Effect of the CRAC Peptide, VLNYYVW, on mPTP Opening in Rat Brain and Liver Mitochondria
Abstract
:1. Introduction
2. Results
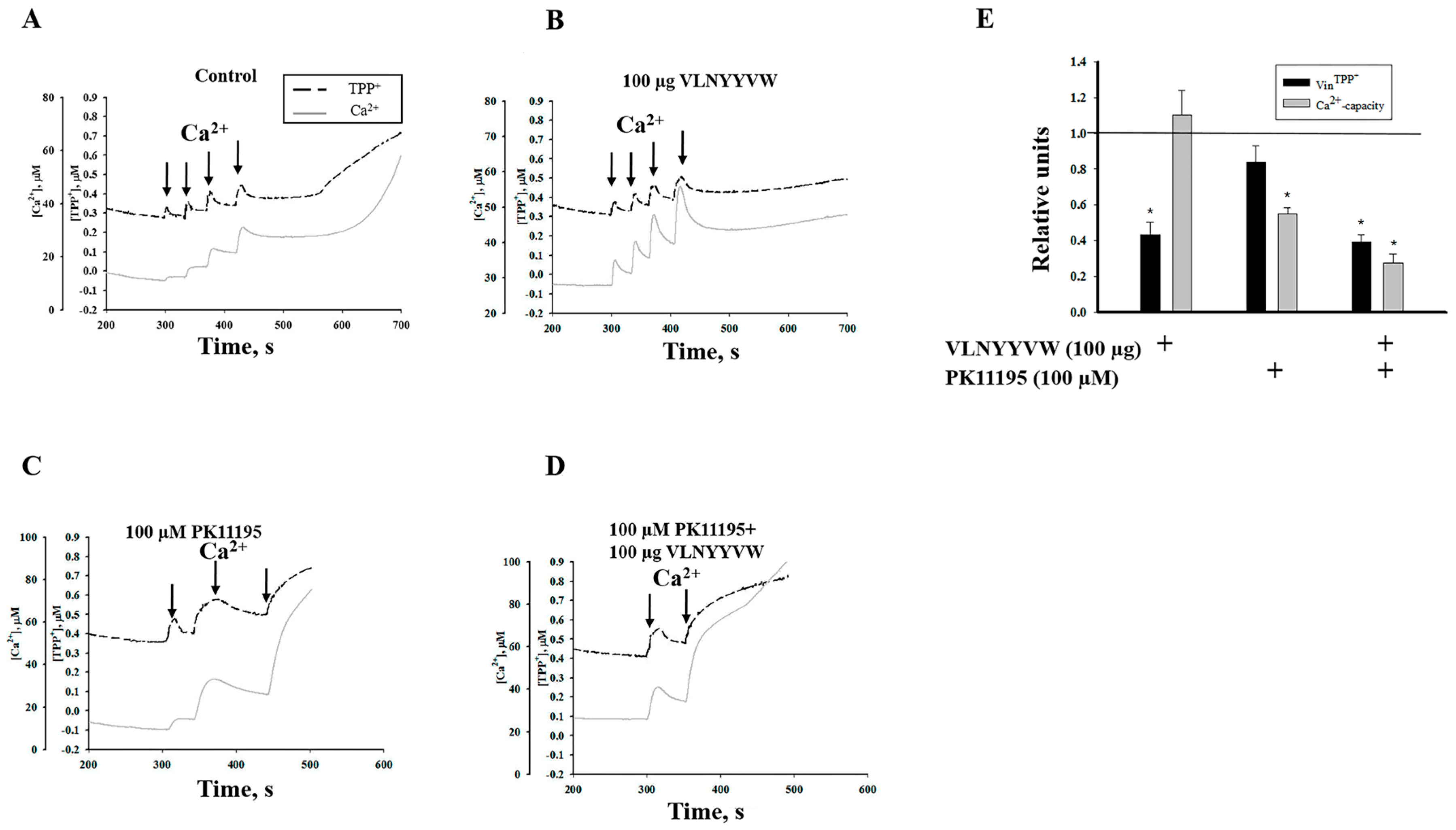
2.1. Effect of the CRAC Peptide (VLNYYVW) and the Combined Effect of the CRAC Peptide with PK 11195 on Calcium Capacity in RBM
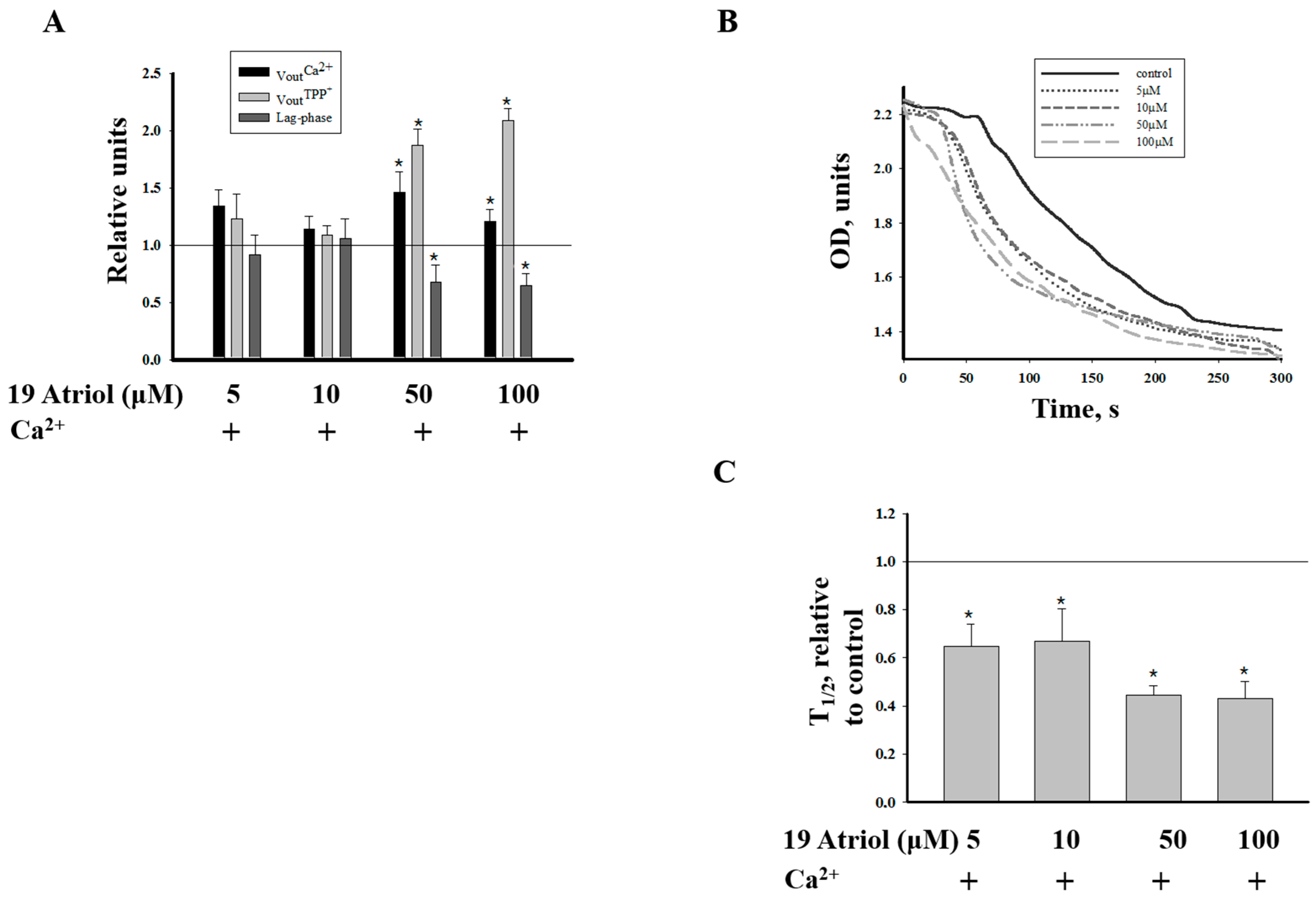
2.2. Effect of 19-Atriol (an Inhibitor of Cholesterol-Binding TSPO) on the Swelling of RLM
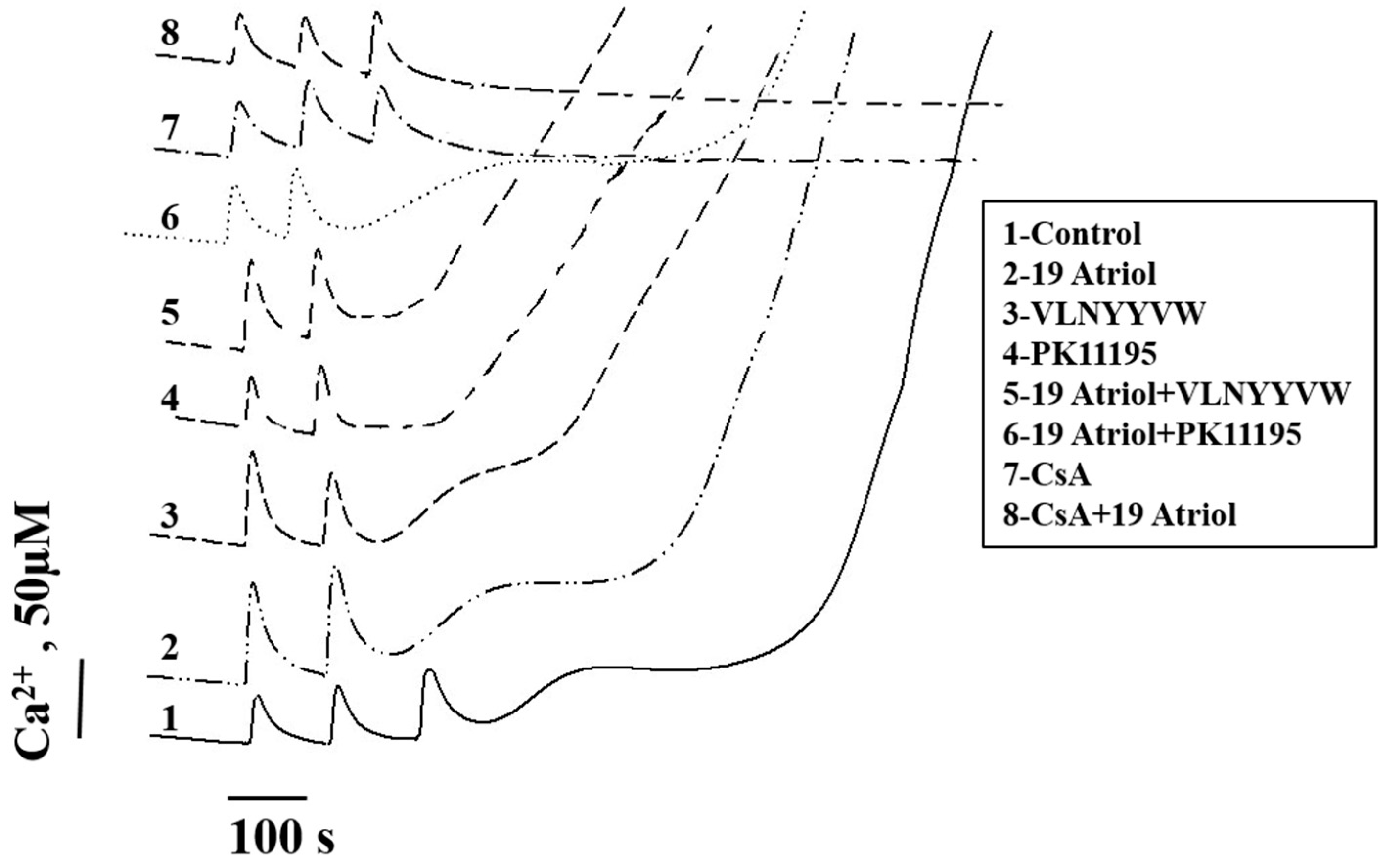
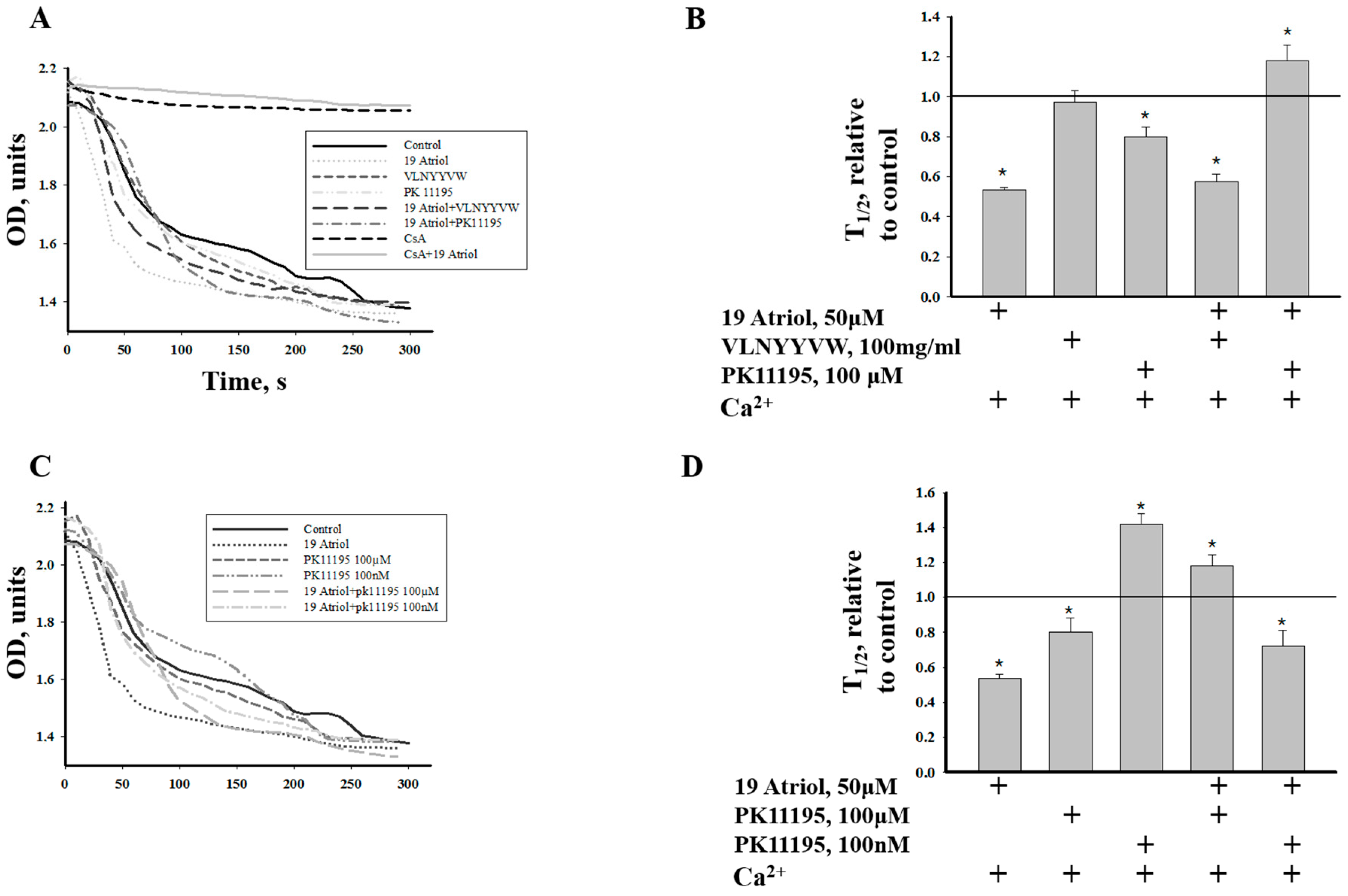
2.3. Combined Effect of 19-Atriol and the CRAC Peptide on Ca2+-Induced RLM Swelling
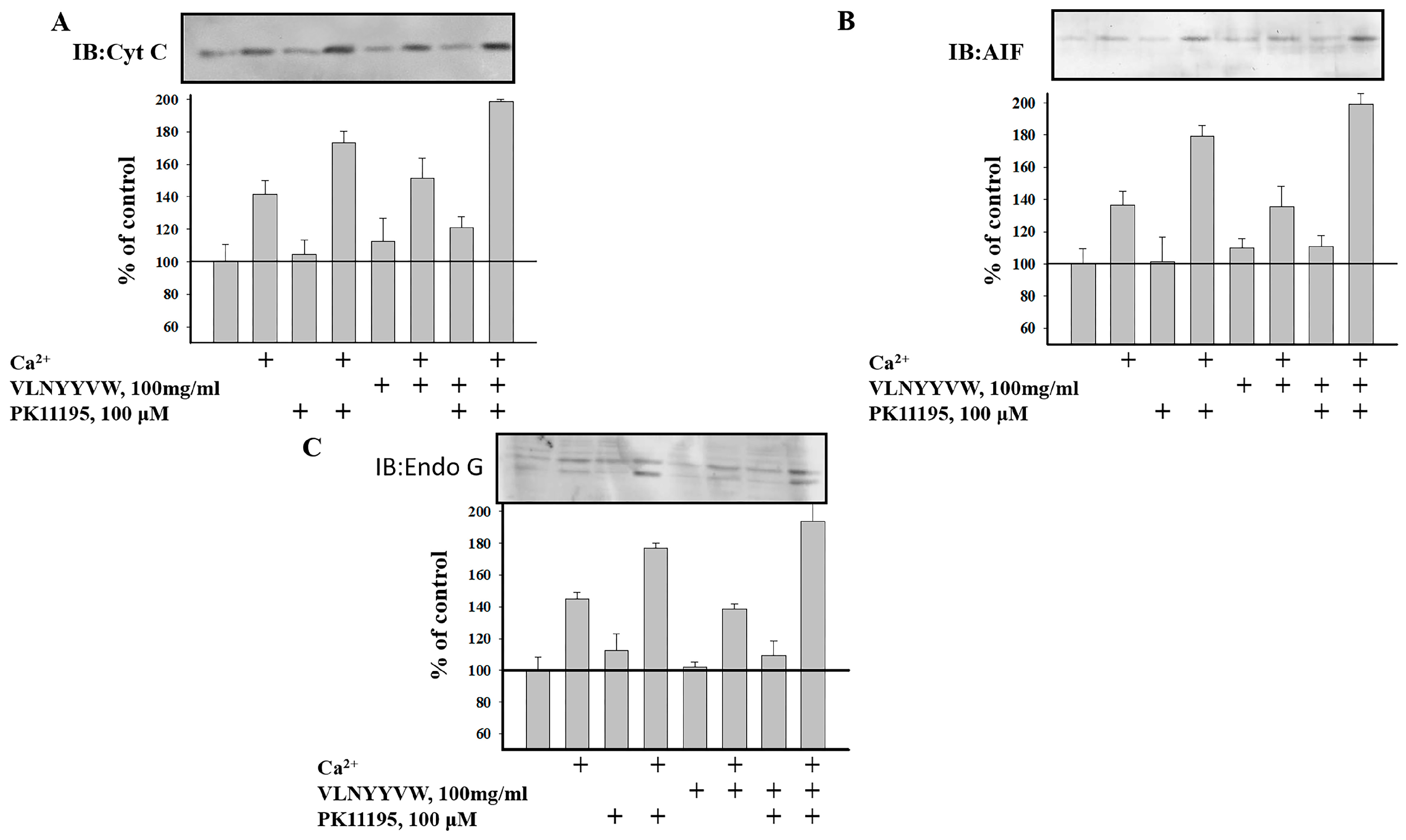
2.4. Effect of the CRAC Peptide (VLNYYVW) on the Release of Apoptotic Factors (Cytochrome c, AIF, and EndoG) from RBM under mPTP Opening
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation of Rat Liver Mitochondria (RLM)
4.3. Isolation of Rat Brain Mitochondria (RBM)
4.4. Evaluation of Mitochondrial Functions
4.5. Electrophoresis and Immunoblotting of Mitochondrial Proteins
4.6. Cytochrome c, AIF, and EndoG Release
4.7. Quantification and Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TSPO | Translocator Protein |
| CRAC | Cholesterol recognition/interaction amino acid consensus |
| mPTP | Mitochondrial permeability transition pore |
References
- Colbeau, A.; Nachbaur, J.; Vignais, P.M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim. Biophys. Acta 1971, 249, 462–492. [Google Scholar] [CrossRef]
- Li, H.; Yao, Z.; Degenhardt, B.; Teper, G.; Papadopoulos, V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc. Natl. Acad. Sci. USA 2001, 98, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Jamin, N.; Neumann, J.M.; Ostuni, M.A.; Vu, T.K.; Yao, Z.X.; Murail, S.; Robert, J.C.; Giatzakis, C.; Papadopoulos, V.; Lacapere, J.J. Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Mol. Endocrinol. 2005, 19, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Murail, S.; Robert, J.C.; Coic, Y.M.; Neumann, J.M.; Ostuni, M.A.; Yao, Z.X.; Papadopoulos, V.; Jamin, N.; Lacapere, J.J. Secondary and tertiary structures of the transmembrane domains of the translocator protein tspo determined by NMR. Stabilization of the TSPO tertiary fold upon ligand binding. Biochim. Biophys. Acta 2008, 1778, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Bernassau, J.M.; Reversat, J.L.; Ferrara, P.; Caput, D.; Lefur, G. A 3D model of the peripheral benzodiazepine receptor and its implication in intra mitochondrial cholesterol transport. J. Mol. Graph. 1993, 11, 236–244. [Google Scholar] [CrossRef]
- Lacapere, J.J.; Papadopoulos, V. Peripheral-type benzodiazepine receptor: Structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids 2003, 68, 569–585. [Google Scholar] [CrossRef]
- Giatzakis, C.; Papadopoulos, V. Differential utilization of the promoter of peripheral-type benzodiazepine receptor by steroidogenic versus nonsteroidogenic cell lines and the role of sp1 and sp3 in the regulation of basal activity. Endocrinology 2004, 145, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Taketani, S.; Kohno, H.; Furukawa, T.; Tokunaga, R. Involvement of peripheral-type benzodiazepine receptors in the intracellular transport of heme and porphyrins. J. Biochem. 1995, 117, 875–880. [Google Scholar] [PubMed]
- Holt, S.A.; Le Brun, A.P.; Majkrzak, C.F.; McGillivray, D.J.; Heinrich, F.; Losche, M.; Lakey, J.H. An ion-channel-containing model membrane: Structural determination by magnetic contrast neutron reflectometry. Soft Matter 2009, 5, 2576–2586. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Newcombe, J.; Gunn, R.N.; Cagnin, A.; Turkheimer, F.; Heppner, F.; Price, G.; Wegner, F.; Giovannoni, G.; Miller, D.H.; et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: Quantitative in vivo imaging of microglia as a measure of disease activity. Brain 2000, 123, 2321–2337. [Google Scholar] [CrossRef] [PubMed]
- Wilms, H.; Claasen, J.; Rohl, C.; Sievers, J.; Deuschl, G.; Lucius, R. Involvement of benzodiazepine receptors in neuroinflammatory and neurodegenerative diseases: Evidence from activated microglial cells in vitro. Neurobiol. Dis. 2003, 14, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Choi, H.B.; McLarnon, J.G. Peripheral benzodiazepine receptor ligand pk11195 reduces microglial activation and neuronal death in quinolinic acid-injected rat striatum. Neurobiol. Dis. 2005, 20, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Holmuhamedov, E. Voltage-dependent anion channel (VDAC) as mitochondrial governator—Thinking outside the box. Biochim. Biophys. Acta 2006, 1762, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Colombini, M. Vdac: The channel at the interface between mitochondria and the cytosol. Mol. Cell. Biochem. 2004, 256–257, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.M.; Chan, S.H. The voltage dependent anion channel affects mitochondrial cholesterol distribution and function. Arch. Biochem. Biophys. 2007, 466, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Boujrad, N.; Vidic, B.; Papadopoulos, V. Acute action of choriogonadotropin on leydig tumor cells: Changes in the topography of the mitochondrial peripheral-type benzodiazepine receptor. Endocrinology 1996, 137, 5727–5730. [Google Scholar] [PubMed]
- Terrones, O.; Antonsson, B.; Yamaguchi, H.; Wang, H.G.; Liu, J.; Lee, R.M.; Herrmann, A.; Basanez, G. Lipidic pore formation by the concerted action of proapoptotic bax and TBID. J. Biol. Chem. 2004, 279, 30081–30091. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Abundis, E.; Correa, F.; Rodriguez, E.; Soria-Castro, E.; Rodriguez-Zavala, J.S.; Pacheco-Alvarez, D.; Zazueta, C. A CRAC-like motif in bax sequence: Relationship with protein insertion and pore activity in liposomes. Biochim. Biophys. Acta 2011, 1808, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Baburina, Y.; Grachev, D.; Krestinina, O.; Papadopoulos, V.; Lemasters, J.J.; Odinokova, I.; Reiser, G. Carbenoxolone induces permeability transition pore opening in rat mitochondria via the translocator protein tspo and connexin43. Arch. Biochem. Biophys. 2014, 558, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Sayer, B.G.; Epand, R.F. Peptide-induced formation of cholesterol-rich domains. Biochemistry 2003, 42, 14677–14689. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.M.; Brown, A.C.; Mittal, J. Disorder in cholesterol-binding functionality of CRAC peptides: A molecular dynamics study. J. Phys. Chem. B 2014, 118, 13169–13174. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Grachev, D.; Krestinina, O.; Evtodienko, Y.; Yurkov, I.; Papadopoulos, V.; Reiser, G. The peripheral-type benzodiazepine receptor is involved in control of Ca2+-induced permeability transition pore opening in rat brain mitochondria. Cell Calcium 2007, 42, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Veenman, L.; Shandalov, Y.; Gavish, M. VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. J. Bioenerg. Biomembr. 2008, 40, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Midzak, A.; Akula, N.; Lecanu, L.; Papadopoulos, V. Novel androstenetriol interacts with the mitochondrial translocator protein and controls steroidogenesis. J. Biol. Chem. 2011, 286, 9875–9887. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Krestinina, O.; Galvita, A.; Grachev, D.; Baburina, Y.; Stricker, R.; Evtodienko, Y.; Reiser, G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2’,3’-cyclic nucleotides and 2’,3’-cyclic nucleotide 3’-phosphodiesterase. Am. J. Physiol. Cell Physiol. 2009, 296, C1428–C1439. [Google Scholar] [CrossRef] [PubMed]
- Baburina, Y.; Azarashvili, T.; Grachev, D.; Krestinina, O.; Galvita, A.; Stricker, R.; Reiser, G. Mitochondrial 2’, 3’-cyclic nucleotide 3’-phosphodiesterase (CNP) interacts with mptp modulators and functional complexes (i-v) coupled with release of apoptotic factors. Neurochem. Int. 2015, 90, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Seydel, J.K.; Coats, E.A.; Cordes, H.P.; Wiese, M. Drug membrane interaction and the importance for drug transport, distribution, accumulation, efficacy and resistance. Arch. Pharm. (Weinheim) 1994, 327, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Lucio, M.; Lima, J.L.; Reis, S. Drug-membrane interactions: Significance for medicinal chemistry. Curr. Med. Chem. 2010, 17, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M. Proteins and cholesterol-rich domains. Biochim. Biophys. Acta 2008, 1778, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Barrantes, F.J. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013, 4, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epand, R.M. Cholesterol and the interaction of proteins with membrane domains. Prog. Lipid Res. 2006, 45, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Hatty, C.R.; Le Brun, A.P.; Lake, V.; Clifton, L.A.; Liu, G.J.; James, M.; Banati, R.B. Investigating the interactions of the 18 kDa translocator protein and its ligand PK11195 in planar lipid bilayers. Biochim. Biophys. Acta 2014, 1838, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, L.; Jaremko, M.; Giller, K.; Becker, S.; Zweckstetter, M. Structure of the mitochondrial translocator protein in complex with a diagnostic ligand. Science 2014, 343, 1363–1366. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Krestinina, O.; Baburina, Y.; Odinokova, I.; Grachev, D.; Papadopoulos, V.; Akatov, V.; Lemasters, J.J.; Reiser, G. Combined effect of G3139 and TSPO ligands on Ca2+-induced permeability transition in rat brain mitochondria. Arch. Biochem. Biophys. 2015, 587, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, T.; Lehtonen, J.Y.; Kinnunen, P.K. Interactions of cyclosporin a with phospholipid membranes: Effect of cholesterol. Mol. Pharmacol. 1999, 55, 32–38. [Google Scholar] [PubMed]
- Veenman, L.; Gavish, M. The role of 18 kDa mitochondrial translocator protein (TSPO) in programmed cell death, and effects of steroids on TSPO expression. Curr. Mol. Med. 2012, 12, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Krestinina, O.; Yurkov, I.; Evtodienko, Y.; Reiser, G. High-affinity peripheral benzodiazepine receptor ligand, PK11195, regulates protein phosphorylation in rat brain mitochondria under control of Ca2+. J. Neurochem. 2005, 94, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Drolle, E.; Kucerka, N.; Hoopes, M.I.; Choi, Y.; Katsaras, J.; Karttunen, M.; Leonenko, Z. Effect of melatonin and cholesterol on the structure of DOPC and DPPC membranes. Biochim. Biophys. Acta 2013, 1828, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, L.; Oudard, S.; Beurdeley-Thomas, A.; Dutrillaux, B.; Poupon, M.F. Effect of 1-(2-chlorophenyl)-n-methyl-n-(1-methylpropyl)-3-isoquinoline carboxamide (PK11195), a specific ligand of the peripheral benzodiazepine receptor, on the lipid fluidity of mitochondria in human glioma cells. Biochem. Pharmacol. 1999, 58, 715–721. [Google Scholar] [CrossRef]
- Montero, J.; Mari, M.; Colell, A.; Morales, A.; Basanez, G.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Cholesterol and peroxidized cardiolipin in mitochondrial membrane properties, permeabilization and cell death. Biochim. Biophys. Acta 2010, 1797, 1217–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Lecanu, L.; Yao, Z.X.; McCourty, A.; Sidahmed el, K.; Orellana, M.E.; Burnier, M.N.; Papadopoulos, V. Control of hypercholesterolemia and atherosclerosis using the cholesterol recognition/interaction amino acid sequence of the translocator protein TSPO. Steroids 2013, 78, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Allshire, A.; Bernardi, P.; Saris, N.E. Manganese stimulates calcium flux through the mitochondrial uniporter. Biochim. Biophys. Acta 1985, 807, 202–209. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azarashvili, T.; Krestinina, O.; Baburina, Y.; Odinokova, I.; Akatov, V.; Beletsky, I.; Lemasters, J.; Papadopoulos, V. Effect of the CRAC Peptide, VLNYYVW, on mPTP Opening in Rat Brain and Liver Mitochondria. Int. J. Mol. Sci. 2016, 17, 2096. https://doi.org/10.3390/ijms17122096
Azarashvili T, Krestinina O, Baburina Y, Odinokova I, Akatov V, Beletsky I, Lemasters J, Papadopoulos V. Effect of the CRAC Peptide, VLNYYVW, on mPTP Opening in Rat Brain and Liver Mitochondria. International Journal of Molecular Sciences. 2016; 17(12):2096. https://doi.org/10.3390/ijms17122096
Chicago/Turabian StyleAzarashvili, Tamara, Olga Krestinina, Yulia Baburina, Irina Odinokova, Vladimir Akatov, Igor Beletsky, John Lemasters, and Vassilios Papadopoulos. 2016. "Effect of the CRAC Peptide, VLNYYVW, on mPTP Opening in Rat Brain and Liver Mitochondria" International Journal of Molecular Sciences 17, no. 12: 2096. https://doi.org/10.3390/ijms17122096





