Hepatoprotective Effect of Cuscuta campestris Yunck. Whole Plant on Carbon Tetrachloride Induced Chronic Liver Injury in Mice
Abstract
:1. Introduction
2. Results and Discussion
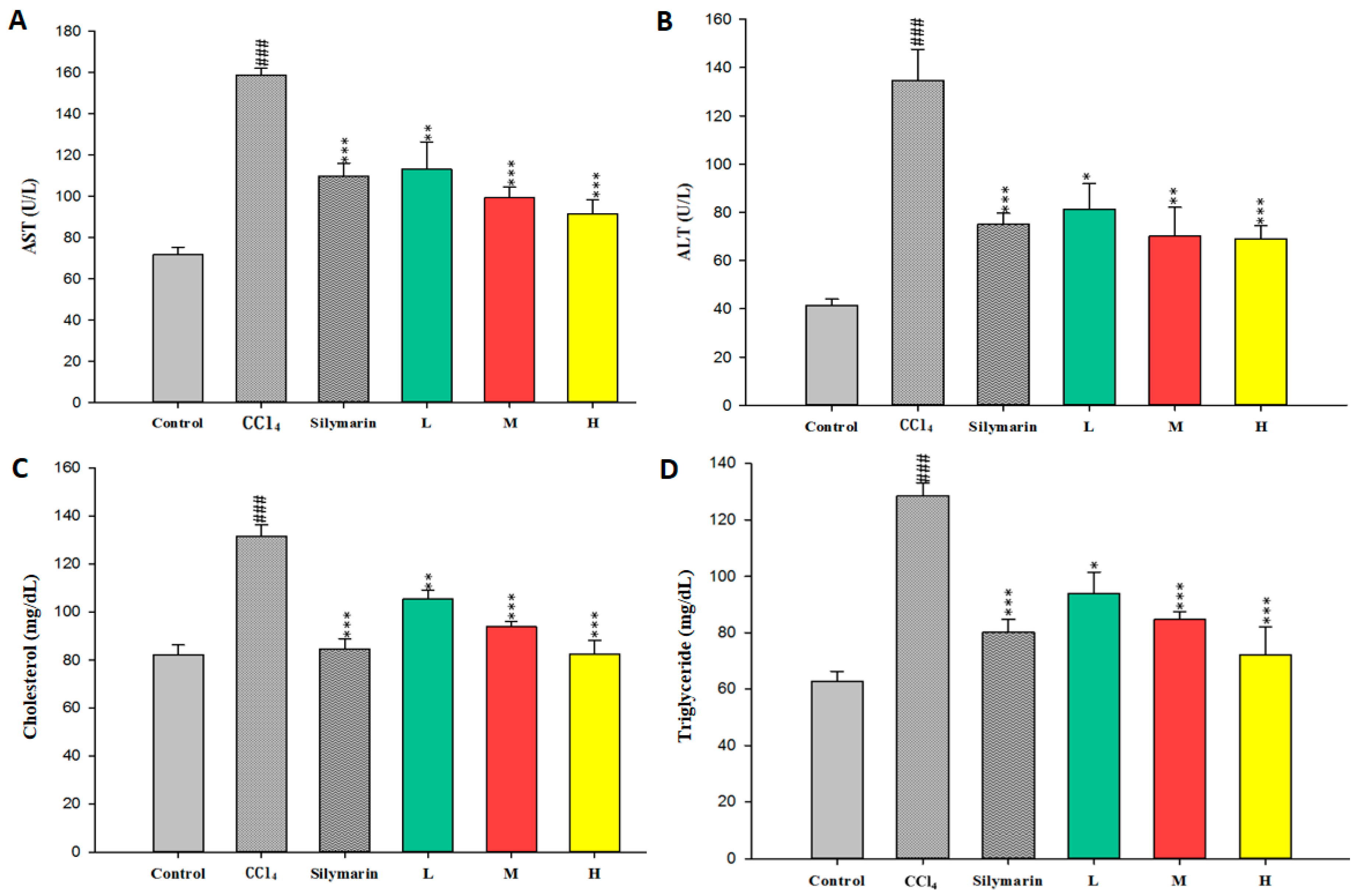
2.1. Effect of CCEtOH on CCl4-Induced Hepatotoxicity
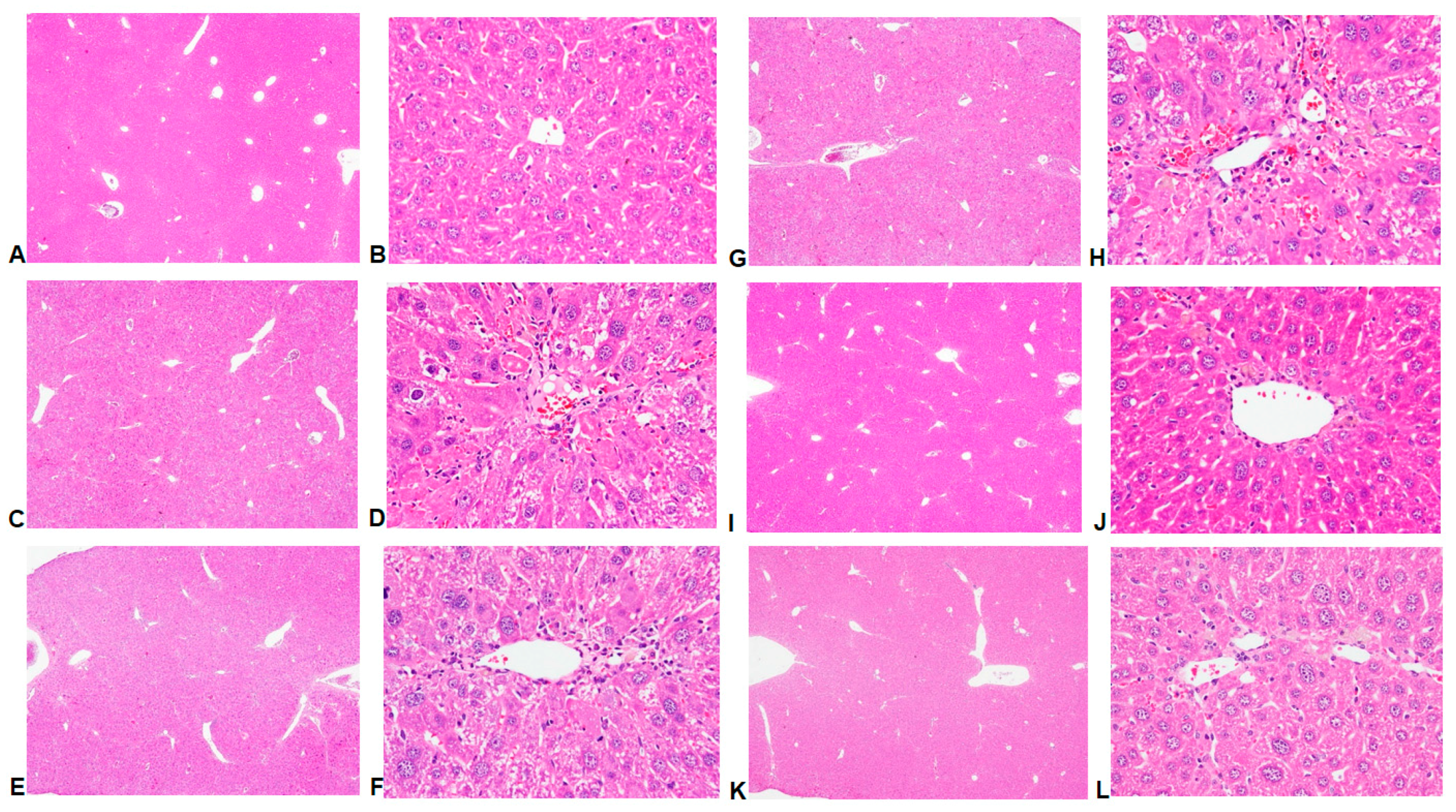
2.2. Histological Analyses
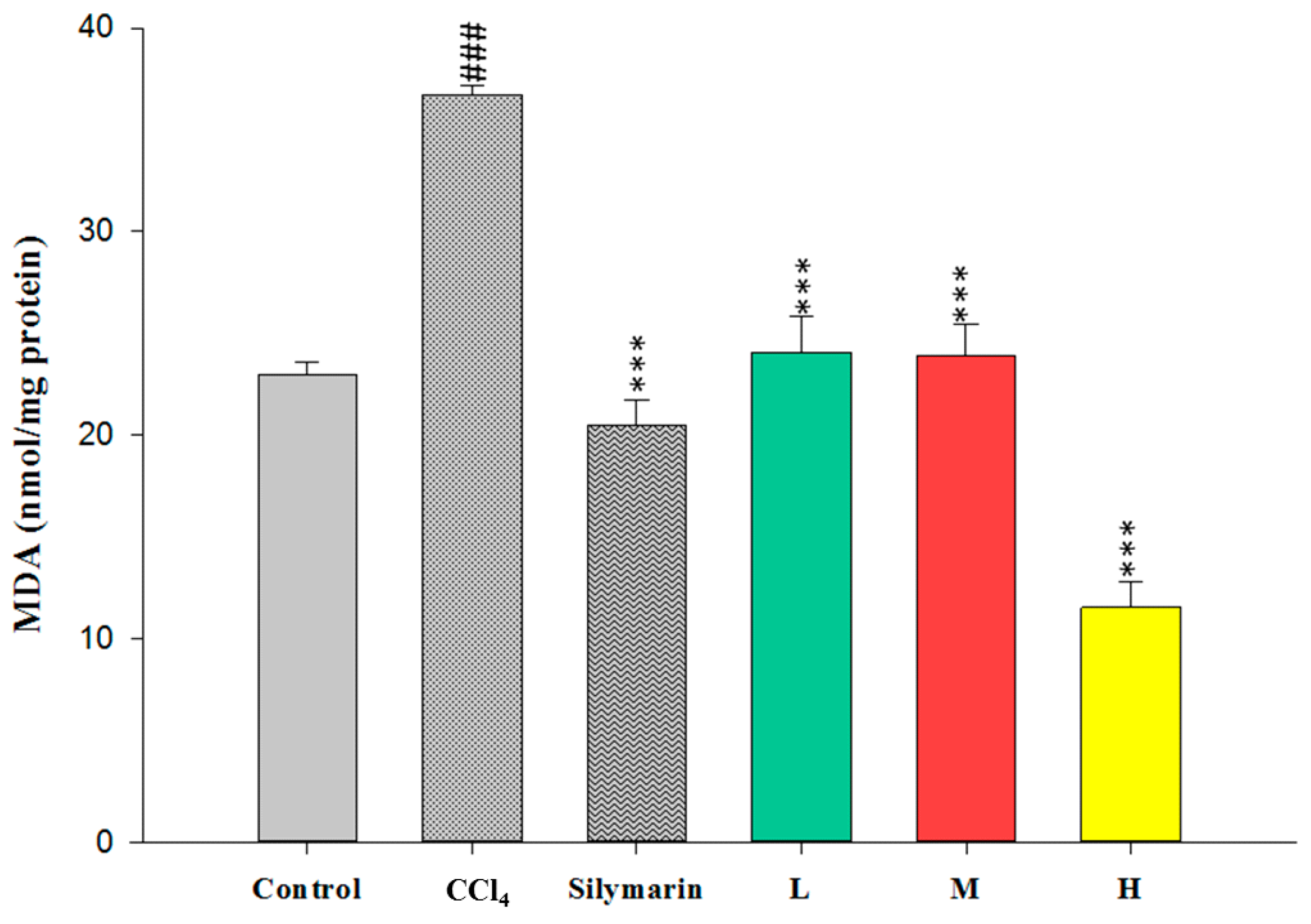
2.3. Effect of CCEtoH on MDA Level
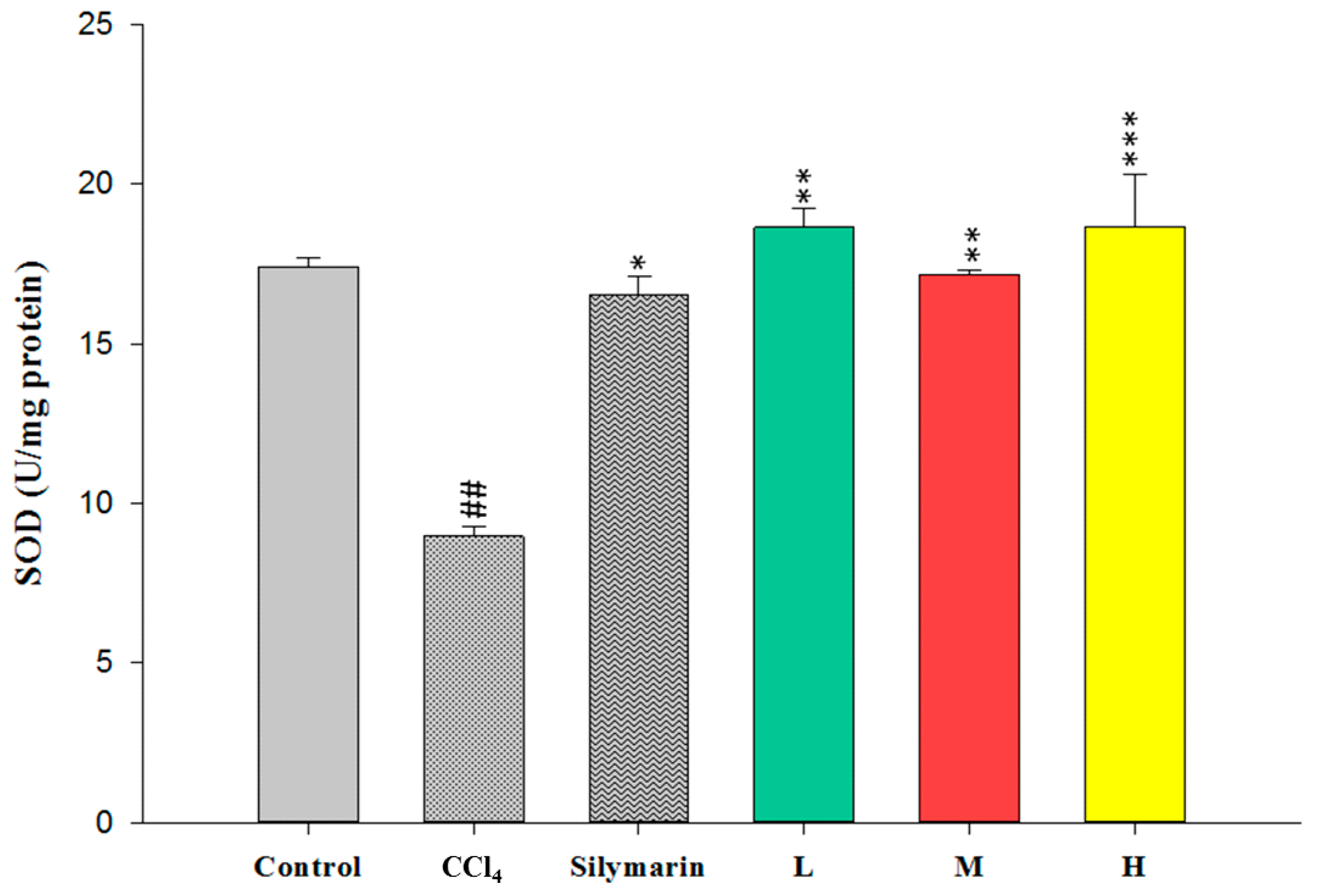
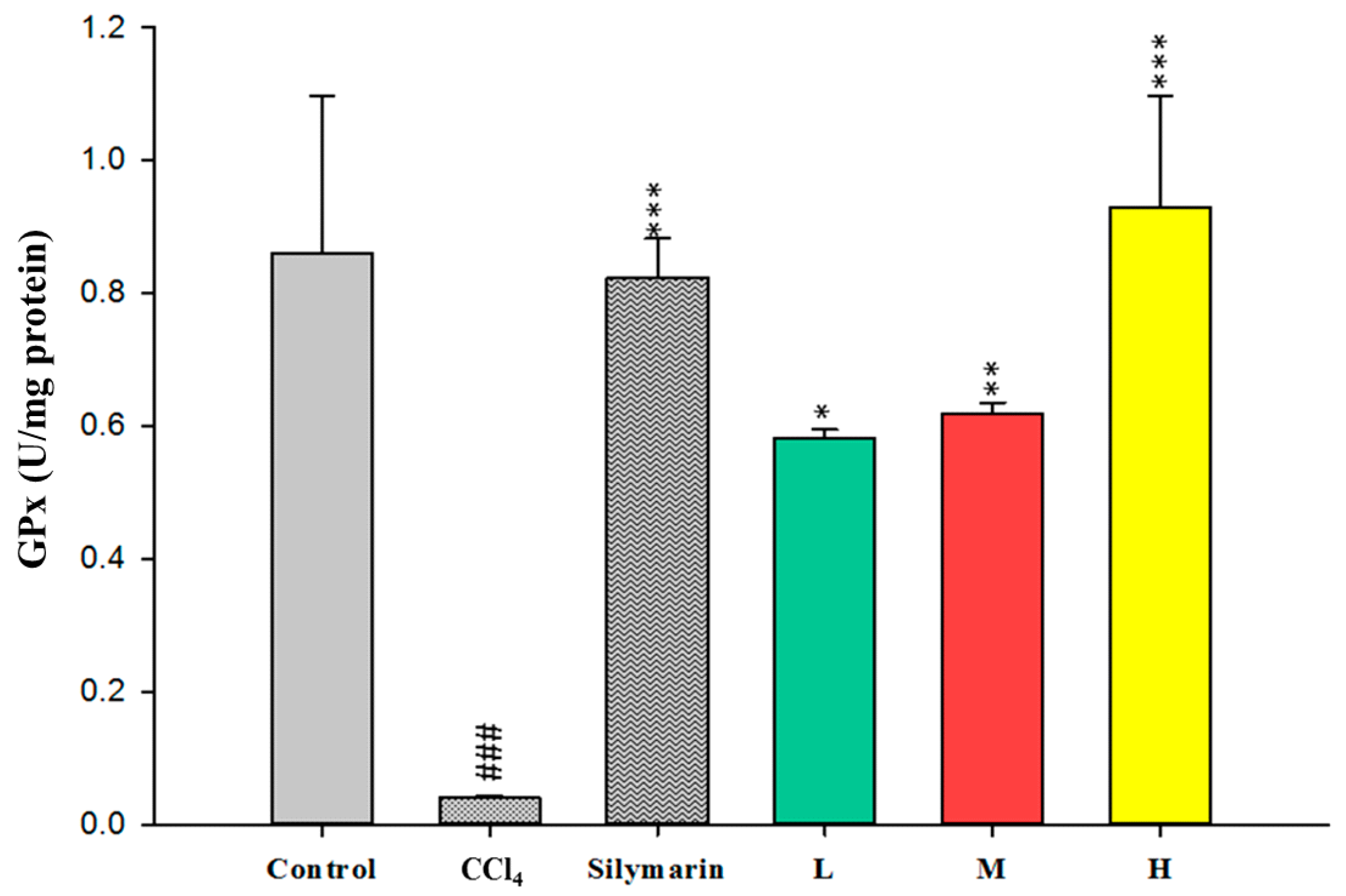
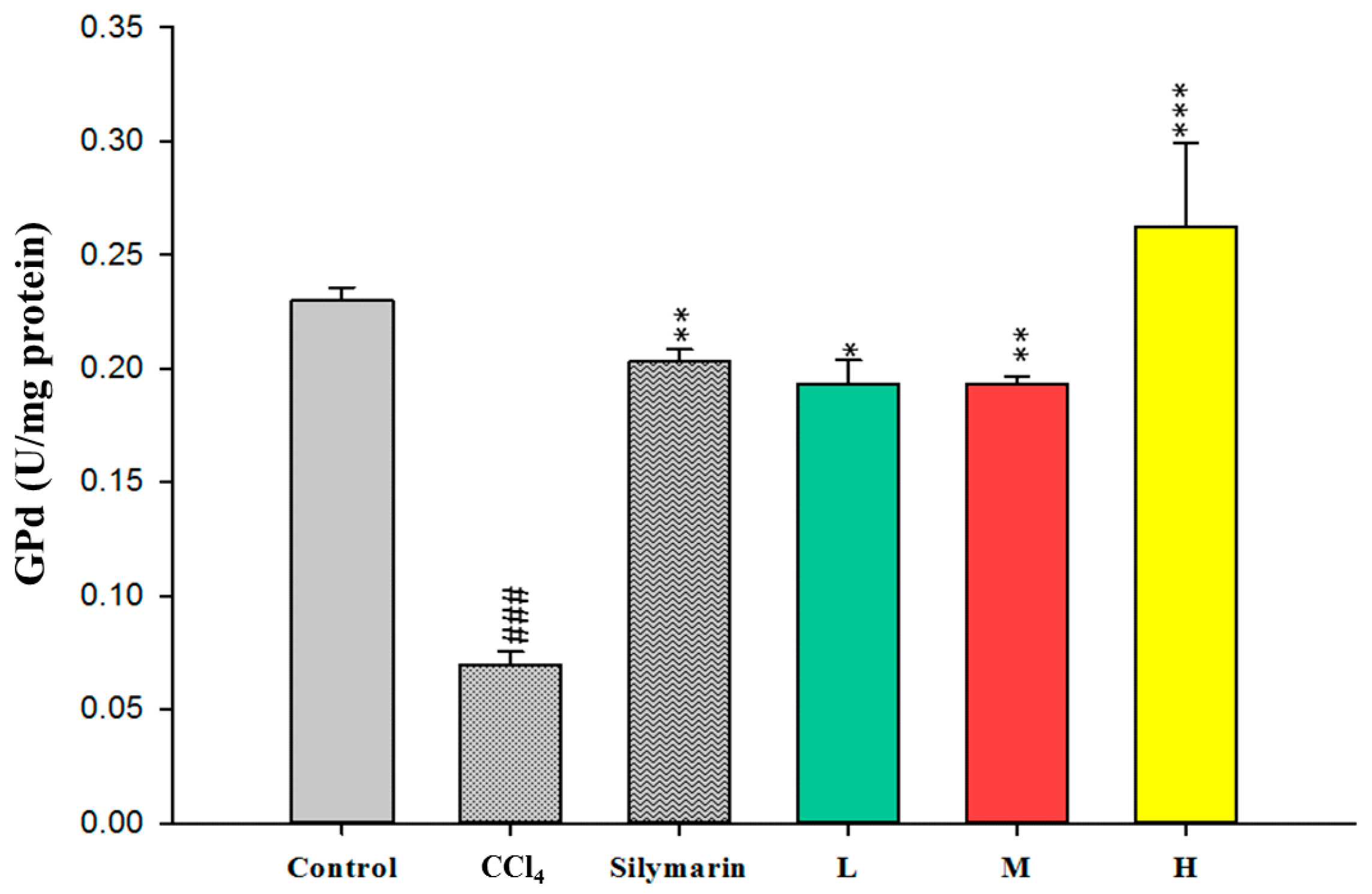
2.4. Effect of CCEtOH on Antioxidant Enzymatic Activities
2.5. Phytochemical Analysis of CCEtOH
3. Materials and Methods
3.1. Plant Materials and Preparation of Plant Extract
3.2. Chemicals
3.3. Experimental Animals
3.4. Experimental Design of CCl4-Induced Hepatotoxicity
3.5. Serum Biochemistry
3.6. Histological Analysis
3.7. MDA Level as Well as Antioxidant Enzymatic Activity Measurement
3.8. Statistical Analyses
3.9. Phytochemical Analysis of CCEtOH by HPLC
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donnapee, S.; Li, J.; Yang, X.; Ge, A.H.; Donkor, P.O.; Gao, X.M.; Chang, Y.X. Cuscuta chinensis Lam.: A systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. J. Ethnopharmacol. 2014, 157, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Bao, Y.; Guo, J. The total flavones from Semen cuscutae reverse the reduction of testosterone level and the expression of androgen receptor gene in kidney-yang deficient mice. J. Ethnopharmacol. 2008, 119, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.L.; Wu, T.H.; Lin, L.T.; Lin, C.C. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J. Ethnopharmacol. 2007, 111, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Chang, W.T.; Lee, M.S.; Chiu, Y.J.; Chao, W.K.; Lin, Y.C.; Lin, M.K.; Peng, W.H. Antinociceptive and anti-inflammatory activities of Cuscuta chinensis seeds in mice. Am. J. Chin. Med. 2014, 42, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wang, Z.; Fang, J.; Li, X. Structural features of an immunostimulating and antioxidant acidic polysaccharide from the seeds of Cuscuta chinensis. Planta Med. 2002, 68, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Heo, S.I.; Li, L.; Lee, M.J.; Wang, M.H. Antioxidant and hepatoprotective activities of Cirsium setidens NAKAI against CCl4-induced liver damage. Am. J. Chin. Med. 2008, 36, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, N.L.; Zhang, W.D.; Li, H.L.; Lu, G.C.; Yuan, B.J.; Jiang, H.; She, J.H.; Zhang, C. Protective effect of dehydrocavidine on carbon tetrachloride-induced acute hepatotoxicity in rats. J. Ethnopharmacol. 2008, 117, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Peng, W.H.; Chiu, T.H.; Huang, S.C.; Huang, T.H.; Lai, S.C.; Lai, Z.R.; Lee, C.Y. Hepatoprotective effect of Scoparia dulcis on carbon tetrachloride induced acute liver injury in mice. Am. J. Chin. Med. 2010, 38, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Brattin, W.J.; Glende, E.A., Jr.; Recknagel, R.O. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J. Free Radic. Biol. Med. 1985, 1, 27–38. [Google Scholar] [CrossRef]
- Basu, S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 2003, 189, 113–127. [Google Scholar] [CrossRef]
- Lee, C.Y.; Peng, W.H.; Cheng, H.Y.; Chen, F.N.; Lai, M.T.; Chiu, T.H. Hepatoprotective effect of Phyllanthus in Taiwan on acute liver damage induced by carbon tetrachloride. Am. J. Chin. Med. 2006, 34, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.W.; Boll, M.; Stampfl, A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Adewole, S.O.; Salako, A.A.; Doherty, O.W.; Naicker, T. Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. Afr. J. Biomed. Res. 2007, 10, 153–164. [Google Scholar] [CrossRef]
- Ruwart, M.J.; Wilkinson, K.F.; Rush, B.D.; Vidmar, T.J.; Peters, K.M.; Henley, K.S.; Appelman, H.D.; Kim, K.Y.; Schuppan, D.; Hahn, E.G. The integrated value of serum procollagen III peptide over time predicts hepatic hydroxyproline content and stainable collagen in a model of dietary cirrhosis in the rat. Hepatology 1989, 10, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Manibusan, M.K.; Odin, M.; Eastmond, D.A. Postulated carbon tetrachloride mode of action: A review. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2007, 25, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, R.O.; Glende, E.A., Jr.; Dolak, J.A.; Waller, R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989, 43, 139–154. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, S.W.; Kim, Y.S.; Kang, S.S.; Kim, J.A.; Lee, S.H.; Lee, S.M. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. Biol. Pharm. Bull. 2007, 30, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.H.; Chien, T.Y.; Tzeng, C.F.; Lin, Y.H.; Wu, C.H.; Wang, C.C. Prevention of hepatic oxidative injury by Xiao-Chen-Chi-Tang in mice. J. Ethnopharmacol. 2007, 111, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.Y.; Fu, T.Y.; Shih, P.H.; Lee, C.P.; Yen, G.C. Du-Zhong (Eucommia ulmoides Oliv.) leaves inhibit CCl4-induced hepatic damage in rats. Food Chem. Toxicol. 2006, 44, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Chen, C.J.; Wan, L.; Koizumi, A.; Chang, W.T.; Yang, M.J.; Lin, W.H.; Tsai, F.J.; Lin, M.K. Quercetin is increased in heat-processed Cuscuta campestris seeds, which enhances the seed’s anti-inflammatory and anti-cancer activities. Process Biochem. 2011, 46, 2248–2254. [Google Scholar] [CrossRef]
- Park, J.Y.; Han, X.; Piao, M.J.; Oh, M.C.; Fernando, P.M.; Kang, K.A.; Ryu, Y.S.; Jung, U.; Kim, I.G.; Hyun, J.W. Hyperoside induces endogenous antioxidant system to alleviate oxidative stress. J. Cancer Prev. 2016, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Antioxidant properties of quercetin. Adv. Exp. Med. Biol. 2011, 701, 283–289. [Google Scholar] [PubMed]
- Choi, J.H.; Kim, D.W.; Yun, N.; Choi, J.S.; Islam, M.N.; Kim, Y.S.; Lee, S.M. Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J. Nat. Prod. 2011, 74, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Jakovac, H.; Vasiljev Marchesi, V.; Vladimir-Knežević, S.; Cvijanović, O.; Tadić, Z.; Romić, Z.; Rahelić, D. Differential hepatoprotective mechanisms of rutin and quercetin in CCl4-intoxicated BALB/cN mice. Acta Pharmacol. Sin. 2012, 33, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1984, 113, 484–490. [Google Scholar]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [PubMed]
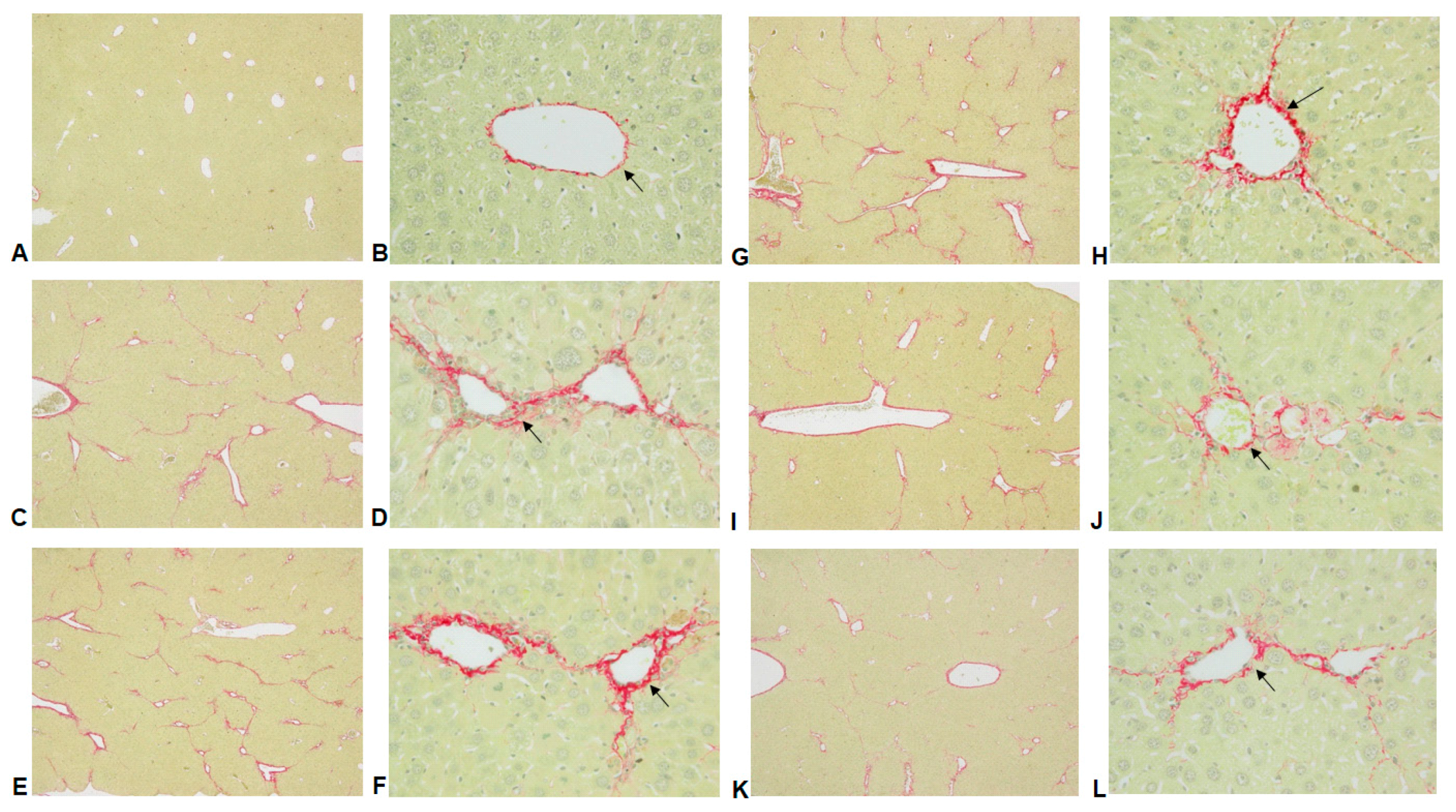
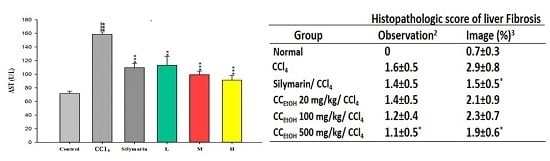
| Group | Histopathologic Score of Liver Fibrosis | |
|---|---|---|
| Observation 2 | Image (%) 3 | |
| Normal | 0 | 0.7 ± 0.3 |
| CCl4 | 1.6 ± 0.5 | 2.9 ± 0.8 |
| Silymarin/CCl4 | 1.4 ± 0.5 | 1.5 ± 0.5 * |
| CCEtOH 20 mg/kg/CCl4 | 1.4 ± 0.5 | 2.1 ± 0.9 |
| CCEtOH 100 mg/kg/CCl4 | 1.2 ± 0.4 | 2.3 ± 0.7 |
| CCEtOH 500 mg/kg/CCl4 | 1.1 ± 0.5 * | 1.9 ± 0.6 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, W.-H.; Chen, Y.-W.; Lee, M.-S.; Chang, W.-T.; Tsai, J.-C.; Lin, Y.-C.; Lin, M.-K. Hepatoprotective Effect of Cuscuta campestris Yunck. Whole Plant on Carbon Tetrachloride Induced Chronic Liver Injury in Mice. Int. J. Mol. Sci. 2016, 17, 2056. https://doi.org/10.3390/ijms17122056
Peng W-H, Chen Y-W, Lee M-S, Chang W-T, Tsai J-C, Lin Y-C, Lin M-K. Hepatoprotective Effect of Cuscuta campestris Yunck. Whole Plant on Carbon Tetrachloride Induced Chronic Liver Injury in Mice. International Journal of Molecular Sciences. 2016; 17(12):2056. https://doi.org/10.3390/ijms17122056
Chicago/Turabian StylePeng, Wen-Huang, Yi-Wen Chen, Meng-Shiou Lee, Wen-Te Chang, Jen-Chieh Tsai, Ying-Chih Lin, and Ming-Kuem Lin. 2016. "Hepatoprotective Effect of Cuscuta campestris Yunck. Whole Plant on Carbon Tetrachloride Induced Chronic Liver Injury in Mice" International Journal of Molecular Sciences 17, no. 12: 2056. https://doi.org/10.3390/ijms17122056