J.; Jia, Y.M.; Li, R.S.; Zhao, R.Q. Microvesicle-shuttled miR-130b reduces fat deposition in recipient primary cultured porcine adipocytes by inhibiting PPARγ expression. J. Cell. Physiol. 2014, 229, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Zhang, Y.; Zhang, Y.; Chen, L.; Mo, D. Up-regulated miR-145 expression inhibits porcine preadipocytes differentiation by targeting irs1. Int. J. Biol. Sci. 2012, 8, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qiu, Y.; Liu, S.; Dong, P.; Ning, X.; Li, Y.; Yang, G.; Sun, S. Over-expressed miR-103 promotes porcine adipocyte differentiation. Chin. J. Biotechnol. 2012, 28, 927–936. (In Chinese) [Google Scholar]
- Jing, J.; Xiong, S.T.; Li, Z.; Wu, J.J.; Zhou, L.; Gui, J.F.; Mei, J. A feedback regulatory loop involving p53/miR-200 and growth hormone endocrine axis controls embryo size of zebrafish. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Schliekelman, M.J.; Gibbons, D.L.; Faca, V.M.; Creighton, C.J.; Rizvi, Z.H.; Zhang, Q.; Wong, C.H.; Wang, H.; Ungewiss, C.; Ahn, Y.H.; et al. Targets of the tumor suppressor miR-200 in regulation of the epithelial-mesenchymal transition in cancer. Cancer Res. 2011, 71, 7670–7682. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jiang, C.; Sun, Q.; Yan, F.; Wang, L.; Fu, Z.; Liu, T.; Hu, F. Downregulation of miR429 and inhibition of cell migration and invasion in nasopharyngeal carcinoma. Mol. Med. Rep. 2016, 13, 3236–3242. [Google Scholar] [PubMed]
- Lei, W.K.; Liu, Y.E.; Zheng, Y.Z.; Qu, L. miR-429 inhibits oral squamous cell carcinoma growth by targeting ZEB1. Med. Sci. Monit. 2015, 21, 383–389. [Google Scholar] [PubMed]
- Gao, H.; Liu, C. miR-429 represses cell proliferation and induces apoptosis in HBV-related HCC. Biomed. Pharmacother. 2014, 68, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.R.; Gao, P.; Zhu, B.Y.; Chen, X.; Lin, F.; Wang, X.; Wei, J.X.; Zhang, H.Z. Downregulation of microRNA-429 inhibits cell proliferation by targeting p27kip1 in human prostate cancer cells. Mol. Med. Rep. 2015, 11, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Liang, Z.; Chen, L.; Tan, G.; Wang, K.; Liu, L.; Liu, J.; Chen, H. MicroRNA-429 suppresses cell proliferation, epithelial-mesenchymal transition, and metastasis by direct targeting of BMI1 and E2f3 in renal cell carcinoma. Urol. Oncol. 2015, 33, 332.e9–332.e18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Li, M.; Zang, W.Q.; Ma, Y.Y.; Wang, N.; Li, P.; Wang, T.; Zhao, G.Q. miR-429 up-regulation induces apoptosis and suppresses invasion by targeting Bcl-2 and SP-1 in esophageal carcinoma. Cell. Oncol. 2013, 36, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Ren, H.; Xu, P.; Cheng, J.; Huang, S.; Zhou, R.; Mu, Y.; Yang, S.; Qi, D.; Wang, Y.; et al. Adipocyte miR-200b/a/429 ablation in mice leads to high-fat-diet-induced obesity. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.S.; Du, Z.Q.; Cheng, B.H.; Wang, Y.X.; Yao, J.; Li, Y.M.; Cao, Z.P.; Luan, P.; Wang, N.; Li, H. Expression profiling of preadipocyte microRNAs by deep sequencing on chicken lines divergently selected for abdominal fatness. PLoS ONE 2015, 10, e0117843. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Li, Y.J.; Li, X.J.; Ning, X.M.; Li, M.H.; Yang, G.S. MicroRNA identity and abundance in developing swine adipose tissue as determined by solexa sequencing. J. Cell. Biochem. 2011, 112, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Yao, Y.; Yang, Y.; Liao, K.; Wu, J.R. Kruppel-like factor KLF9 regulates PPARγ transactivation at the middle stage of adipogenesis. Cell Death Differ. 2011, 18, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Ma, L.; Pledger, W.J. P27kip1 inhibits the cell cycle through non-canonical G1/S phase-specific gatekeeper mechanism. Cell Cycle 2015, 14, 3954–3964. [Google Scholar] [CrossRef] [PubMed]
- Gubelmann, C.; Schwalie, P.C.; Raghav, S.K.; Roder, E.; Delessa, T.; Kiehlmann, E.; Waszak, S.M.; Corsinotti, A.; Udin, G.; Holcombe, W.; et al. Identification of the transcription factor ZEB1 as a central component of the adipogenic gene regulatory network. Elife 2014, 3, e03346. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Ho, J.Y.; Chou, S.C.; Yu, D.S. miR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget 2016, 7, 26593–26603. [Google Scholar] [CrossRef] [PubMed]
- Krek, A.; Grun, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.N.; Wang, S.Q. Role of kruppel-like transcription factors in adipogenesis. Dev. Biol. 2013, 373, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Wei, H.K.; Song, T.X.; Yang, Y.; Zhang, F.; Zhou, Y.F.; Peng, J.; Jiang, S.W. KLF13 promotes porcine adipocyte differentiation through PPARγ activation. Cell. Biosci. 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.J.; Lee, Y.J.; Lee, M.Y.; Choi, H.; Lee, H.; Kim, J.W. Kruppel-like factor KLF8 plays a critical role in adipocyte differentiation. PLoS ONE 2012, 7, e52474. [Google Scholar] [CrossRef] [PubMed]
- Escalona-Nandez, I.; Guerrero-Escalera, D.; Estanes-Hernandez, A.; Ortiz-Ortega, V.; Tovar, A.R.; Perez-Monter, C. The activation of peroxisome proliferator-activated receptor gamma is regulated by kruppel-like transcription factors 6 & 9 under steatotic conditions. Biochem. Biophys. Res. Commun. 2015, 458, 751–756. [Google Scholar] [PubMed]
- Kimura, H.; Fujimori, K. Activation of early phase of adipogenesis through kruppel-like factor KLF9-mediated, enhanced expression of CCAAT/enhancer-binding protein β in 3T3-L1 cells. Gene 2014, 534, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Panda, H.; Pelakh, L.; Chuang, T.D.; Luo, X.P.; Bukulmez, O.; Chegini, N. Endometrial miR-200c is altered during transformation into cancerous states and targets the expression of ZEBs, VEGFA, FLT1, IKK B, KLF9, and FBLN5. Reprod. Sci. 2012, 19, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, H.; Hunter, T. P27, a novel inhibitor of G1 cyclin-CDK protein-kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef]
- Katner, A.L.; Gootam, P.; Hoang, Q.B.L.; Gnarra, J.R.; Rayford, W. A recombinant adenovirus expressing p27(kip1) induces cell cycle arrest and apoptosls in human 786–0 renal carcinoma cells. J. Urol. 2002, 168, 766–773. [Google Scholar] [CrossRef]
- Wang, S.B.; Zhou, G.X.; Shu, G.; Wang, L.N.; Zhu, X.T.; Gao, P.; Xi, Q.Y.; Zhang, Y.L.; Yuan, L.; Jiang, Q.Y. Glucose utilization, lipid metabolism and BMP-Smad signaling pathway of porcine intramuscular preadipocytes compared with subcutaneous preadipocytes. Cell. Physiol. Biochem. 2013, 31, 981–996. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Wei, H.K.; Song, T.X.; Yang, Y.; Peng, J.; Jiang, S.W. Transcriptome comparison between porcine subcutaneous and intramuscular stromal vascular cells during adipogenic differentiation. PLoS ONE 2013, 8, e77094. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.X.; Wang, S.B.; Wang, Z.G.; Zhu, X.T.; Shu, G.; Liao, W.Y.; Yu, K.F.; Gao, P.; Xi, Q.Y.; Wang, X.Q.; et al. Global comparison of gene expression profiles between intramuscular and subcutaneous adipocytes of neonatal landrace pig using microarray. Meat Sci. 2010, 86, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Lu, J.X.; Chen, Y.; Zhao, Y.Q.; Guo, P.H.; Yang, J.T.; Zang, R.X. Comparison of the adipogenesis in intramuscular and subcutaneous adipocytes from bamei and landrace pigs. Biochem. Cell Biol. 2014, 92, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Nobusue, H.; Kano, K. Establishment and characteristics of porcine preadipocyte cell lines derived from mature adipocytes. J. Cell. Biochem. 2010, 109, 542–552. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| PPARγ | AGGACTACCAAAGTGCCATCAAA | GAGGCTTTATCCCCACAGACAC |
| aP2 | GAGCACCATAACCTTAGATGGA | AAATTCTGGTAGCCGTGACA |
| C/EBPβ | GCACAGCGACGAGTACAAGA | TATGCTGCGTCTCCAGGTTG |
| FAS | CCCCGAATCTGCACTACCAC | AGTTGGGCTGAAGGATGACG |
| ATGL | CCTCATTCCACCTGCTCTCC | GTGATGGTGCTCTTGAGTTCGT |
| cyclinB | AATCCCTTCTTGTGGTTA | CTTAGATGTGGCATACTTG |
| CDKN2B | AGTGGCGGCGGTGGAGAT | GGGTGAGGGTGGCAGGGT |
| KLF9 | CGAATCTGGGTCGAGTCCTT | GGGCTTTGAGATGGGAGGAT |
| p27 | GGAGGAAGATGTCAAACGTGAG | TCTGCAGTGCTTCTCCAAGTC |
| GAPDH | AGGTCGGAGTGAACGGATTTG | ACCATGTAGTGGAGGTCAATGAAG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Chen, F.-F.; Ge, J.; Zhu, J.-Y.; Shi, X.-E.; Li, X.; Yu, T.-Y.; Chu, G.-Y.; Yang, G.-S. miR-429 Inhibits Differentiation and Promotes Proliferation in Porcine Preadipocytes. Int. J. Mol. Sci. 2016, 17, 2047. https://doi.org/10.3390/ijms17122047
Peng Y, Chen F-F, Ge J, Zhu J-Y, Shi X-E, Li X, Yu T-Y, Chu G-Y, Yang G-S. miR-429 Inhibits Differentiation and Promotes Proliferation in Porcine Preadipocytes. International Journal of Molecular Sciences. 2016; 17(12):2047. https://doi.org/10.3390/ijms17122047
Chicago/Turabian StylePeng, Ying, Fen-Fen Chen, Jing Ge, Jia-Yu Zhu, Xin-E Shi, Xiao Li, Tai-Yong Yu, Gui-Yan Chu, and Gong-She Yang. 2016. "miR-429 Inhibits Differentiation and Promotes Proliferation in Porcine Preadipocytes" International Journal of Molecular Sciences 17, no. 12: 2047. https://doi.org/10.3390/ijms17122047