Mutations in the Mitochondrial ND1 Gene Are Associated with Postoperative Prognosis of Localized Renal Cell Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of Study Subjects
2.2. Nucleotide Differentiation Index Analysis (NST)
2.3. Analysis of ND1 Mutations
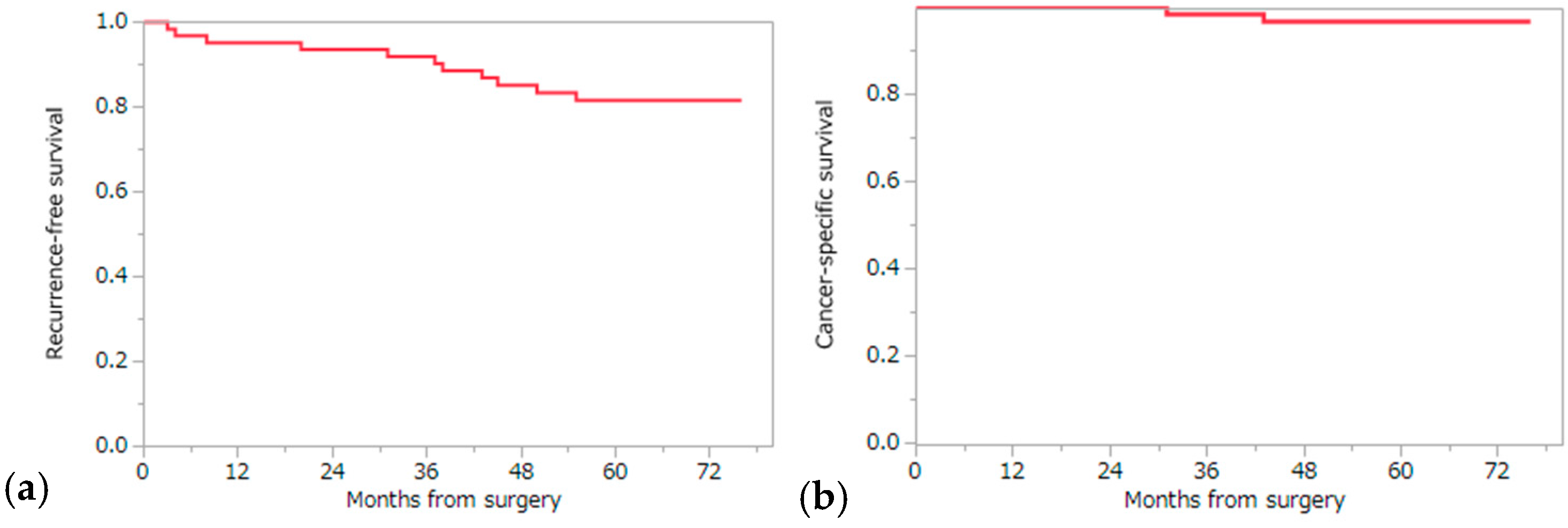
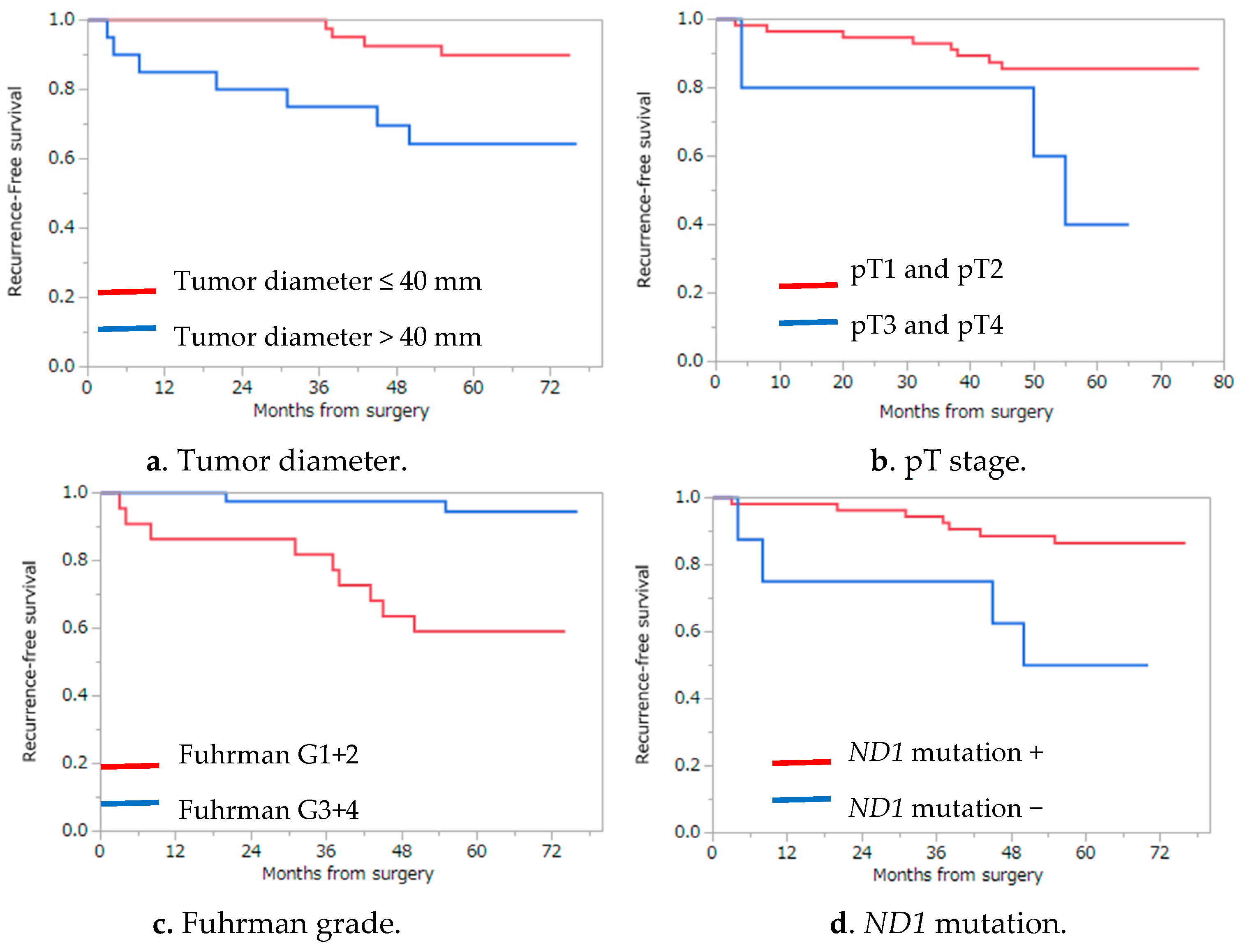
2.4. Analysis of Recurrence Risk Factors and Cancer-Specific and Overall Survival
3. Discussion
4. Materials and Methods
4.1. Patients and Tumor Specimens
4.2. Assessment of Formalin-Fixed Paraffin-Embedded Tissue Specimens
4.3. Amplification of mtDNA with Polymerase Chain Reaction
- F3274 (5′-ACAGTCAGAGGTTCAATTCCTCTTCT-3′),
- R3590 (5′-ATAGGAGGCCTAGGTTGAGGTTGACCA-3′),
- F3590 (5′-TGGTCAACCTCAACCTAGGCCTCCTAT-3′),
- R3725 (5′-GATGGCTAGGGTGACTTCATATGAGA-3′),
- F3731 (5′-ATGAAGTCACCCTAGCCATCATTCTACTA-3′),
- R4021 (5′-TCATATGTTGTTCCTAGGAAGATTGTAGT-3′),
- ND1-F (5′-TCCGAACTAGTCTCAGGCTTCA-3′),
- ND1-R (5′-CACGGAGAATTTTGGATTCTCAG-3′).
4.4. Nucleotide Differentiation Index Analysis
4.5. Detection of Somatic Mutations in ND1 Gene
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, L.; Tarone, R.E.; McLaughlin, J.K. The epidemiology of renal cell carcinoma. J. Urol. 2006, 176, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.M.; Gill, I.S.; Ellison, L.M. The evolving presentation of renal carcinoma in the United States: Trends from the Surveillance, Epidemiology, and End Results program. J. Urol. 2006, 176, 2397–2400. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Tiwari, R.C.; Murray, T.; Ghafoor, A.; Samuels, A.; Ward, E.; Feuer, E.J.; Thun, M.J.; American Cancer Society. Cancer statistics, 2004. CA Cancer J. Clin. 2004, 54, 8–29. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Janzen, N.K.; Kim, H.L.; Figlin, R.A.; Belldegrun, A.S. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol. Clin. N. Am. 2003, 30, 843–852. [Google Scholar] [CrossRef]
- Hollingsworth, J.M.; Miller, D.C.; Daignault, S.; Hollenbeck, B.K. Rising incidence of small renal masses: A need to reassess treatment effect. J. Natl. Cancer Inst. 2006, 98, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Sorbellini, M.; Kattan, M.W.; Snyder, M.E.; Reuter, V.; Motzer, R.; Goetzl, M.; McKiernan, J.; Russo, P. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J. Urol. 2005, 173, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Frank, I.; Blute, M.L.; Cheville, J.C.; Lohse, C.M.; Weaver, A.L.; Zincke, H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: The Ssign score. J. Urol. 2002, 168, 2395–2400. [Google Scholar] [CrossRef]
- Antonelli, A.; Cozzoli, A.; Zani, D.; Zanotelli, T.; Nicolai, M.; Cunico, S.C.; Simeone, C. The follow-up management of non-metastatic renal cell carcinoma: Definition of a surveillance protocol. BJU Int. 2007, 99, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Eichelberg, C.; Junker, K.; Ljungberg, B.; Moch, H. Diagnostic and prognostic molecular markers for renal cell carcinoma: A critical appraisal of the current state of research and clinical applicability. Eur. Urol. 2009, 55, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.L.; Palleschi, G.; Silvestri, L.; Moschese, D.; Ricci, S.; Petrozza, V.; Carbone, A.; di Carlo, A. Serum and urine biomarkers for human renal cell carcinoma. Dis. Markers 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Schon, E.A.; DiMauro, S.; Hirano, M. Human mitochondrial DNA: Roles of inherited and somatic mutations. Nat. Rev. Genet. 2012, 13, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Croteau, D.L.; Bohr, V.A. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J. Biol. Chem. 1997, 272, 25409–25412. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yin, P.H.; Lin, J.C.; Wu, C.C.; Chen, C.Y.; Wu, C.W.; Chi, C.W.; Tam, T.N.; Wei, Y.H. Mitochondrial genome instability and mtDNA depletion in human cancers. Ann. N. Y. Acad. Sci. 2005, 1042, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Mambo, E.; Sidransky, D. Mitochondrial DNA mutations in human cancer. Oncogene 2006, 25, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Guo, Z.; Xu, J.; Zhang, J.; Cui, L.; Zhang, H.; Zhang, S. The 9-bp deletion at position 8272 in region V of mitochondrial DNA is associated with renal cell carcinoma outcome. Mitochondrial DNA A 2016, 27, 1973–1975. [Google Scholar] [CrossRef] [PubMed]
- Grzybowska-Szatkowska, L.; Slaska, B. Mitochondrial NADH dehydrogenase polymorphisms are associated with breast cancer in Poland. J. Appl. Genet. 2014, 55, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Gasparre, G.; Iommarini, L.; Porcelli, A.M.; Lang, M.; Ferri, G.G.; Kurelac, I.; Zuntini, R.; Mariani, E.; Pennisi, L.F.; Pasquini, E.; et al. An inherited mitochondrial DNA disruptive mutation shifts to homoplasmy in oncocytic tumor cells. Hum. Mutat. 2009, 30, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Yusnita, Y.; Norsiah, M.D.; Rahman, A.J. Mutations in mitochondrial NADH dehydrogenase subunit 1 (mtND1) gene in colorectal carcinoma. Malays. J. Pathol. 2010, 32, 103–110. [Google Scholar] [PubMed]
- Komiyama, T.; Iwama, H.; Osada, N.; Nakamura, Y.; Kobayashi, H.; Tateno, Y.; Gojobori, T. Dopamine receptor genes and evolutionary differentiation in the domestication of fighting cocks and long-crowing chickens. PLoS ONE 2014, 9, e101778. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K. Mitochondrial DNA disorders. Eur. J. Pediatr. 2000, 159, S219–S226. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, J.; Dressman, D.C.; Iacobuzio-Donahue, C.; Markowitz, S.D.; Velculescu, V.E.; Diaz, L.A., Jr.; Kinzler, K.W.; Vogelstein, B.; Papadopoulos, N. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature 2010, 464, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C.; Chalkia, D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Vocke, C.D.; Merino, M.J.; Schmidt, L.S.; Linehan, W.M. Mitochondrial DNA mutations distinguish bilateral multifocal renal oncocytomas from familial Birt-Hogg-Dube tumors. Mod. Pathol. 2015, 28, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Gasparre, G.; Porcelli, A.M.; Bonora, E.; Pennisi, L.F.; Toller, M.; Iommarini, L.; Ghelli, A.; Moretti, M.; Betts, C.M.; Martinelli, G.N.; et al. Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 9001–9006. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, A.M.; Ghelli, A.; Ceccarelli, C.; Lang, M.; Cenacchi, G.; Capristo, M.; Pennisi, L.F.; Morra, I.; Ciccarelli, E.; Melcarne, A.; et al. The genetic and metabolic signature of oncocytic transformation implicates HIF1α destabilization. Hum. Mol. Genet. 2010, 19, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.J.; Lunetta, K.L.; van Orsouw, N.J.; Moore, F.D., Jr.; Mutter, G.L.; Vijg, J.; Dahia, P.L.; Eng, C. Somatic mitochondrial DNA (mtDNA) mutations in papillary thyroid carcinomas and differential mtDNA sequence variants in cases with thyroid tumours. Oncogene 2000, 19, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Guinan, P.D.; Vogelzang, N.J.; Fremgen, A.M.; Chmiel, J.S.; Sylvester, J.L.; Sener, S.F.; Imperato, J.P. Renal cell carcinoma: Tumor size, stage and survival. Members of the Cancer Incidence and End Results Committee. J. Urol. 1995, 153, 901–903. [Google Scholar] [CrossRef]
- Teng, J.; Gao, Y.; Chen, M.; Wang, K.; Cui, X.; Liu, Y.; Xu, D. Prognostic value of clinical and pathological factors for surgically treated localized clear cell renal cell carcinoma. Chin. Med. J. 2014, 127, 1640–1644. [Google Scholar] [PubMed]
- Kovacs, G. Molecular cytogenetics of renal cell tumors. Adv. Cancer Res. 1993, 62, 89–124. [Google Scholar] [PubMed]
- Long, J.P.; Anglard, P.; Gnarra, J.R.; Walther, M.M.; Merino, M.J.; Liu, S.; Lerman, M.I.; Zbar, B.; Linehan, W.M. The use of molecular genetic analysis in the diagnosis of renal cell carcinoma. World J. Urol. 1994, 12, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Lisztwan, J.; Imbert, G.; Wirbelauer, C.; Gstaiger, M.; Krek, W. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999, 13, 1822–1833. [Google Scholar] [CrossRef] [PubMed]
- Shiao, Y.H.; Resau, J.H.; Nagashima, K.; Anderson, L.M.; Ramakrishna, G. The von Hippel-Lindau tumor suppressor targets to mitochondria. Cancer Res. 2000, 60, 2816–2819. [Google Scholar] [PubMed]
- Ganesamoni, R.; Bhattacharyya, S.; Kumar, S.; Chauhan, A.; Mete, U.K.; Agarwal, M.M.; Mavuduru, R.; Kaushik, G.; Mandal, A.K.; Singh, S.K. Status of oxidative stress in patients with renal cell carcinoma. J. Urol. 2012, 187, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Sazuka, T.; Nihei, N.; Nakamura, K.; Sakamoto, S.; Fukasawa, S.; Komaru, A.; Ueda, T.; Igarashi, T.; Ichikawa, T. Interferon treatment for Japanese patients with favorable-risk metastatic renal cell carcinoma in the era of targeted therapy. Korean J. Urol. 2015, 56, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Takada, N.; Takase, S.; Takada, A.; Date, T. Differences in the hepatitis C virus genotypes in different countries. J. Hepatol. 1993, 17, 277–283. [Google Scholar] [CrossRef]
- Tomita, Y.; Shinohara, N.; Yuasa, T.; Fujimoto, H.; Niwakawa, M.; Mugiya, S.; Miki, T.; Uemura, H.; Nonomura, N.; Takahashi, M.; et al. Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn. J. Clin. Oncol. 2010, 40, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Sharma, L.K.; Li, H.; Xiang, R.; Holstein, D.; Wu, J.; Lechleiter, J.; Naylor, S.L.; Deng, J.J.; Lu, J.; et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum. Mol. Genet. 2009, 18, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]

| Age (years), Median (range) | 62 (39–80) |
| Sex (male/female) | 48/14 |
| Nephrectomy (total/partial) | 42/20 |
| Observation (months), median (range) | 66.5 (29–76) |
| Tumor diameter (mm), median, range | 32 (12–105) |
| Histology type | |
| clear | 55 |
| papillary | 2 |
| chromophobe | 3 |
| multilocular | 1 |
| acquired cystic disease (ACD)-associated | 1 |
| Pathological T stage | |
| T1a | 40 |
| T1b | 14 |
| T2a | 3 |
| T2b | 0 |
| T3a | 1 |
| T3b | 3 |
| T4 | 1 |
| Fuhrman | |
| G1 | 1 |
| G2 | 53 |
| G3 | 6 |
| G4 | 2 |
| Microvessel invasion | |
| V0 | 50 |
| V1 | 12 |
| Patient | Base Change | AA Change | Heteroplasmy | Histology Type | Outcome |
|---|---|---|---|---|---|
| hk347 | C4197Y * | + | Clear cell | No recurrence | |
| T4248Y | + | ||||
| hk355 | C3497T | A64V | Clear cell | No recurrence | |
| hk357 | C4197Y * | + | Clear cell | No recurrence | |
| hk362 | C3970Y | + | Clear cell | No recurrence | |
| hk363 | T4248Y | + | Clear cell | No recurrence | |
| hk364 | C3497T | A64V | Clear cell | No recurrence | |
| G3635A | S110N | ||||
| hk368 | C4197Y * | + | Clear cell | No recurrence | |
| T4248Y | + | ||||
| hk372 | A4200W | + | Clear cell | Recurrence | |
| T4216Y | Y304H | + | |||
| hk382 | A4200W | + | Clear cell | No recurrence | |
| T4216Y | Y304H | + | |||
| hk383 | C3572ins | L89P (Frameshift) | Clear cell | Recurrence | |
| hk385 | G3496T | A64S | Clear cell | Recurrence/Cancer Death | |
| C4141Y * | R279W | + | |||
| T4248Y | + | ||||
| hk387 | C4197Y * | + | Clear cell | Recurrence | |
| T4248Y | + | ||||
| hk392 | G4048R | D248N | + | ACD-associated RCC | Recurrence |
| C4071Y | + | ||||
| hk393 | T3368Y | M21T | + | Clear cell | No recurrence |
| hk394 | A4200W | + | Clear cell | Recurrence | |
| T4216Y | Y304H | + | |||
| hk399 | C3970Y | + | Clear cell | Recurrence | |
| hk401 | T4117Y | + | Clear cell | No recurrence (Figure 1) | |
| hk403 | C3328Y * | L8F | + | Clear cell | Recurrence/Cancer Death |
| C3970T | |||||
| hk405 | G4113R | + | Clear cell | No recurrence |
| Variables | No. of Cases | 5-Year RFS Rate (%) | p Value |
|---|---|---|---|
| Age (years) | 0.1736 | ||
| ≤60 | 28 | 88.7 | |
| >60 | 34 | 75.7 | |
| Sex | 0.2690 | ||
| Female | 14 | 91.7 | |
| Male | 48 | 78.7 | |
| Tumor localization | 0.7795 | ||
| Left | 24 | 83.1 | |
| Right | 38 | 80.4 | |
| Nephrectomy | 0.2951 | ||
| Total | 42 | 78.1 | |
| Partial | 20 | 89.2 | |
| Tumor diameter (mm) | 0.0091 ** | ||
| ≤40 | 42 | 89.9 | |
| >40 | 20 | 64.3 | |
| Histological type | 0.9142 | ||
| clear | 56 | 81.6 | |
| non-clear | 6 | 80.0 | |
| pT stage | 0.0122 ** | ||
| ≤pT2 | 57 | 85.6 | |
| ≥pT3 | 5 | 40.0 | |
| Fuhrman grade | 0.0070 ** | ||
| I/II | 54 | 86.6 | |
| III/IV | 8 | 50.0 | |
| Vessel invasion | 0.1389 | ||
| Presence | 50 | 66.7 | |
| Absence | 12 | 85.5 | |
| ND1 somatic mutation | 0.0006 ** | ||
| Presence | 19 | 57.9 | |
| Absence | 43 | 92.4 |
| Variables | Lower 0.95 | Upper 0.95 | Likelihood Ratio of Chi-Square | p Value |
|---|---|---|---|---|
| Tumor diameter | −1.425707 | 0.1053829 | 2.90251154 | 0.0884 |
| Pathological T stage | −1.420865 | 0.2705481 | 1.90465633 | 0.1676 |
| Fuhrman grade | −0.704015 | 1.1331817 | 0.16594524 | 0.6837 |
| Somatic mutation in ND | 0.3042389 | 1.8077773 | 8.11445592 | 0.0044 ** |
| Variables | No. of Cases | 5-Year CSS Rate (%) | p Value |
|---|---|---|---|
| Age (years) | 0.2085 | ||
| ≤60 | 28 | 100 | |
| >60 | 34 | 94.1 | |
| Sex | 0.4682 | ||
| Female | 14 | 100 | |
| Male | 48 | 95.8 | |
| Tumor localization | 0.2481 | ||
| Left | 24 | 100 | |
| Right | 38 | 94.5 | |
| Nephrectomy | 0.3356 | ||
| Total | 42 | 95.2 | |
| Partial | 20 | 100 | |
| Tumor diameter (mm) | 0.0415 ** | ||
| ≤40 | 42 | 100 | |
| >40 | 20 | 90.0 | |
| Histological type | 0.6373 | ||
| clear | 56 | 96.3 | |
| non-clear | 6 | 100 | |
| pT stage | 0.0330 ** | ||
| ≤pT2 | 57 | 98.2 | |
| ≥pT3 | 5 | 80.0 | |
| Fuhrman grade | 0.1272 | ||
| I/II | 54 | 98.1 | |
| III/IV | 8 | 87.5 | |
| Vessel invasion | 0.2890 | ||
| Presence | 50 | 91.7 | |
| Absence | 12 | 98.0 | |
| ND1 somatic mutation | 0.0341 ** | ||
| Presence | 19 | 89.5 | |
| Absence | 43 | 100 |
| Variables | Lower 0.95 | Upper 0.95 | Likelihood Ratio of Chi-Square | p Value |
|---|---|---|---|---|
| Tumor diameter | −43,798.79 | 43,776.674 | 2.20091038 | 0.1379 |
| Pathological T stage | −2.119379 | 1.1119271 | 0.48646922 | 0.4855 |
| Somatic mutation in ND1 | −24,003.11 | 24,024.239 | 3.0118406 | 0.0827 |
| Variables | Lower 0.95 | Upper 0.95 | Likelihood Ratio of Chi-Square | p Value |
|---|---|---|---|---|
| Tumor diameter | −1.474545 | 0.0066134 | 3.41616475 | 0.0646 |
| Fuhrman grade | −0.87471 | 0.7815922 | 0.01212236 | 0.9123 |
| Somatic mutation in ND1 | 0.0945703 | 1.5221494 | 5.33970621 | 0.0208 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Komiyama, T.; Inomoto, C.; Kamiguchi, H.; Kajiwara, H.; Kobayashi, H.; Nakamura, N.; Terachi, T. Mutations in the Mitochondrial ND1 Gene Are Associated with Postoperative Prognosis of Localized Renal Cell Carcinoma. Int. J. Mol. Sci. 2016, 17, 2049. https://doi.org/10.3390/ijms17122049
Kim H, Komiyama T, Inomoto C, Kamiguchi H, Kajiwara H, Kobayashi H, Nakamura N, Terachi T. Mutations in the Mitochondrial ND1 Gene Are Associated with Postoperative Prognosis of Localized Renal Cell Carcinoma. International Journal of Molecular Sciences. 2016; 17(12):2049. https://doi.org/10.3390/ijms17122049
Chicago/Turabian StyleKim, Hakushi, Tomoyoshi Komiyama, Chie Inomoto, Hiroshi Kamiguchi, Hiroshi Kajiwara, Hiroyuki Kobayashi, Naoya Nakamura, and Toshiro Terachi. 2016. "Mutations in the Mitochondrial ND1 Gene Are Associated with Postoperative Prognosis of Localized Renal Cell Carcinoma" International Journal of Molecular Sciences 17, no. 12: 2049. https://doi.org/10.3390/ijms17122049





