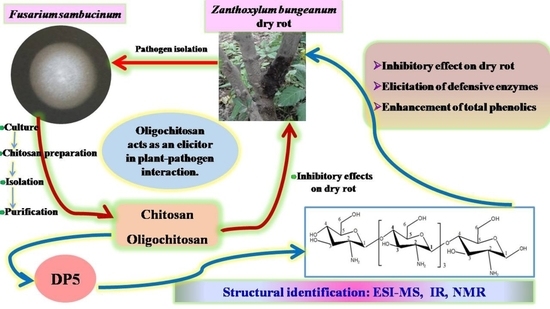
Structural Characterization of Oligochitosan Elicitor from Fusarium sambucinum and Its Elicitation of Defensive Responses in Zanthoxylum bungeanum
Abstract
:1. Introduction
2. Results and Discussions
2.1. Isolation of Chitosan and Oligochitosan from Fusarium sambucinum
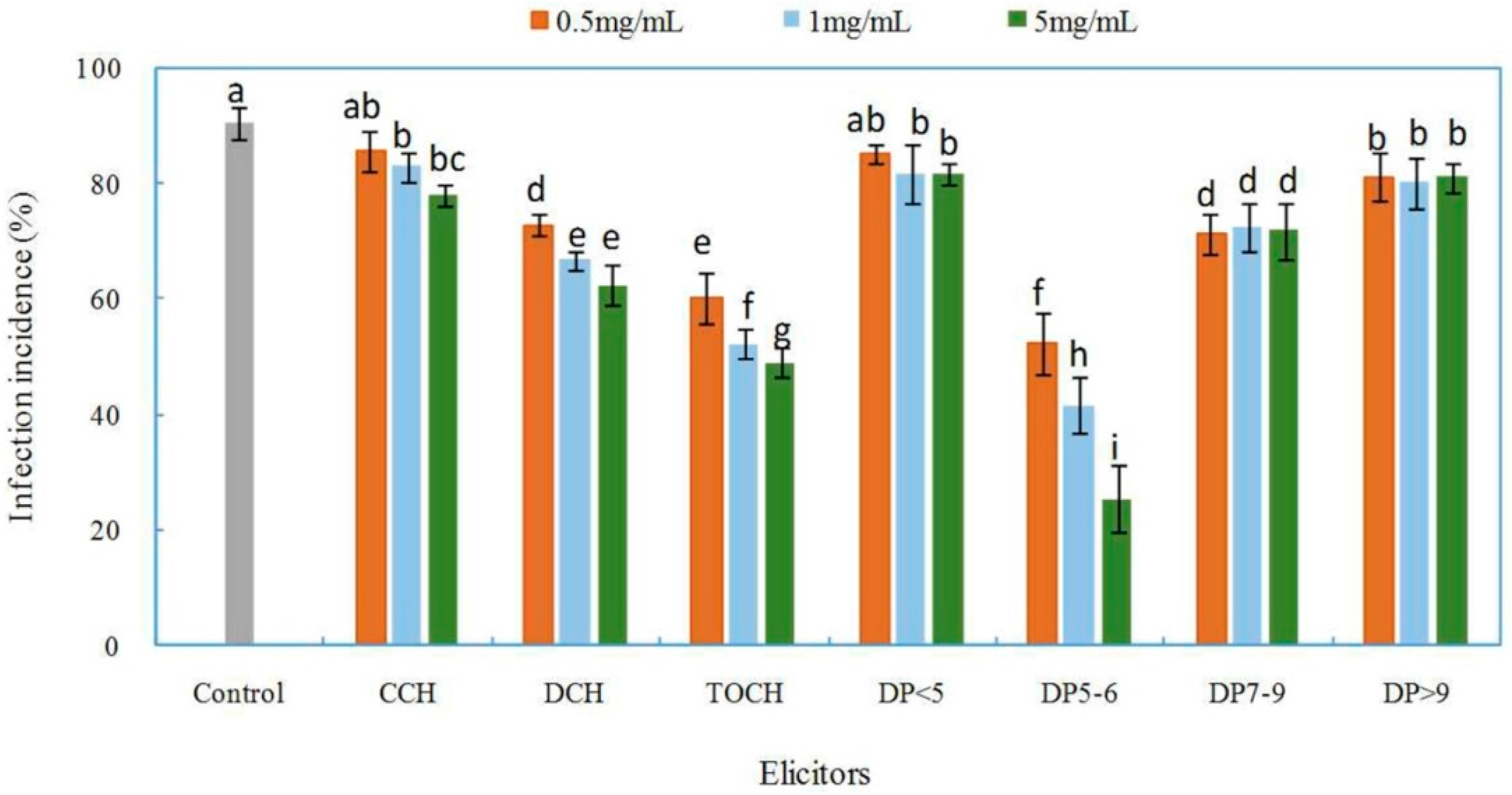
2.2. Effects of Chitosan and Oligochitosan on the Infection of the Pathogen on Z. bungeanum Stems
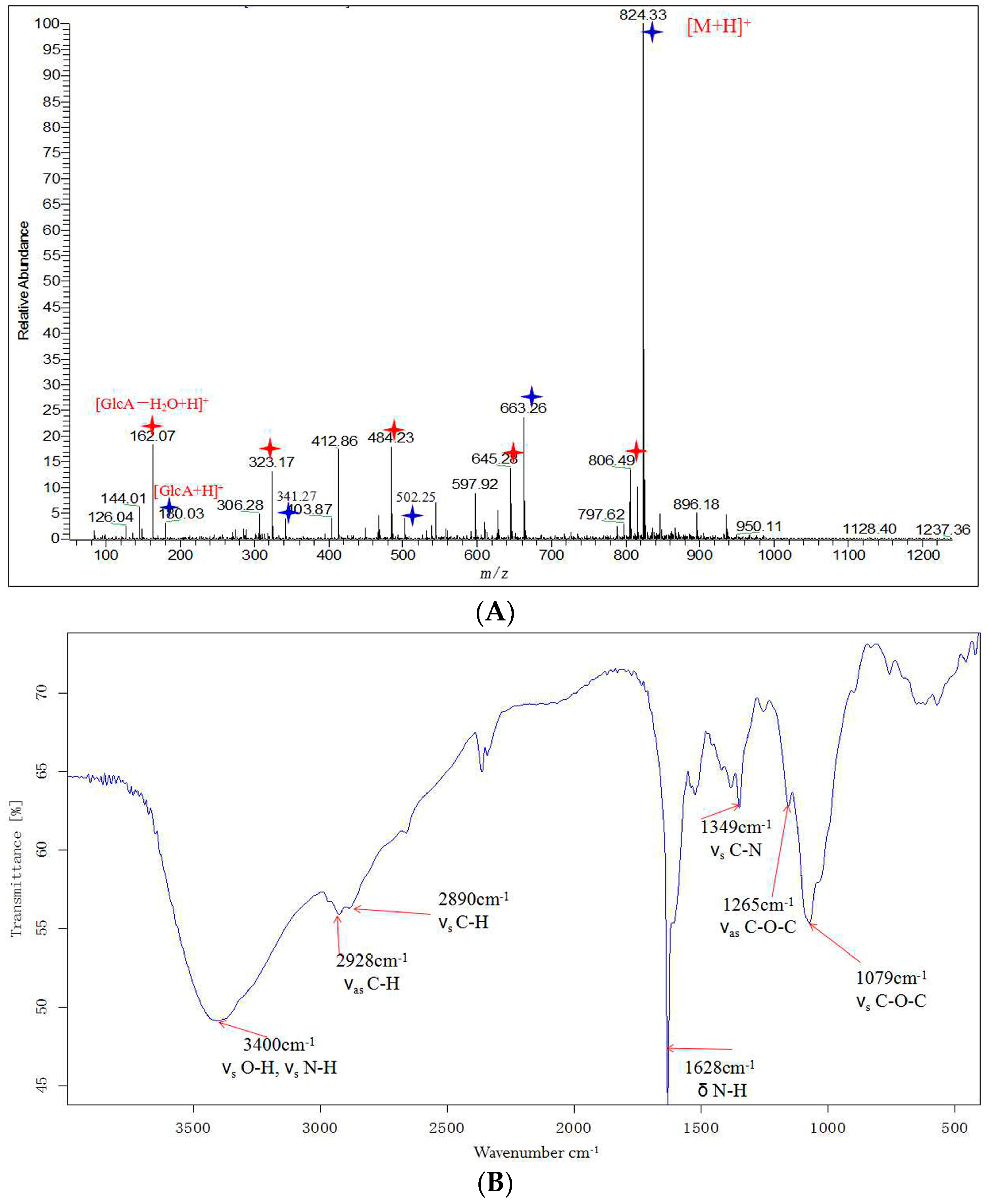
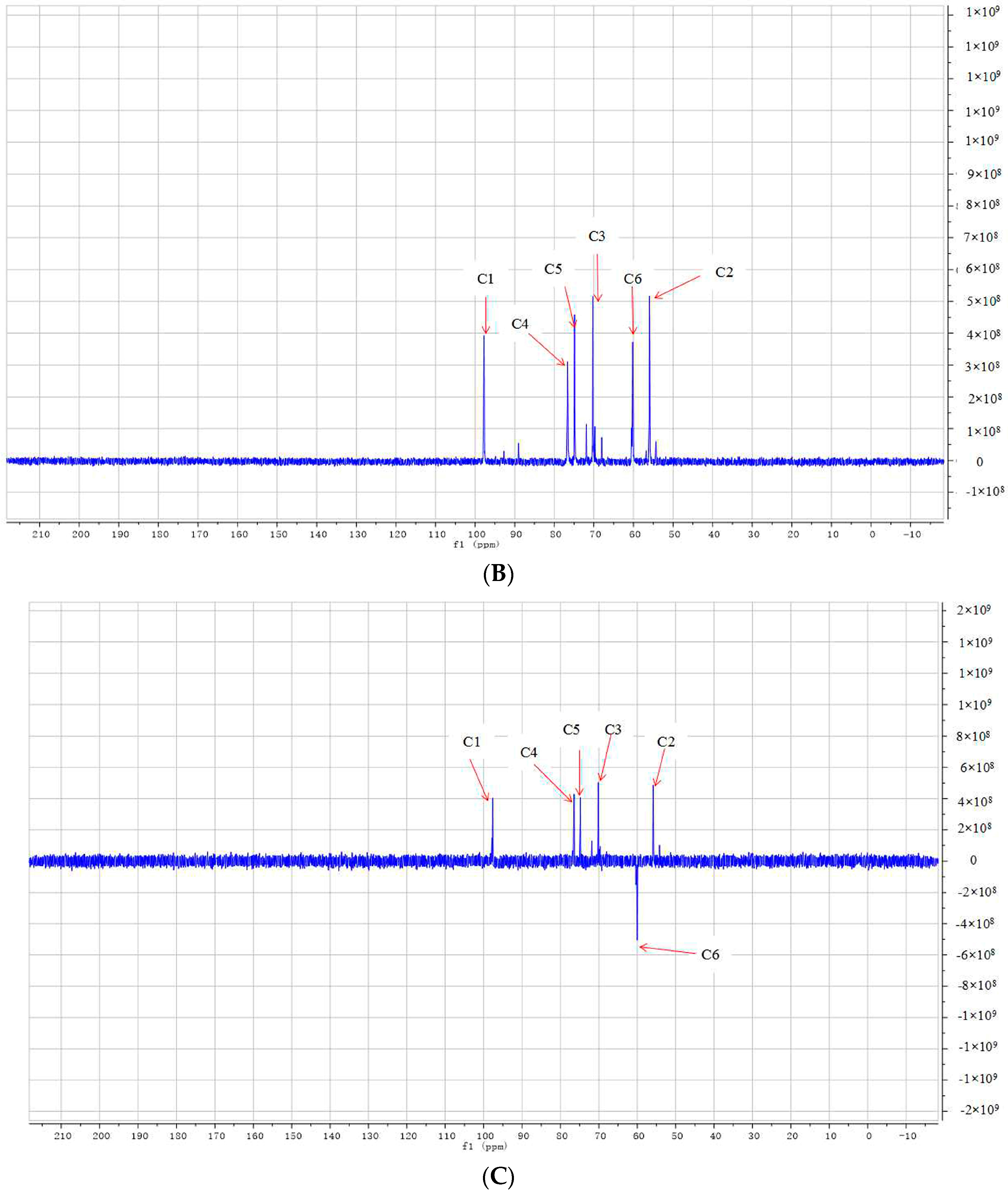
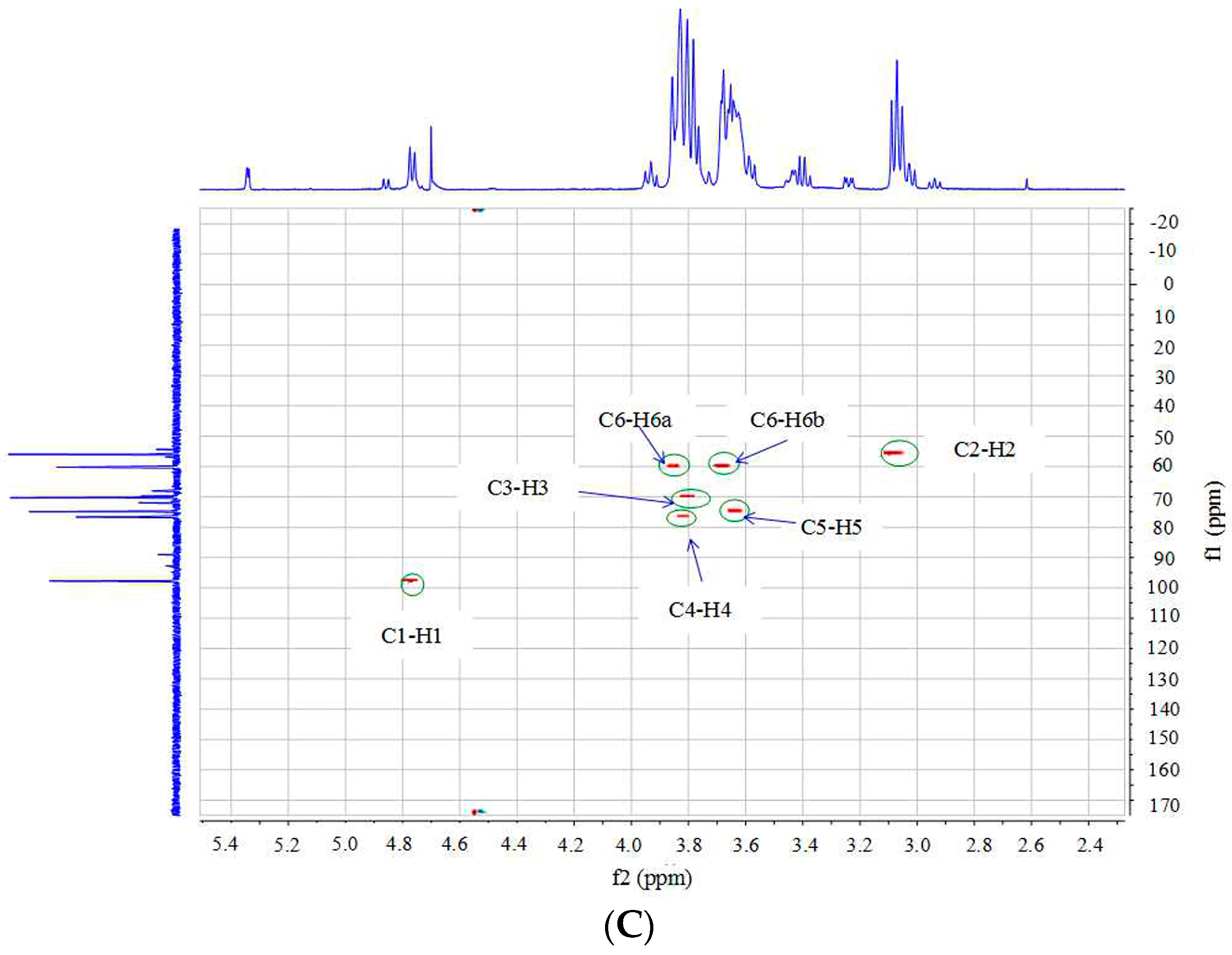
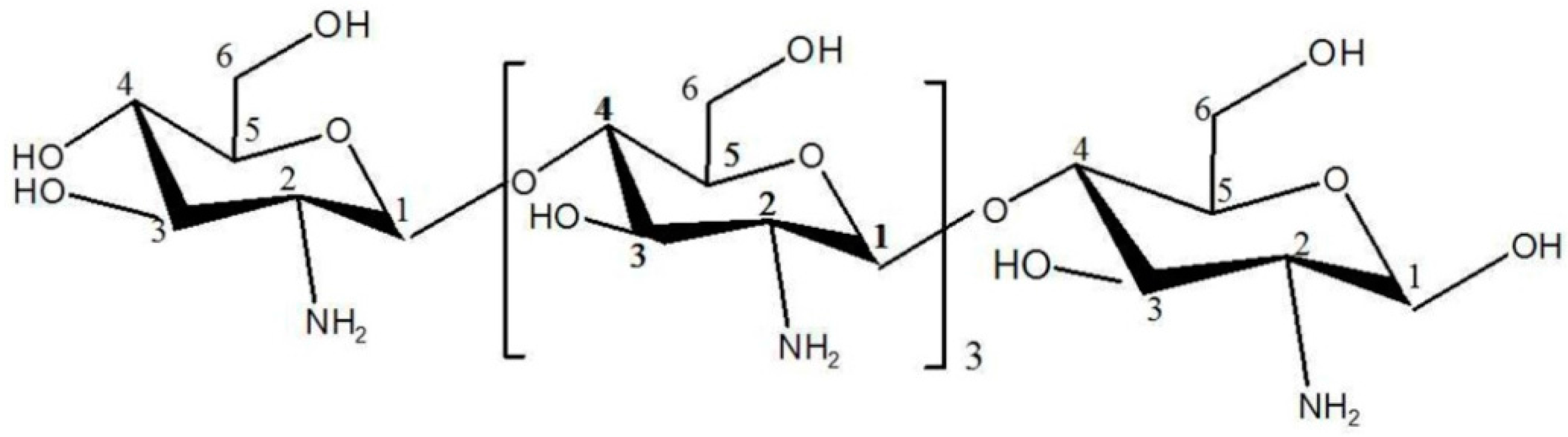
2.3. Structural Analysis of DP5
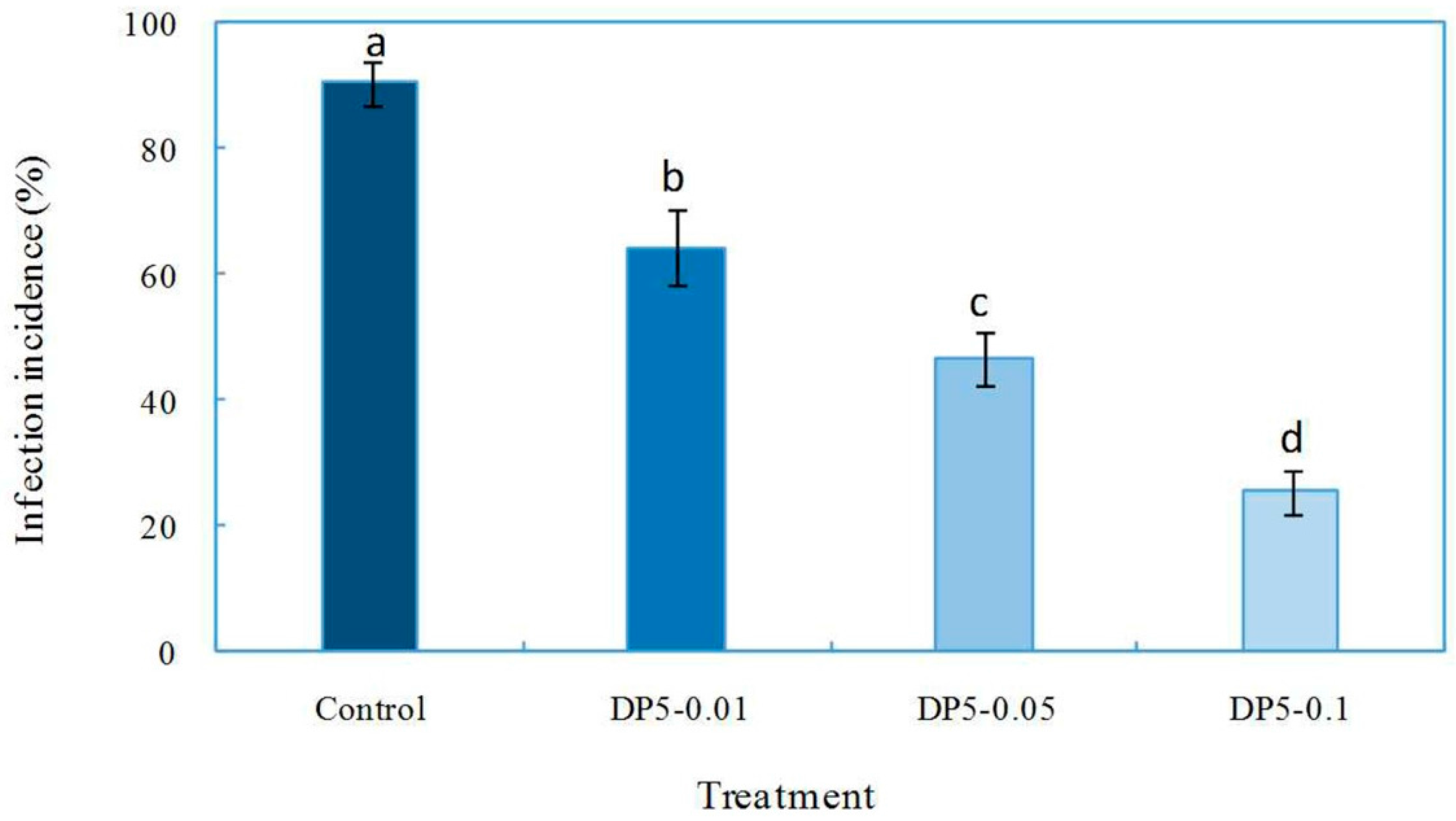
2.4. Effects of Oligochitosan DP5 on the Infection of the Pathogen on Z. bungeanum Stems
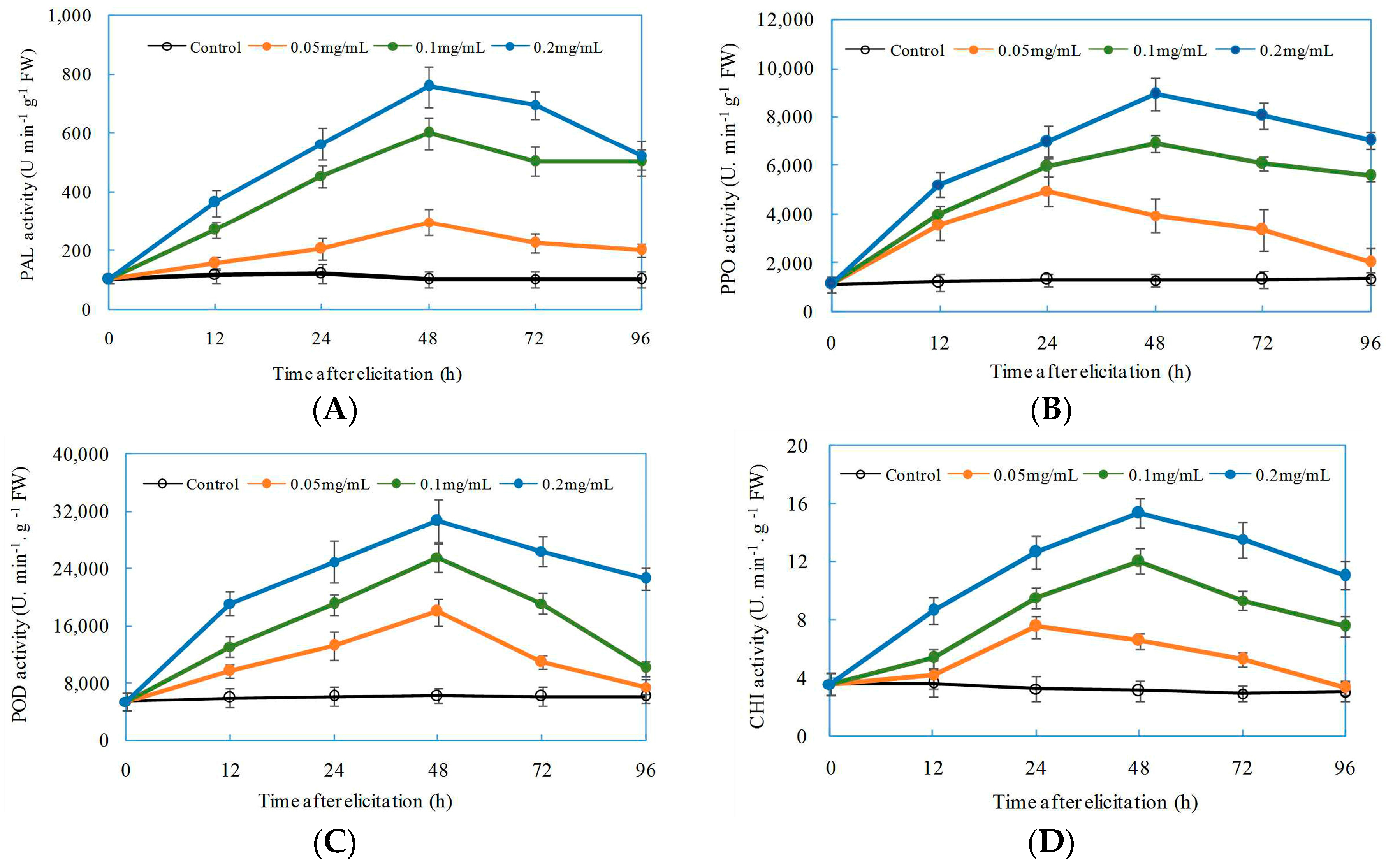
2.5. Induced Effects of Oligochitosan DP5 on Defensive Enzymes in Z. bungeanum
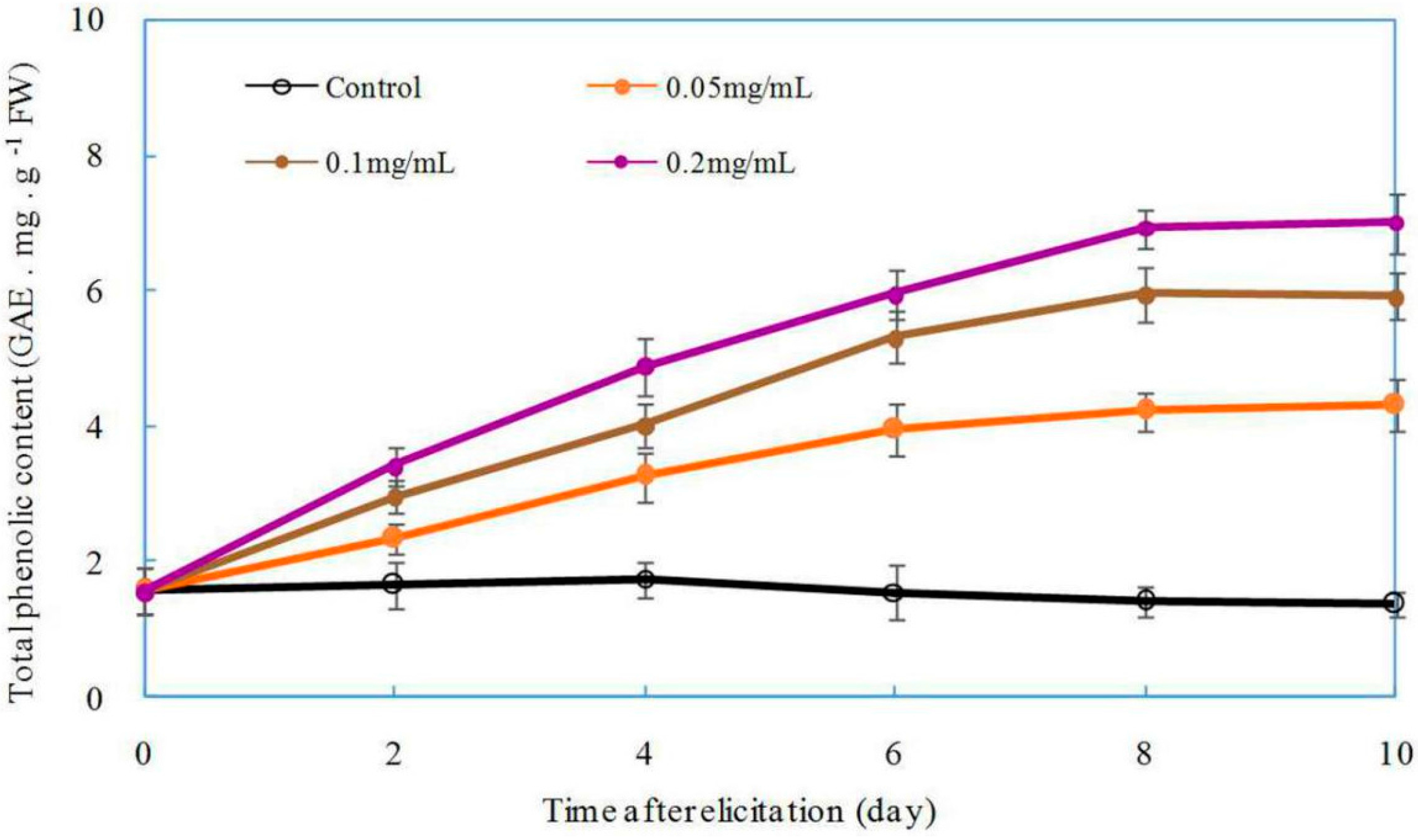
2.6. Effect of DP5 on the Production of Total Phenolics in Z. bungeanum
3. Materials and Methods
3.1. Materials and Reagents
3.2. Cultivation of F. sambuciunum
3.3. Preparation of Chitosan and Oligochitosan from F. sambucinum Mycelia
3.4. Isolation and Purification of Oligochitosan
3.5. Structural Identification of DP5
3.6. Effect of Chitosan and Oligochitosan on Pathogen Infection of Z. bungeanum Stems
3.7. Effects of Oligochitosan DP5 on the Infection of the Pathogen on Z. bungeanum Stems
3.8. Elicitation of Defensive Enzymes in Z. bungeanum by Oligochitosan DP5
3.9. Effect of Oligochitosan DP5 on the Accumulation of Total Phenolics in Z. bungeanum
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ESI-MS | Electrospray ionization mass spectrometry |
| FT-IR | Fourier transform infrared spectroscopy |
| NMR | Nuclear magnetic resonance |
| DA | Degree of acetylation |
| DP | Degree of polymerization |
| PAL | Phenylalanine ammonia lyase |
| PPO | Polyphenoloxidase |
| POD | Peroxidase |
| CHI | Chitinase |
| CCH | Crude chitosan |
| TOCH | Total oligochitosan |
| DCH | Deacetyated chitosan |
| DP < 5 | Oligosaccharide fraction with a degree of polymerization less than five |
| DP5–6 | Oligosaccharide fraction with a degree of polymerization between five and six |
| DP7–9 | Oligosaccharide fraction with a degree of polymerization between seven and nine |
| DP > 9 | Oligosaccharide fraction with a degree of polymerization greater than nine |
| DP5 | Oligochitosan with a degree of polymerization of five |
| DEPT-135 | Distortionless enhancement by polarization transfer (135°) |
| COSY | Correlation spectroscopy |
| NOESY | Rotating frame nuclear Overhauser effect spectroscopy |
| HSQC | Heteronuclear single-quantum correlation spectroscopy |
| GlcN | Glucosamine |
References
- Albersheim, P.; Valent, B.S. Host-pathogen interaction in plant: Plants, when exposed to oligosaccharide of fungao origin, defend themselves by accumulation antibiotics. J. Cell Biol. 1978, 78, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Dubery, I.A.; Slater, V. Induced defence responses in cotton leaf disks by elicitors from Verticillium dahliae. Phytochemistry 1997, 44, 1429–1434. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Analysis of defensive responses activated by volatile allo-ocimene treatment in Arabidopsis thaliana. Phytochemistry 2006, 67, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Kuć, J. Phytoalexins, stress metabolism, and disease resistance in plants. Annu. Rev. Phytopathol. 1995, 33, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Sharan, M.; Taguchi, G.; Gonda, K.; Jouke, T.; Shimosaka, M.; Hayashida, N.; Okazaki, M. Effects of methyl jasmonate and elicitor on the activation of phenylalanine ammonia-lyase and the accumulation of scopoletin and scopolin in tobacco cell cultures. Plant Sci. 1998, 132, 13–19. [Google Scholar] [CrossRef]
- Sharp, J.K.; McNeil, M.; Albersheim, P. The primary structures of one elicitor-active and seven elicitor-inactive hexa (β-d-glucopyranosyl)-d-glucitols isolated from the mycelial walls of Phytophthora megasperma f. sp. glycinea. J. Biol. Chem. 1984, 259, 11321–11336. [Google Scholar] [PubMed]
- Nothnagel, E.A.; McNeil, M.; Albersheim, P.; Dell, A. Host-pathogen interactions. XXII. A galacturonic acid oligosaccharide from plant cell walls elicits phytoalexins. Plant Physiol. 1983, 71, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Waldmüller, T.; Cosio, E.G.; Grisebach, H.; Ebel, J. Release of highly elicitor-active glucans by germinating zoospores of Phytophthora megasperma f. sp. glycinea. Planta 1992, 188, 498–505. [Google Scholar] [PubMed]
- Benhamou, N. Elicitor-induced plant defence pathways. Trends Plant Sci. 1996, 1, 233–240. [Google Scholar] [CrossRef]
- Dutsadee, C.; Nunta, C. Induction of peroxidase, scopoletin, phenolic compounds and resistance in Hevea brasiliensisby elicitin and a novel protein elicitor purified from Phytophthora palmivora. Physiol. Mol. Plant Pathol. 2008, 72, 179–187. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Kucera, J. Fungal mycelium—The source of chitosan for chromatography. J. Chromatogr. B 2004, 808, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Walker-Simmons, M.; Hadwiger, L.A.; Ryan, C.A. Chitosans and pectic polysaccharides both induce the accumulation of the antifungal phytoalexin pisatin in pea pods and antinutrient proteinase inhibitors in tomato leaves. Biochem. Biophys. Res. Commun. 1983, 110, 194–199. [Google Scholar] [CrossRef]
- Barber, M.S.; Bertram, R.E.; Ride, J.P. Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiol. Mol. Plant Pathol. 1989, 34, 3–12. [Google Scholar] [CrossRef]
- Dixon, R.A.; Harrison, M.J. Early events in the activation of plant defense responses. Annu. Rev. Phytopathol. 1994, 32, 479–501. [Google Scholar] [CrossRef]
- Hadwiger, L.A.; Ogawa, T.; Kuyama, H. Chitosan polymer sizes effective in inducing phytoalexin accumulation and fungal suppression are verified with synthesized oligomers. Mol. Plant-Microbe Interact. 1994, 7, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Vander, P.; Varum, K.M.; Domard, A.; El Gueddari, N.E.; Moerschbacher, B.M. Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves. Plant Physiol. 1998, 118, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Kauss, H.; Jeblick, W.; Domard, A. The degrees of polymerization and N-acetylation of chitosan determine its ability to elicit callose formation in suspension cells and protoplasts of Catharanthus roseus. Planta 1989, 178, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Baturo-Ciesniewska, A.; Lenc, L.; Grabowski, A.; Lukanowski, A. Characteristics of polish isolates of Fusarium sambucinum: Molecular identification, pathogenicity, diversity and reaction to control agents. Am. J. Potato Res. 2015, 92, 49–61. [Google Scholar] [CrossRef]
- Cao, Z.; Liang, Y. The research of Zanthoxylum bungeanum trunk-rot. J. Northwest For. Coll. 1992, 7, 58–62. [Google Scholar]
- Cao, Z.; Ma, X. A preliminary study on the trunk-rot disease of pricklyash. Shaanxi For. Sci. Technol. 1990, 2, 36–38, 40. [Google Scholar]
- Cao, Z.; Ming, Y.; Chen, D.; Zhang, H. Resistance of prichly ash to stem rot and pathogenicity differentiation of Fusarium sambuciunum. J. Northwest For. Univ. 2010, 25, 115–118. [Google Scholar]
- Cheng, J.; Lee, X.; Theng, B.K.G.; Zhang, L.; Fang, B.; Li, F. Biomass accumulation and carbon sequestration in an age-sequence of Zanthoxylum bungeanum plantations under the Grain for Green Program in karst regions, Guizhou province. Agric. For. Meteorol. 2015, 203, 88–95. [Google Scholar] [CrossRef]
- Li, J.; Wang, F.; Li, S.; Peng, Z. Effects of pepper (Zanthoxylum bungeanum Maxim.) leaf extract on the antioxidant enzyme activities of salted silver carp (Hypophthalmichthys molitrix) during processing. J. Funct. Foods 2015, 18, 1179–1190. [Google Scholar] [CrossRef]
- Li, P.; Cao, Z.; Wu, Z.; Wang, X.; Li, X. The effect and action mechanisms of oligochitosan on control of stem dry rot of Zanthoxylum bungeanum. Int. J. Mol. Sci. 2016, 17, 1044. [Google Scholar] [CrossRef] [PubMed]
- Yonni, F.; Moreira, M.T.; Fasoli, H.; Grandi, L.; Cabral, D. Simple and easy method for the determination of fungal growth and decolourative capacity in solid media. Int. Biodeterior. Biodegrad. 2004, 54, 283–287. [Google Scholar] [CrossRef]
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Yui, T.; Imada, K.; Okuyama, K.; Obata, Y.; Suzuki, K.; Ogawa, K. Molecular and crystal structure of the anhydrous form of chitosan. Macromolecules 1994, 27, 7601–7605. [Google Scholar] [CrossRef]
- Kendra, D.F.; Hadwiger, L.A. Characterization of the smallest chitosan oligomer that is maximally antifungal to Fusarium solani and elicits pisatin formation by Pisum sativum. Exp. Mycol. 1984, 8, 276–281. [Google Scholar] [CrossRef]
- Plascencia-Jatomea, M.; Viniegra, G.; Olayo, R.; Castillo-Ortega, M.M.; Shirai, K. Effect of chitosan and temperature on spore germination of Aspergillus niger. Macromol. Biosci. 2003, 3, 582–586. [Google Scholar] [CrossRef]
- Reddy, M.V.B.; Barka, E.A.; Castaigne, F.; Arul, J. Effect of chitosan on growth and toxin production by Alternaria alternata f. sp. lycopersici. HortScience 1997, 32, 467–468. [Google Scholar]
- Sathiyabama, M.; Balasubramanian, R. Chitosan induces resistance components in Arachis hypogaea against leaf rust caused by Puccinia arachidis Speg. Crop Prot. 1998, 17, 307–331. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, X.; Du, Y. Oligochitosan: A plant diseases vaccine—A review. Carbohydr. Polym. 2010, 82, 1–8. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Desbrières, J.; Roberts, G.; Rinaudo, M. Characterization of chitosan by steric exclusion chromatography. Polymer 2001, 42, 9921–9927. [Google Scholar] [CrossRef]
- De Velde, K.V.; Kiekens, P. Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT-IR spectroscopy and solid state 13C NMR. Carbohydr. Polym. 2004, 58, 409–416. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Muniz, E.C.; Hsieh, Y.L. 1H-NMR and 1H–13C HSQC surface characterization of chitosan—Chitin sheath-core nanowhiskers. Carbohydr. Polym. 2015, 123, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K.; Kojima, T.; Nishiyama, Y.; Shimojoh, M. Synthesis and some properties of nonnatural amino polysaccharides: Branched chitin and chitosan. Macromolecules 2000, 33, 4711–4716. [Google Scholar] [CrossRef]
- Kasaai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohydr. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Rinaudo, M. Synthesis and characterization of chitosan alkyl phosphate. J. Chil. Chem. Soc. 2006, 51, 815–820. [Google Scholar] [CrossRef]
- El-Mekawy, A.; Hegab, H.M.; El-Baz, A.; Hudson, M. Fabrication and characterization of fungal chitosan-SAP membranes for hemostatic application. Curr. Biochem. Eng. 2014, 1, 75–82. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H-NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Sieval, A.B.; Thanou, M.; Kotze, A.F.; Verhoef, J.C.; Brussee, J.; Junginger, H.E. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr. Polym. 1998, 36, 157–165. [Google Scholar] [CrossRef]
- Fernandez-Megia, E.; Novoa-Carballal, R.; Quiñoá, E.; Riguera, R. Optimal routine conditions for the determination of the degree of acetylation of chitosan by 1H NMR. Carbohydr. Polym. 2005, 61, 155–161. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernandez-Lauzardoa, A.N.; Velazquez-del Vallea, M.G.; Hernandez-Lópeza, M.; Ait Barkab, E.; Bosquez-Molinac, E.; Wilsond, C.L. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Chitosan and chitin oligomers increase phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities in soybean leaves. J. Plant Physiol. 2003, 160, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, S.P.; Meng, X.H.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Badawy, M.E.; Rabea, E.I.; Steurbaut, W.; Rogge, T.M.; Stevens, C.V.; Smagghe, G.; Hofte, M. Fungicidal activity of some O-acyl chitosan derivatives against grey mould Botrytis cinerea and rice leaf blast Pyricularia grisea. Commun. Agric. Appl. Biol. Sci. 2005, 70, 215–218. [Google Scholar] [PubMed]
- Meng, X.; Yang, L.; Kennedy, J.F.; Tian, S. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr. Polym. 2010, 81, 70–75. [Google Scholar] [CrossRef]
- Anand, T.; Chandrasekaran, A.; Raguchander, T.; Prakasam, T.; Samiyappan, R. Chemical and biological treatments for enhancing resistance in chili against Collectotrichum capsici and Leveillula taurica. Arch. Phytopathol. Plant Prot. 2009, 42, 533–551. [Google Scholar] [CrossRef]
- Shadle, G.L.; Wesley, S.V.; Korth, K.L.; Chen, F.; Lamb, C.; Dixon, R.A. Phenylpropanoid compounds and disease resistance in transgenic tobacco with altered expression of l-phenylalanine ammonia-lyase. Phytochemistry 2003, 64, 153–161. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar] [CrossRef]
- Vaughn, K.C.; Duke, S.O. Function of polyphenol oxidase in higher plants. Physiol. Plant. 1984, 60, 106–112. [Google Scholar] [CrossRef]
- Ben-Shalom, N.; Ardi, R.; Pinto, R.; Aki, C.; Fallik, E. Controlling gray mould caused by Botrytis cinerea in cucumber plants by means of chitosan. Crop Prot. 2003, 22, 285–290. [Google Scholar] [CrossRef]
- Graham, M.Y.; Graham, T.L. Rapid accumulation of anionic peroxidases and phenolic polymers in soybean cotyledon tissues following treatment with Phytophthora megasperma f. sp. glycinea wall glucan. Plant Physiol. 1991, 97, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Hoffland, E.; Pieterse, C.M.J.; Bik, L.; van Pelt, J.A. Induced systemic resistance in radish is not associated with accumulation of pathogenesis-related proteins. Physiol. Mol. Plant Pathol. 1995, 46, 309–320. [Google Scholar] [CrossRef]
- Jain, S. The pathogenesis related class 10 proteins in plant defense against biotic and abiotic stresses. Adv. Plants Agric. Res. 2015, 2, 77. [Google Scholar] [CrossRef]
- Kiirika, L.M.; Stahl, F.; Wydra, K. Phenotypic and molecular characterization of resistance induction by single and combined application of chitosan and silicon in tomato against Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 2013, 81, 1–12. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, K.; Shao, X.; Jing, W.; Su, Z. Effects of the yeast Pichia guilliermondii against Rhizopus nigricans on tomato fruit. Postharvest Biol. Technol. 2008, 49, 113–120. [Google Scholar] [CrossRef]
- Barros, L.; Alves, C.T.; Dueñas, M.; Silva, S.; Oliveira, R.; Carnalho, A.M.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterization of phenolic compounds in wild medicinal flowers from Portugal by HPLC–DAD–ESI/MS and evaluation of antifungal properties. Ind. Crop. Prod. 2013, 44, 104–110. [Google Scholar] [CrossRef]
- Rashed, K.; Ćirić, A.; Glamočlija, J.; Soković, M. Antibacterial and antifungal activities of methanol extract and phenolic compounds from Diospyros virginiana L. Ind. Crop. Prod. 2014, 59, 210–215. [Google Scholar] [CrossRef]
- Tajdini, F.; Amini, M.A.; Nafissi-Varcheh, N.; Faramarzi, M.A. Production, physiochemical and antimicrobial properties of fungal chitosan from Rhizomucor miehei and Mucor racemosus. Int. J. Biol. Macromol. 2010, 47, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.T.; Mau, J.L. Physico-chemical characterization of fungal chitosan from shiitake stipes. LWT Food Sci. Technol. 2007, 40, 472–479. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.; Opwis, K.; Knittel, D.; Schollmeyer, E.; Nichisch-Hartfiel, A. Inhibition of microbial pathogens by fungal chitosan. Int. J. Biol. Macromol. 2010, 47, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Pieterse, C.M.J.; Mauch-Man, B. Priming in plant pathogen interactions. Trends Plant Sci. 2002, 7, 210–216. [Google Scholar] [CrossRef]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in systemic plant immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Ayers, A.R.; Ebel, J.; Valent, B.; Albersheim, P. Host-pathogen interactions. X. The fractionation and biological activity of an elicitor isolated from the mycelia walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976, 57, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, D.; Muthumeena, K.; Lavanya, N.; Suresh, S.; Rajendran, L.; Raguchander, T.; Samiyappan, R. Pseudomonas-induced defence molecules in rice plants against leaf folder (Cnaphalocrocis medinalis) pest. Pest Manag. Sci. 2007, 63, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Viswanathan, R.; Raguchander, T.; Prakasam, V.; Samiyappan, R. Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pest and diseases. Crop Prot. 2001, 20, 1–11. [Google Scholar] [CrossRef]
- Ilnicka, A.; Walczyk, M.; Lukaszewicz, J.P. The fungicidal properties of the carbon materials obtained from chitin and chitosan promoted by copper salts. Mater. Sci. Eng. C 2015, 52, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.K.; Valent, B.; Albersheim, P. Purification and partial characterization of a β-glucan fragment that elicits phytoalexin accumulation in soybean. J. Biol. Chem. 1984, 259, 11312–11320. [Google Scholar] [PubMed]
- Streit, F.; Koch, F.; Laranjeira, M.C.M.; Ninow, J.L. Production of fungal chitosan in liquid cultivation using apple pomace as substrate. Braz. J. Microbiol. 2009, 40, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kassai, M.R.; Arul, J.; Charlet, G. Fragmentation of chitosan by acids. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mao, Z.; Lou, J.; Li, Y.; Mou, Y.; Lu, S.; Peng, Y.; Zhou, L. Enhancement of diosgenin production in Dioscorea zingiberensis cell cultures by oligosaccharides from its endophytic fungus Fusarium oxysporum Dzf17. Molecules 2011, 16, 10631–10644. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Jia, Z.; Huang, J.; Zhang, C. Preparation of low molecular weight chitosan by complex enzymes hydrolysis. Int. J. Chem. 2011, 3, 180–186. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuc, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Tian, S.; John, N.; Hershkovitz, V. Effect of heat treatment on inhibition of Monilinia fructicola and induction of disease resistance in peach fruit. Postharvest Biol. Technol. 2012, 65, 61–68. [Google Scholar] [CrossRef]
- Nantawanit, N.; Chanchaichaovivat, A.; Panijpan, B.; Ruenwongsa, P. Induction of defense response against Colletotrichum capsici in chili fruit by the yeast Pichia guilliermondii strain R13. Biol. Control 2010, 52, 145–152. [Google Scholar] [CrossRef]
- Chen, F.; Long, X.; Yu, M.; Liu, Z.; Liu, L.; Shao, H. Phenolics and antifungal activities analysis in industrial crop Jerusalem artichoke (Helianthus tuberosus L.) leaves. Ind. Crop. Prod. 2013, 47, 339–345. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Michel, I.; Tessaro, I.C.; Marczak, L.D.F. Optimization of phenolics extraction from sesame seed cake. Sep. Purif. Technol. 2014, 122, 506–514. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, C.; Liang, J. Effect of esterification condensation on the Folin-Ciocalteu method for the quantitative measurement of total phenols. Food Chem. 2015, 170, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid microplate high-throughput methodology for assessment of Folin-Ciocalteu reducing capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Linhardt, R.J.; Cao, Z. Structural Characterization of Oligochitosan Elicitor from Fusarium sambucinum and Its Elicitation of Defensive Responses in Zanthoxylum bungeanum. Int. J. Mol. Sci. 2016, 17, 2076. https://doi.org/10.3390/ijms17122076
Li P, Linhardt RJ, Cao Z. Structural Characterization of Oligochitosan Elicitor from Fusarium sambucinum and Its Elicitation of Defensive Responses in Zanthoxylum bungeanum. International Journal of Molecular Sciences. 2016; 17(12):2076. https://doi.org/10.3390/ijms17122076
Chicago/Turabian StyleLi, Peiqin, Robert J. Linhardt, and Zhimin Cao. 2016. "Structural Characterization of Oligochitosan Elicitor from Fusarium sambucinum and Its Elicitation of Defensive Responses in Zanthoxylum bungeanum" International Journal of Molecular Sciences 17, no. 12: 2076. https://doi.org/10.3390/ijms17122076