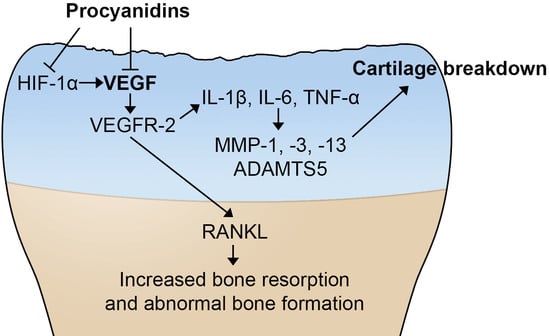
Procyanidins Mitigate Osteoarthritis Pathogenesis by, at Least in Part, Suppressing Vascular Endothelial Growth Factor Signaling
Abstract
:1. Introduction
2. Results
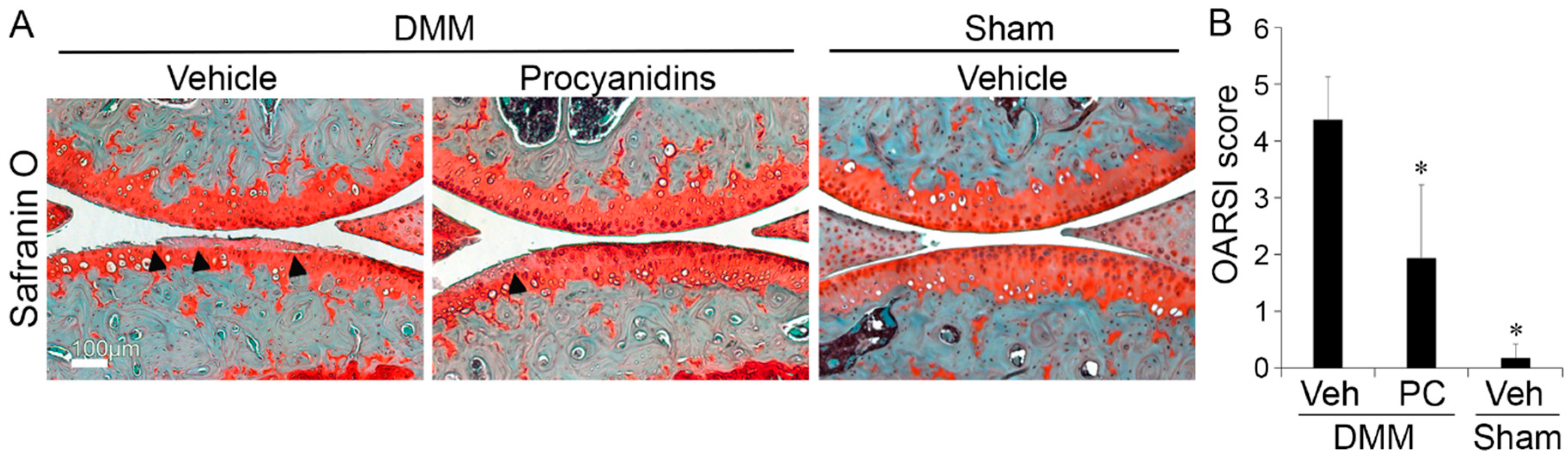
2.1. Procyanidins Slowed Destabilization of the Medial Meniscus (DMM)-Induced Osteoarthritis (OA) Disease Progression in Mice
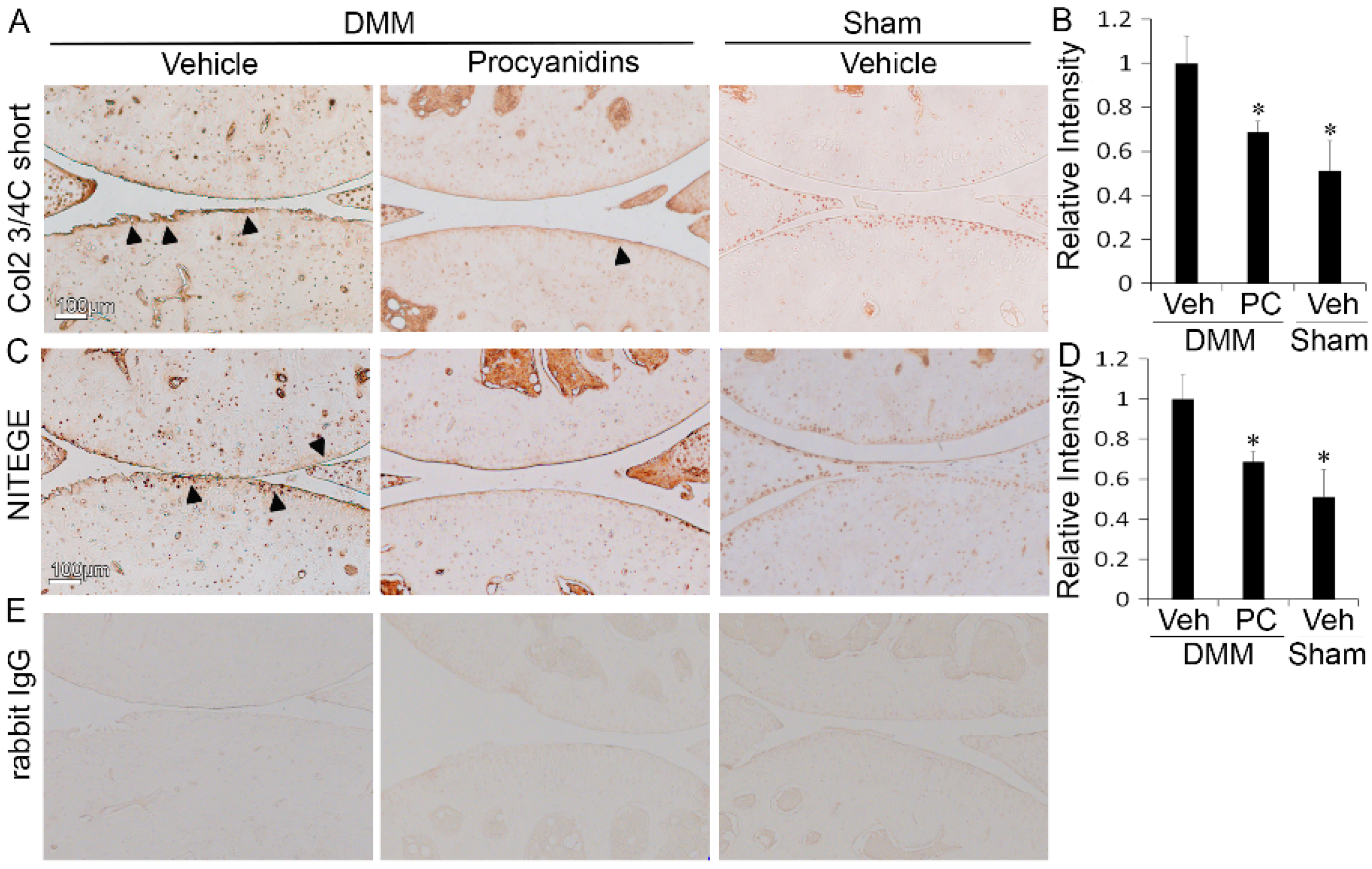
2.2. Procyanidins Reduced Levels of Cleaved Type II Collagen and Cleaved Aggrecan in the Articular Cartilage of Mice Subjected to DMM Surgery
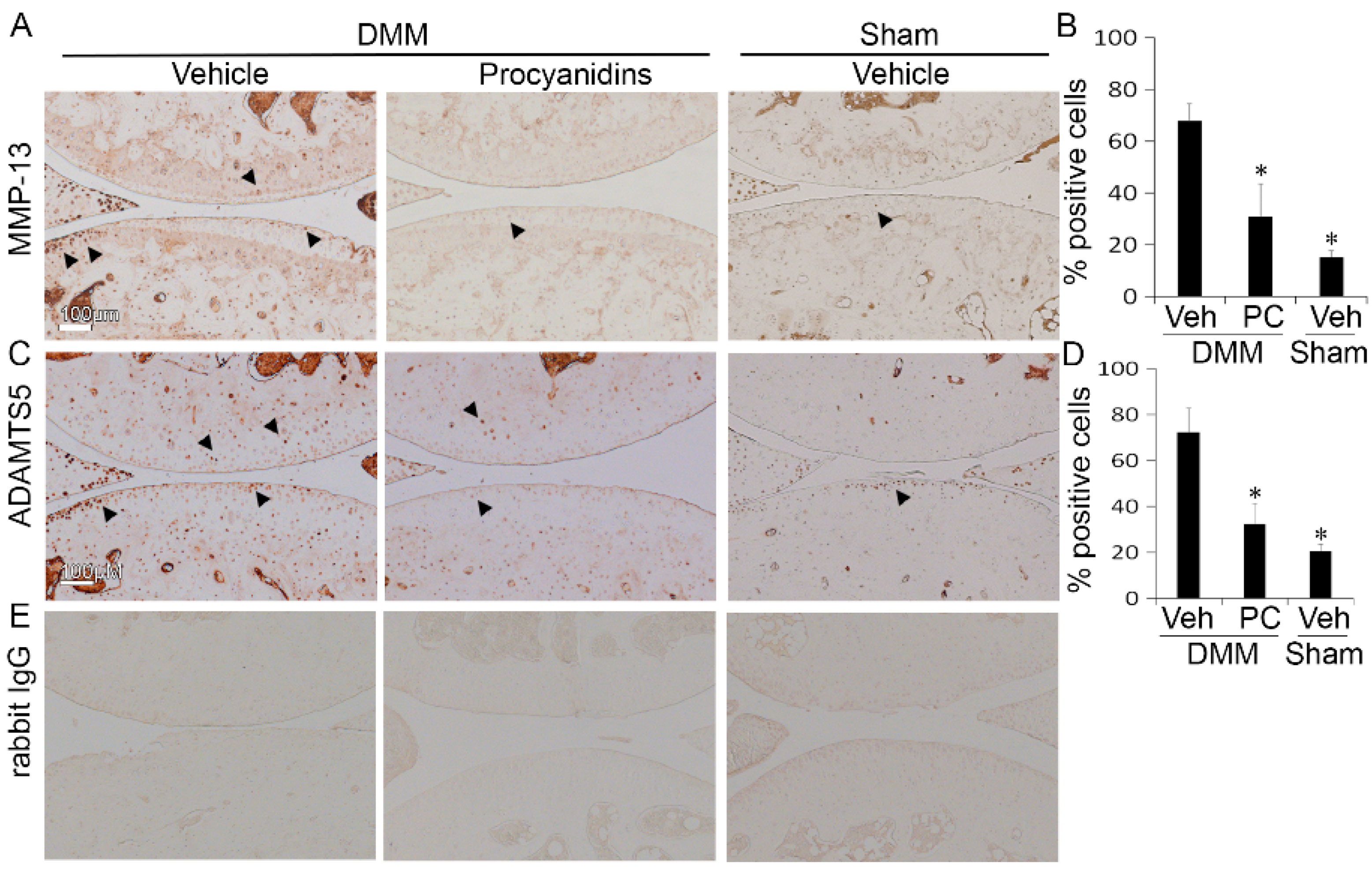
2.3. Procyanidins Reduced Protein Levels of Matrix Metalloproteinase 13 (MMP-13) and a Disintegrin and Metalloproteinase with Thrombospondin Motifs 5 (ADAMTS5) in Cartilage of Mice Subjected to DMM Surgery
2.4. Procyanidins Suppressed Expression of Vascular Endothelial Growth Factor (VEGF) and Genes that May Be Regulated by VEGF and Involved in OA Pathogenesis in Cartilage of Mice with DMM-Induced OA
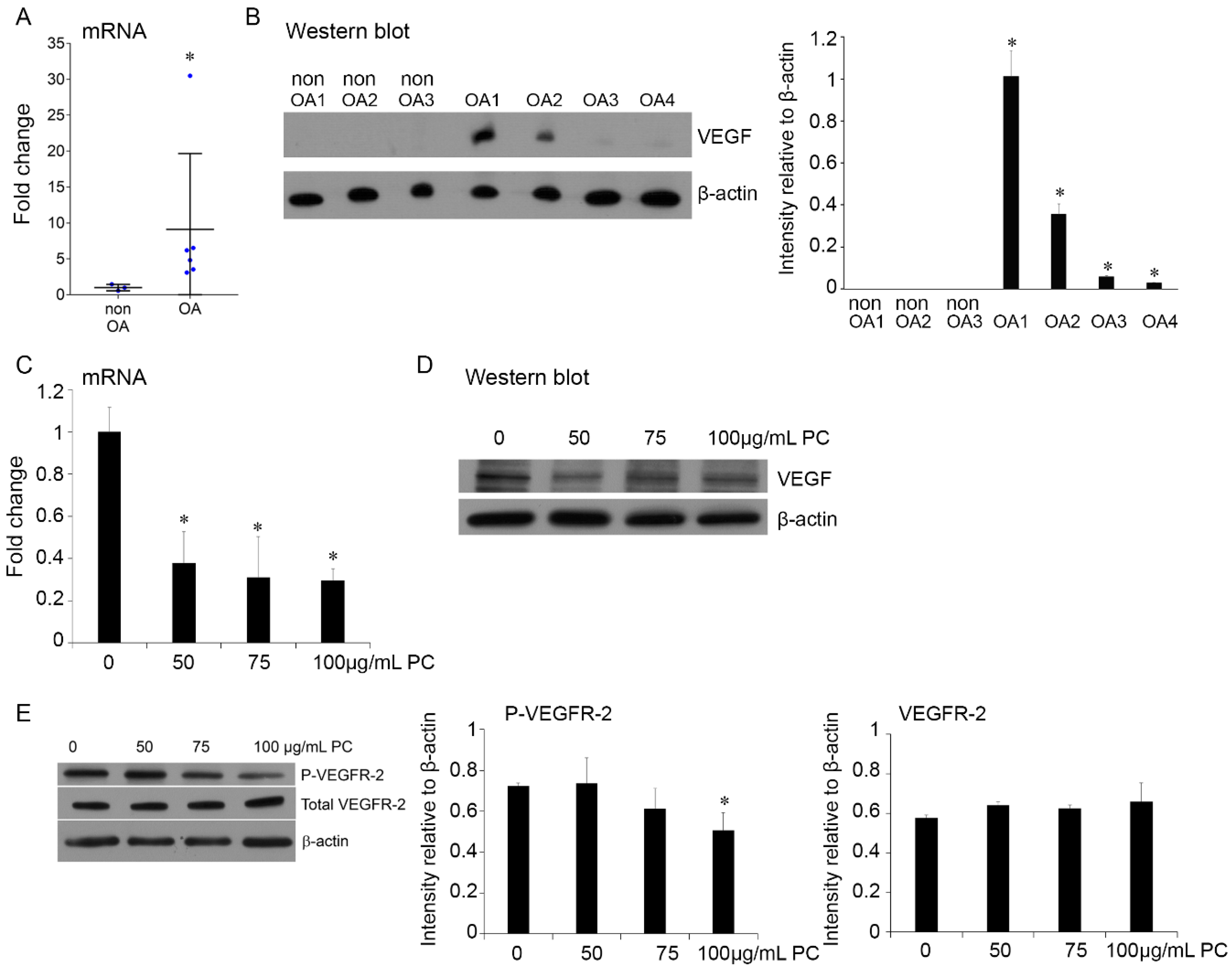
2.5. Procyanidins Reduced Expression of VEGF and Phosphorylatison of VEGF Receptor in Primary Human OA Chondrocytes
2.6. Procyanidins B2 and B3 in Procyanidins Were Primarily Responsible for Reduction in VEGF Signaling
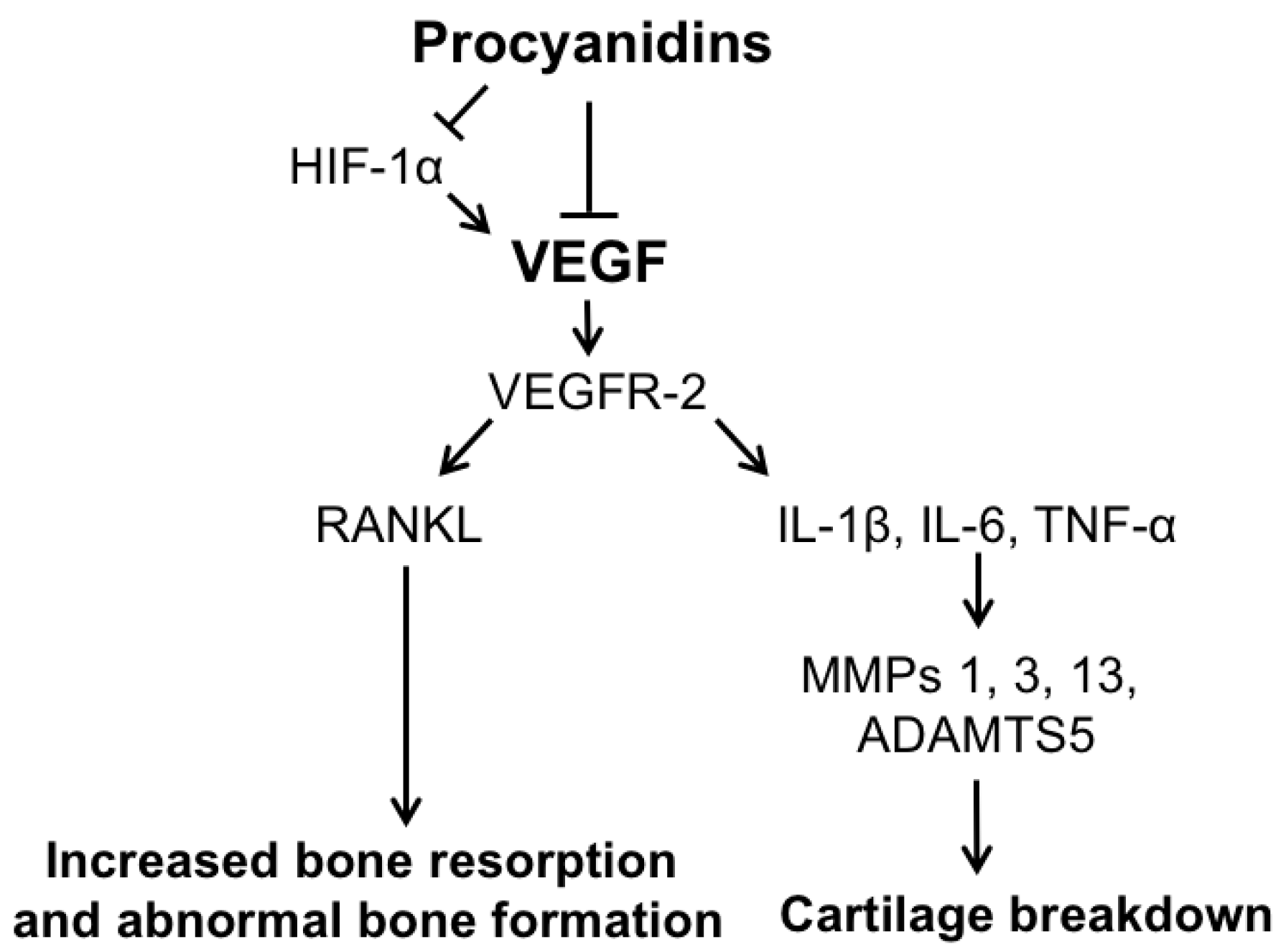
3. Discussion
4. Materials and Methods
4.1. Primary Human Chondrocytes Culture
4.2. Procyanidins Preparation and Treatment In Vitro
4.3. DMM Mouse Model and Procyanidins Treatment In Vivo
4.4. Safranin-O and Immunohistochemistry
4.5. RNA Isolation and Real-Time PCR
4.6. Protein Extraction and Western Blot
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACAN | aggrecan |
| ADAMTS5 | A disintegrin and metalloproteinase with thrombospondin motifs 5 |
| Col2a1 | type II collagen |
| DMM | destabilization of the medial meniscus |
| ECM | extracellular matrix |
| GRAS | generally recognized as safe |
| Hif1-α | hypoxia-inducible factor 1-alpha |
| IL | interleukin |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| MMP | matrix metalloproteinase |
| OA | osteoarthritis |
| PC | procyanidins |
| PCR | polymerase chain reaction |
| RANKL | receptor activator of nuclear factor κ-B ligand |
| ROS | reactive oxygen species |
| TNF-α | tumor necrosis factor α |
| VEGF | vascular endothelial growth factor |
| VEGFR-2 | vascular endothelial growth factor receptor 2 |
References
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Benderdour, M.; Martel-Pelletier, J.; Pelletier, J.P.; Kapoor, M.; Zunzunegui, M.V.; Fahmi, H. Cellular aging, senescence and autophagy processes in osteoarthritis. Curr. Aging Sci. 2015, 8, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Rodeo, S. A review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J. Orthop. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C.R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Mascarenhas, R.; Saltzman, B.M.; Rollins, M.; Bach, B.R., Jr.; MacDonald, P. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv. Orthop. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst Century: Risk factors and behaviours that influence disease onset and progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Hunter, D.J. Emerging drugs for the treatment of knee osteoarthritis. Expert Opin. Emerg. Drugs 2015, 20, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Le Graverand-Gastineau, M.P. Disease modifying osteoarthritis drugs: Facing development challenges and choosing molecular targets. Curr. Drug Targets 2010, 11, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Wildi, L.M.; Pelletier, J.P. Future therapeutics for osteoarthritis. Bone 2012, 51, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Haqqi, T.M. Current nutraceuticals in the management of osteoarthritis: A review. Ther. Adv. Musculoskelet. Dis. 2012, 4, 181–207. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Choudhury, M.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Nutraceuticals: Potential for chondroprotection and molecular targeting of osteoarthritis. Int. J. Mol. Sci. 2013, 14, 23063–23085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, S.J.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model. Arthritis Res. Ther. 2014, 16, 508. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Kitadate, K.; Nishioka, H.; Fujii, H.; Kizaki, T.; Kondoh, Y.; Izawa, T.; Ishida, H.; Radak, Z.; Ohno, H. Oligomerized grape seed polyphenols attenuate inflammatory changes due to antioxidative properties in coculture of adipocytes and macrophages. J. Nutr. Biochem. 2010, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Hsu, Y.M.; Jhan, Y.L.; Tsai, S.J.; Lin, S.X.; Su, C.H.; Chou, C.H. structure elucidation of procyanidins isolated from rhododendron formosanum and their anti-oxidative and anti-bacterial activities. Molecules 2015, 20, 12787–12803. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, Y.A.; Yokozawa, T. Pycnogenol modulates apoptosis by suppressing oxidative stress and inflammation in high glucose-treated renal tubular cells. Food Chem. Toxicol. 2011, 49, 2196–2201. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; Park, S.; Lim, B.J.; Seong, A.R.; Lee, Y.H.; Shiota, M.; Yokomizo, A.; Naito, S.; Na, Y.; Yoon, H.G. Procyanidin B3, an inhibitor of histone acetyltransferase, enhances the action of antagonist for prostate cancer cells via inhibition of p300-dependent acetylation of androgen receptor. Biochem. J. 2011, 433, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.T.; Xue, B.; Smoake, J.; Lu, Q.Y.; Park, H.; Henning, S.M.; Burns, W.; Bernabei, A.; Elashoff, D.; Serio, K.J.; et al. MicroRNA-19a/b mediates grape seed procyanidin extract-induced anti-neoplastic effects against lung cancer. J. Nutr. Biochem. 2016, 34, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, V.; Singh, T.; Katiyar, S.K. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008, 269, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Du, P.; Meiser, P.; Jacob, C. Proanthocyanidins: Oligomeric structures with unique biochemical properties and great therapeutic promise. Nat. Prod. Commun. 2012, 7, 381–388. [Google Scholar] [PubMed]
- Iravani, S.; Zolfaghari, B. Pharmaceutical and nutraceutical effects of Pinus pinaster bark extract. Res. Pharm. Sci. 2011, 6, 1–11. [Google Scholar] [PubMed]
- Tokoudagba, J.M.; Auger, C.; Breant, L.; N’Gom, S.; Chabert, P.; Idris-Khodja, N.; Gbaguidi, F.; Gbenou, J.; Moudachirou, M.; Lobstein, A.; et al. Procyanidin-rich fractions from Parkia biglobosa (Mimosaceae) leaves cause redox-sensitive endothelium-dependent relaxation involving NO and EDHF in porcine coronary artery. J. Ethnopharmacol. 2010, 132, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; Chen, Y.; Agarwal, R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation–promotion protocol and identification of procyanidin B5–3’-gallate as the most effective antioxidant constituent. Carcinogenesis 1999, 20, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.M.; Cardile, V.; Avondo, S.; Garufi, F.; Gentile, B.; Puglia, C.; Bonina, F.; Santagati, N.A.; Ronsisvalle, G. The in vitro effect of a lyophilized extract of wine obtained from Jacquez grapes on human chondrocytes. Phytomedicine 2006, 13, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.J.; Joo, Y.B.; Jung, Y.O.; Ju, J.H.; Cho, M.L.; Oh, H.J.; Jhun, J.Y.; Park, M.K.; Park, J.S.; Kang, C.M.; et al. Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate-induced osteoarthritis. Exp. Mol. Med. 2011, 43, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Bang, J.; Son, C.N.; Baek, W.K.; Kim, J.M. Grape seed proanthocyanidin extract ameliorates murine autoimmune arthritis through regulation of TLR4/MyD88/NF-κB signaling pathway. Korean J. Intern. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Aini, H.; Ochi, H.; Iwata, M.; Okawa, A.; Koga, D.; Okazaki, M.; Sano, A.; Asou, Y. Procyanidin B3 prevents articular cartilage degeneration and heterotopic cartilage formation in a mouse surgical osteoarthritis model. PLoS ONE 2012, 7, e37728. [Google Scholar] [CrossRef] [PubMed]
- Takano, F.; Takata, T.; Yoshihara, A.; Nakamura, Y.; Arima, Y.; Ohta, T. Aqueous extract of peanut skin and its main constituent procyanidin A1 suppress serum IgE and IgG1 levels in mice-immunized with ovalbumin. Biol. Pharm. Bull. 2007, 30, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Tomochika, K.; Shimizu-Ibuka, A.; Tamura, T.; Mura, K.; Abe, N.; Onose, J.; Arai, S. Effects of peanut-skin procyanidin A1 on degranulation of RBL-2H3 cells. Biosci. Biotechnol. Biochem. 2011, 75, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Inoue, N.; Ozawa, M.; Shimizu-Ibuka, A.; Arai, S.; Abe, N.; Koshino, H.; Mura, K. Peanut-skin polyphenols, procyanidin A1 and epicatechin-(4 β→6)-epicatechin-(2 β→O→7, 4 β→8)-catechin, exert cholesterol micelle-degrading activity in vitro. Biosci. Biotechnol. Biochem. 2013, 77, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.L.; Kruger, M.C.; Sawyer, G.M.; Hurst, R.D. Procyanidin A2 Modulates IL-4-Induced CCL26 Production in Human Alveolar Epithelial Cells. Int. J. Mol. Sci. 2016, 17, 1888. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Afshari, G.; Mard, S.A.; Khodadadi, A.; Hashemitabar, M. Preventive effects of procyanidin A2 on glucose homeostasis, pancreatic and duodenal homebox 1, and glucose transporter 2 gene expression disturbance induced by bisphenol A in male mice. J. Physiol. Pharmacol. 2016, 67, 243–252. [Google Scholar] [PubMed]
- Xing, J.; Li, R.; Li, N.; Zhang, J.; Li, Y.; Gong, P.; Gao, D.; Liu, H.; Zhang, Y. Anti-inflammatory effect of procyanidin B1 on LPS-treated THP1 cells via interaction with the TLR4-MD-2 heterodimer and p38 MAPK and NF-κB signaling. Mol. Cell. Biochem. 2015, 407, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kodama, E.N.; Inoue, Y.; Tani, H.; Matsuura, Y.; Zhang, J.; Tanaka, T.; Hattori, T. Procyanidin B1 purified from Cinnamomi cortex suppresses hepatitis C virus replication. Antivir. Chem. Chemother. 2010, 20, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Ishihara, S.; Okamoto, T.; Doi, S.; Harui, K.; Higashino, Y.; Kawasaki, T.; Nakajima, N.; Saito, A. Inhibitory activity of synthesized acetylated Procyanidin B1 analogs against HeLa S3 cells proliferation. Molecules 2014, 19, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, B.Y.; Li, X.L.; Wang, Y.J.; Zhang, Z.; Pei, F.; Wang, Q.Z.; Zhang, J.; Cai, Y.W.; Cheng, M.; et al. Restoration of Mimecan Expression by Grape Seed Procyanidin B2 Through Regulation of Nuclear Factor-κB in Mice With Diabetic Nephropathy. Iran. J. Kidney Dis. 2016, 10, 325–331. [Google Scholar] [PubMed]
- Cai, X.; Bao, L.; Ren, J.; Li, Y.; Zhang, Z. Grape seed procyanidin B2 protects podocytes from high glucose-induced mitochondrial dysfunction and apoptosis via the AMPK-SIRT1-PGC-1α axis in vitro. Food Funct. 2016, 7, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiao, L.; Yuan, Y.; Luo, X.; Jiang, M.; Ni, J.; Wang, N. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem. Pharmacol. 2014, 92, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Avelar, M.M.; Gouvea, C.M. Procyanidin B2 cytotoxicity to MCF-7 human breast adenocarcinoma cells. Indian J. Pharm. Sci. 2012, 74, 351–355. [Google Scholar] [PubMed]
- Oizumi, Y.; Mohri, Y.; Hirota, M.; Makabe, H. Synthesis of procyanidin B3 and its anti-inflammatory activity. The effect of 4-alkoxy group of catechin electrophile in the Yb(OTf)3-catalyzed condensation with catechin nucleophile. J. Org. Chem. 2010, 75, 4884–4886. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Fernandes, V.C.; Araujo, P.; Mateus, N.; de Freitas, V. Synthesis, characterisation and antioxidant features of procyanidin B4 and malvidin-3-glucoside stearic acid derivatives. Food Chem. 2015, 174, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Barnor, J.; Huu, T.N.; Morinaga, O.; Hamano, A.; Ndzinu, J.; Frimpong, A.; Minta-Asare, K.; Amoa-Bosompem, M.; Brandful, J.; et al. Procyanidin trimer C1 derived from Theobroma cacao reactivates latent human immunodeficiency virus type 1 provirus. Biochem. Biophys. Res. Commun. 2015, 459, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Nishiyama, C.; Tokura, T.; Nagasako-Akazome, Y.; Ohtake, Y.; Okumura, K.; Ogawa, H. Procyanidin C1 from apple extracts inhibits FcεRI-mediated mast cell activation. Int. Arch. Allergy Immunol. 2008, 147, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.B.; Sung, N.Y.; Yang, M.S.; Song, D.S.; Byun, E.H.; Kim, J.K.; Park, J.H.; Song, B.S.; Lee, J.W.; Park, S.H.; et al. Procyanidin C1 causes vasorelaxation through activation of the endothelial NO/cGMP pathway in thoracic aortic rings. J. Med. Food 2014, 17, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Palozza, P.; Fernandez-Larrea, J.; Ardevol, A.; Blade, C.; Pujadas, G.; Salvado, J.; Arola, L.; Blay, M.T. Procyanidin dimer B1 and trimer C1 impair inflammatory response signalling in human monocytes. Free Radic. Res. 2011, 45, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Kin, R.; Kato, S.; Kaneto, N.; Sakurai, H.; Hayakawa, Y.; Li, F.; Tanaka, K.; Saiki, I.; Yokoyama, S. Procyanidin C1 from Cinnamomi cortex inhibits TGF-β-induced epithelial-to-mesenchymal transition in the A549 lung cancer cell line. Int. J. Oncol. 2013, 43, 1901–1906. [Google Scholar] [PubMed]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; van Oosterom, A.T.; de Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.J. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J. Bone Miner. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Lingaraj, K.; Poh, C.K.; Wang, W. Vascular endothelial growth factor (VEGF) is expressed during articular cartilage growth and re-expressed in osteoarthritis. Ann. Acad. Med. Singap. 2010, 39, 399–403. [Google Scholar] [PubMed]
- Pufe, T.; Petersen, W.; Tillmann, B.; Mentlein, R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001, 44, 1082–1088. [Google Scholar] [CrossRef]
- Enomoto, H.; Inoki, I.; Komiya, K.; Shiomi, T.; Ikeda, E.; Obata, K.; Matsumoto, H.; Toyama, Y.; Okada, Y. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am. J. Pathol. 2003, 162, 171–181. [Google Scholar] [CrossRef]
- Pfander, D.; Kortje, D.; Zimmermann, R.; Weseloh, G.; Kirsch, T.; Gesslein, M.; Cramer, T.; Swoboda, B. Vascular endothelial growth factor in articular cartilage of healthy and osteoarthritic human knee joints. Ann. Rheum. Dis. 2001, 60, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Yudoh, K.; Masuko, K. The potential role of vascular endothelial growth factor (VEGF) in cartilage: How the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthr. Cartil. 2008, 16, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ludin, A.; Sela, J.J.; Schroeder, A.; Samuni, Y.; Nitzan, D.W.; Amir, G. Injection of vascular endothelial growth factor into knee joints induces osteoarthritis in mice. Osteoarthr. Cartil. 2013, 21, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Jiao, Z.; Zheng, J.S.; Xu, W.F.; Zhang, S.Y.; Qin, A.; Yang, C. Injecting vascular endothelial growth factor into the temporomandibular joint induces osteoarthritis in mice. Sci. Rep. 2015, 5, 16244. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Sato, M.; Kobayashi, M.; Yokoyama, M.; Tani, Y.; Mochida, J. Bevacizumab, an anti-vascular endothelial growth factor antibody, inhibits osteoarthritis. Arthritis Res. Ther. 2014, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, K.; Chen, S.; Wen, W. Grape seed extract inhibits VEGF expression via reducing HIF-1α protein expression. Carcinogenesis 2009, 30, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Tyagi, A.K.; Dhanalakshmi, S.; Agarwal, R.; Agarwal, C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int. J. Cancer 2004, 108, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Moyle, C.W.; Cerezo, A.B.; Winterbone, M.S.; Hollands, W.J.; Alexeev, Y.; Needs, P.W.; Kroon, P.A. Potent inhibition of VEGFR-2 activation by tight binding of green tea epigallocatechin gallate and apple procyanidins to VEGF: Relevance to angiogenesis. Mol. Nutr. Food Res. 2015, 59, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007, 19, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Rohdewald, P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int. J. Clin. Pharmacol. Ther. 2002, 40, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Funck-Brentano, T.; Bouaziz, W.; Marty, C.; Geoffroy, V.; Hay, E.; Cohen-Solal, M. Dkk-1-mediated inhibition of Wnt signaling in bone ameliorates osteoarthritis in mice. Arthritis Rheumatol. 2014, 66, 3028–3039. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor and age-related macular degeneration: From basic science to therapy. Nat. Med. 2010, 16, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Warner, S. The genetics of osteoarthritis: A review. J. Funct. Morphol. Kinesiol. 2016, 1, 140–153. [Google Scholar] [CrossRef]
- Rodriguez-Fontenla, C.; Calaza, M.; Evangelou, E.; Valdes, A.M.; Arden, N.; Blanco, F.J.; Carr, A.; Chapman, K.; Deloukas, P.; Doherty, M.; et al. Assessment of osteoarthritis candidate genes in a meta-analysis of nine genome-wide association studies. Arthritis Rheumatol. 2014, 66, 940–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamba, T.; McDonald, D.M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer 2007, 96, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G. Pycnogenol: A blend of procyanidins with multifaceted therapeutic applications? Fitoterapia 2010, 81, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, K.; Nam, S.; Anderson, R.A.; Jove, R.; Wen, W. Novel angiogenesis inhibitory activity in cinnamon extract blocks VEGFR2 kinase and downstream signaling. Carcinogenesis 2010, 31, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mackle, S.; Sussmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Rimbach, G.; Virgili, F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic. Biol. Med. 1999, 27, 704–724. [Google Scholar] [CrossRef]
- Cisar, P.; Jany, R.; Waczulikova, I.; Sumegova, K.; Muchova, J.; Vojtassak, J.; Durackova, Z.; Lisy, M.; Rohdewald, P. Effect of pine bark extract (Pycnogenol®) on symptoms of knee osteoarthritis. Phytother. Res. 2008, 22, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Errichi, S.; Zulli, C.; Errichi, B.M.; Vinciguerra, G.; Ledda, A.; di Renzo, A.; Stuard, S.; Dugall, M.; et al. Treatment of osteoarthritis with Pycnogenol®. The SVOS (San Valentino Osteo-arthrosis Study). Evaluation of signs, symptoms, physical performance and vascular aspects. Phytother. Res. 2008, 22, 518–523. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Leong, D.J.; Zhuo, Z.; Majeska, R.J.; Cardoso, L.; Spray, D.C.; Goldring, M.B.; Cobelli, N.J.; Sun, H.B. Strain-induced mechanotransduction through primary cilia, extracellular ATP, purinergic calcium signaling, and ERK1/2 transactivates CITED2 and downregulates MMP-1 and MMP-13 gene expression in chondrocytes. Osteoarthr. Cartil. 2015, 24, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Conesa, M.T.; Tribolo, S.; Guyot, S.; Tomas-Barberan, F.A.; Kroon, P.A. Oligomeric procyanidins inhibit cell migration and modulate the expression of migration and proliferation associated genes in human umbilical vascular endothelial cells. Mol. Nutr. Food Res. 2009, 53, 266–276. [Google Scholar] [CrossRef] [PubMed]


| Known Procyanidins | Therapeutic Effects or Potential Effects |
|---|---|
| A-Type Dimers | |
| Procyanidin A1 | immune modulation [33,34], cholesterol modulation [35], anti-oxidative effects [20] |
| Procyanidin A2 | anti-inflammation [36], anti-diabetic effect [37] |
| B-Type Dimers (of interest in pine bark) | |
| Procyanidin B1 | anti-inflammation [38], anti-hepatitis-C [39], anti-cancer [40], anti-oxidative effects [19] |
| Procyanidin B2 | anti-diabetic effects [41,42], anti-inflammation [43], anti-cancer [44], anti-oxidative effects [19] |
| Procyanidin B3 | arthritis modulation [32], anti-cancer [22], anti-inflammation [45], anti-oxidative effects [20] |
| Procyanidin B4 | anti-oxidative effects [46] |
| Procyanidin B5 | anti-cancer [28] |
| Procyanidin B6 | |
| Procyanidin B7 | |
| Procyanidin B8 | |
| Trimeric B-type | |
| Procyanidin C1 | immune modulation [47,48], cardiovascular-protective effects [49], anti-inflammation [50], anti-cancer [51] |
| Procyanidin C2 |
| Gene in Mice | Sequence of Selected Primer Pairs | Gene in Humans | Sequence of Selected Primer Pairs |
|---|---|---|---|
| Vegf | F: GCAGACTATTCAGCGGACTCA | VEGF | F: AGGGCAGAATCATCACGAAGT |
| R: GGGAGTGAAGAACCAACCTCC | R: AGGGTCTCGATTGGATGGCA | ||
| MMP-1 | F: CCTCGTTGGACCAAAACACA | ADAMTS-5 | F: GAACATCGACCAACTCTACTCCG |
| R: GCGATGGCATCTTCCACAA | R: CAATGCCCACCGAACCATCT | ||
| MMP-3 | F: TCCTGATGTTGGTGGCTTCA | IL-1β | F: CTCCGGGACTCACAGCAAAA |
| R: CACACTCTGTCTTGGCAAATCC | R: GCCCAAGGCCACAGGTATT | ||
| MMP-13 | F: TCACCTGATTCTTGCGTGCTA | IL-6 | F: CCTGAACCTTCCAAAGATGGC |
| R: CAGATGGACCCCATGTTTGC | R: TTCACCAGGCAAGTCTCCTCA | ||
| Adamts-5 | F: GCTGCTGGTAGCATCGTTACTG | RANKL | F: CAACATATCGTTGGATCACAGCA |
| R: GAGTGTAGCGCGCATGCTT | R: GACAGACTCACTTTATGGGAACC | ||
| Il-1β | F: GCTTCCTTGTGCAAGTGTCTGA | HIF-1α | F: GAACGTCGAAAAGAAAAGTCTCG |
| R: TCAAAAGGTGGCATTTCACAGT | R: CCTTATCAAGATGCGAACTCACA | ||
| Tnf-α | F: AGGGATGAGAAGTTCCCAAATG | ACAN | F: GTGCCTATCAGGA |
| R: GGCTTGTCACTCGAATTTTGAGA | R: GATGCCTTTCACCACGACTTC | ||
| Il-6 | F: TCGGAGGCTTAATTACACATGTTC | MMP-1 | F: TCCACAAATGGTGGGTACAA |
| R: TGCCATTGCACAACTCTTTTCT | R: GGTGACACCAGTGACTGCAC | ||
| Rankl | F: AGGCTCATGGTTGGATGTGG | MMP-13 | F: ACTGAGAGGCTCCGAGAAATG |
| R: GCATTGATGGTGAGGTGTGC | R: GAACCCCGCATCTTGGCTT | ||
| Hif-1α | F: GGATGAGTTCTGAACGTCGAAAAG | TNF-α | F: CGAACATCCAACCTTCCCAAAC |
| R: GTGGCAACTGATGAGCAAGC | R: TGGTGGTCTTGTTGCTTAAAGTTC | ||
| Col2a1 | F: AGCAGGAATTCGGTGTGGAC | MMP-3 | F: TCACAGTTGGAGTTTGACCC |
| R: GACATCAGGTCAGGTCAGCC | R: AAGTGCCCATATTGTGCCTTC | ||
| Acan | F: TGGGATCTACCGCTGTGAAGT | COL2a1 | F: AGCAGGAATTCGGTGTGGAC |
| R: CTCGTCCTTGTCACCATAGCAA | R: GACATCAGGTCAGGTCAGCC | ||
| Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) | F: AGGTCGGTGTGAACGGATTTG | GAPDH | F: CATGGAGAAGGCTGGGGCTCATTTG |
| R: TGTAGACCATGTAGTTGAGGTCA | R: GGGGTGCTAAGCAGTTGGTGGT |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Leong, D.J.; He, Z.; Xu, L.; Liu, L.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Procyanidins Mitigate Osteoarthritis Pathogenesis by, at Least in Part, Suppressing Vascular Endothelial Growth Factor Signaling. Int. J. Mol. Sci. 2016, 17, 2065. https://doi.org/10.3390/ijms17122065
Wang A, Leong DJ, He Z, Xu L, Liu L, Kim SJ, Hirsh DM, Hardin JA, Cobelli NJ, Sun HB. Procyanidins Mitigate Osteoarthritis Pathogenesis by, at Least in Part, Suppressing Vascular Endothelial Growth Factor Signaling. International Journal of Molecular Sciences. 2016; 17(12):2065. https://doi.org/10.3390/ijms17122065
Chicago/Turabian StyleWang, Angela, Daniel J. Leong, Zhiyong He, Lin Xu, Lidi Liu, Sun Jin Kim, David M. Hirsh, John A. Hardin, Neil J. Cobelli, and Hui B. Sun. 2016. "Procyanidins Mitigate Osteoarthritis Pathogenesis by, at Least in Part, Suppressing Vascular Endothelial Growth Factor Signaling" International Journal of Molecular Sciences 17, no. 12: 2065. https://doi.org/10.3390/ijms17122065