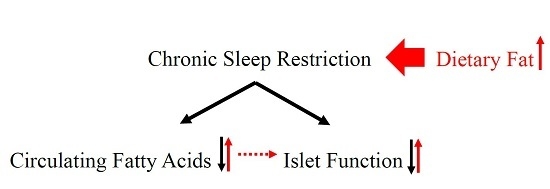
Decrease in Circulating Fatty Acids Is Associated with Islet Dysfunction in Chronically Sleep-Restricted Rats
Abstract
:1. Introduction
2. Results
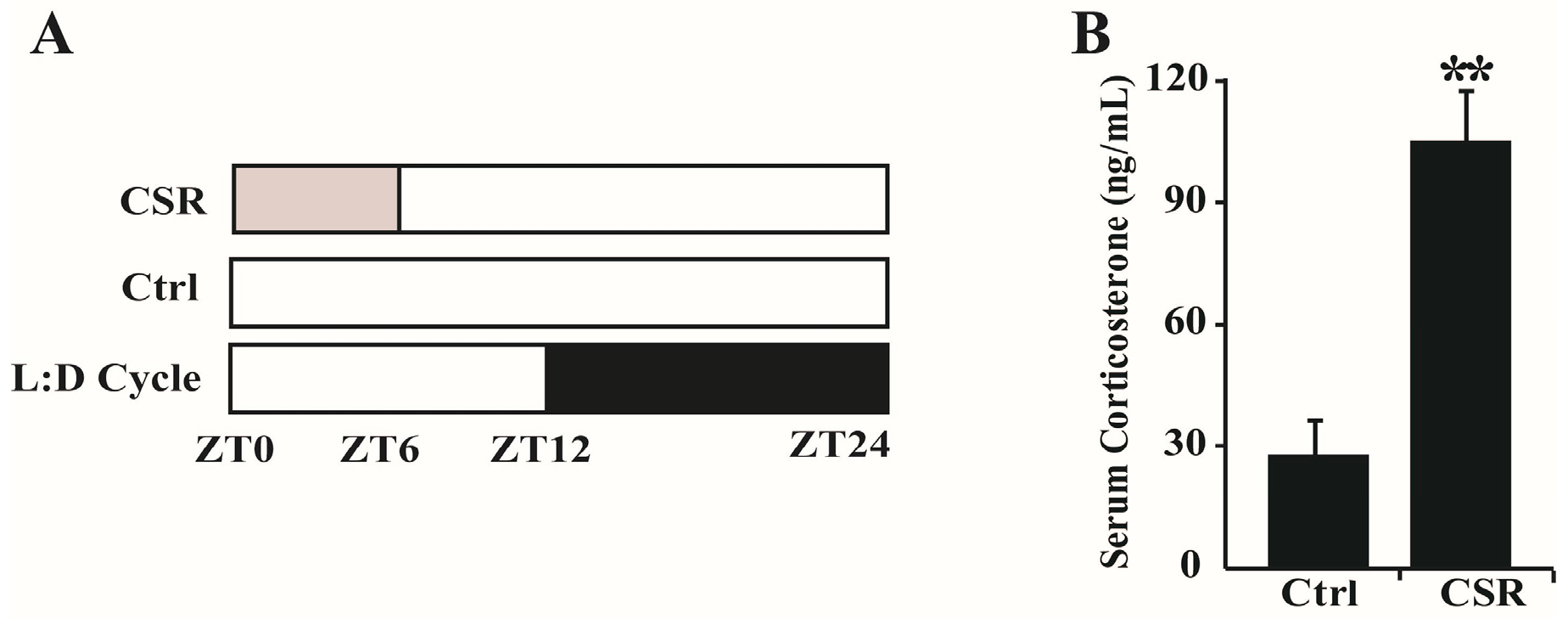
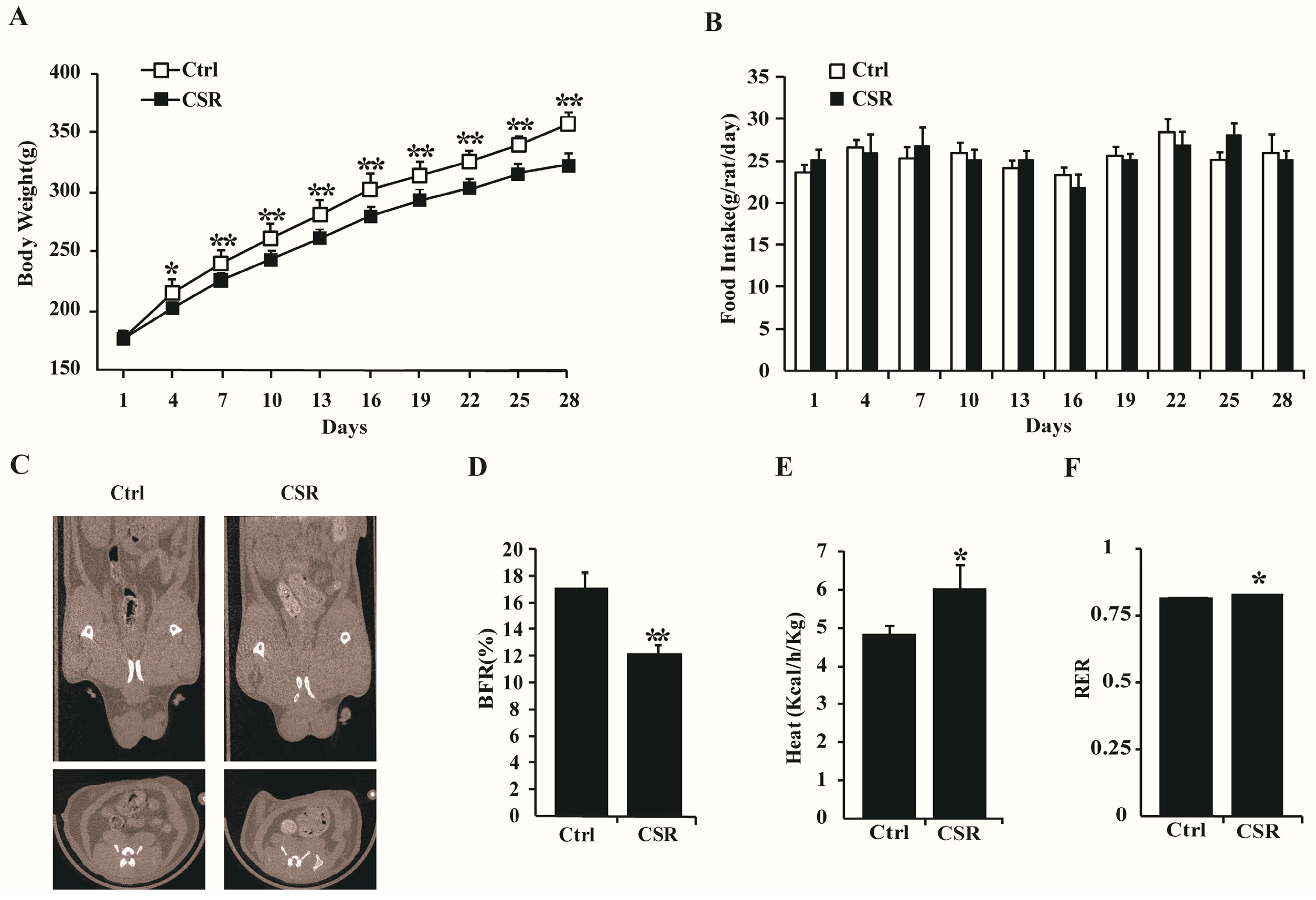
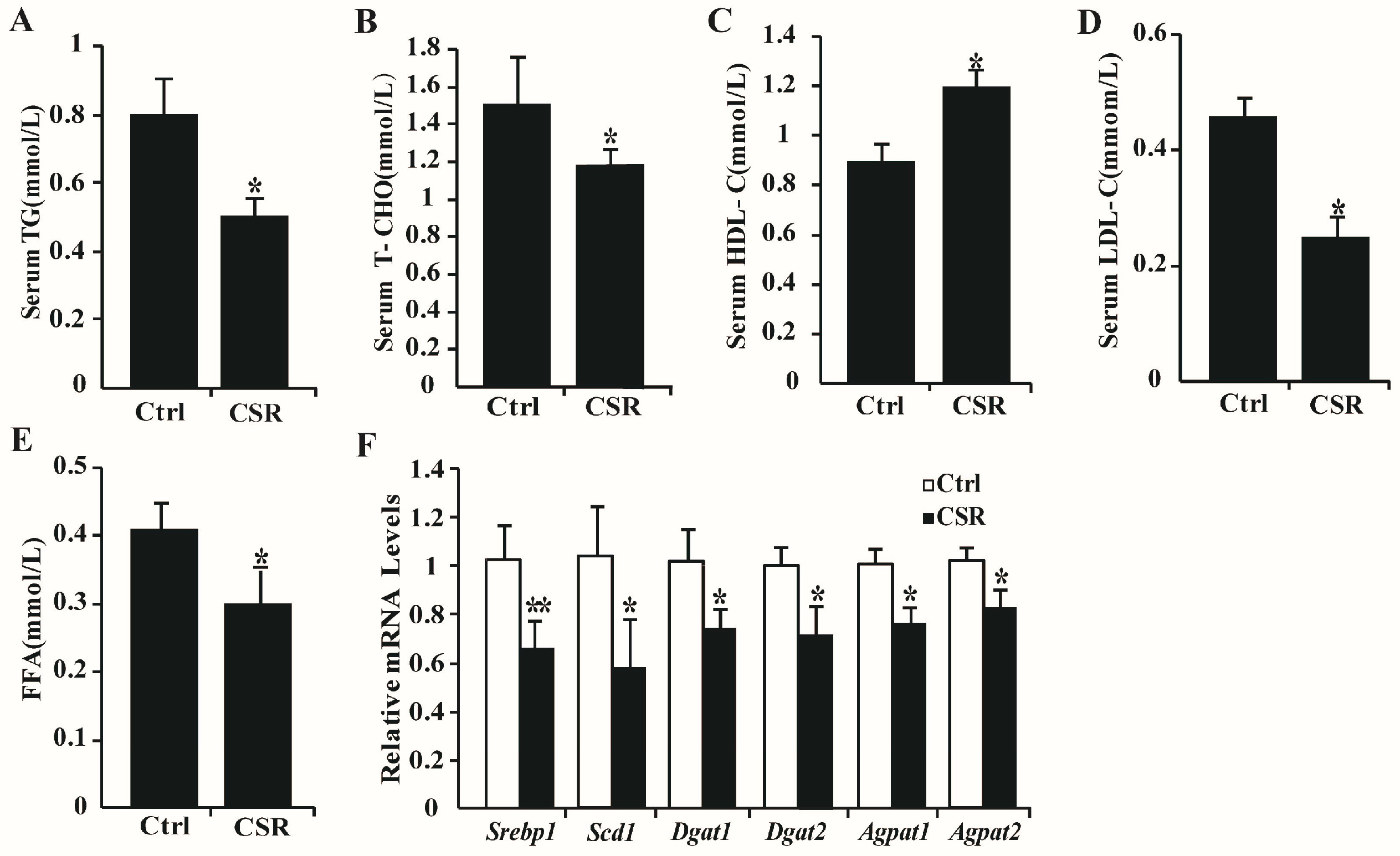
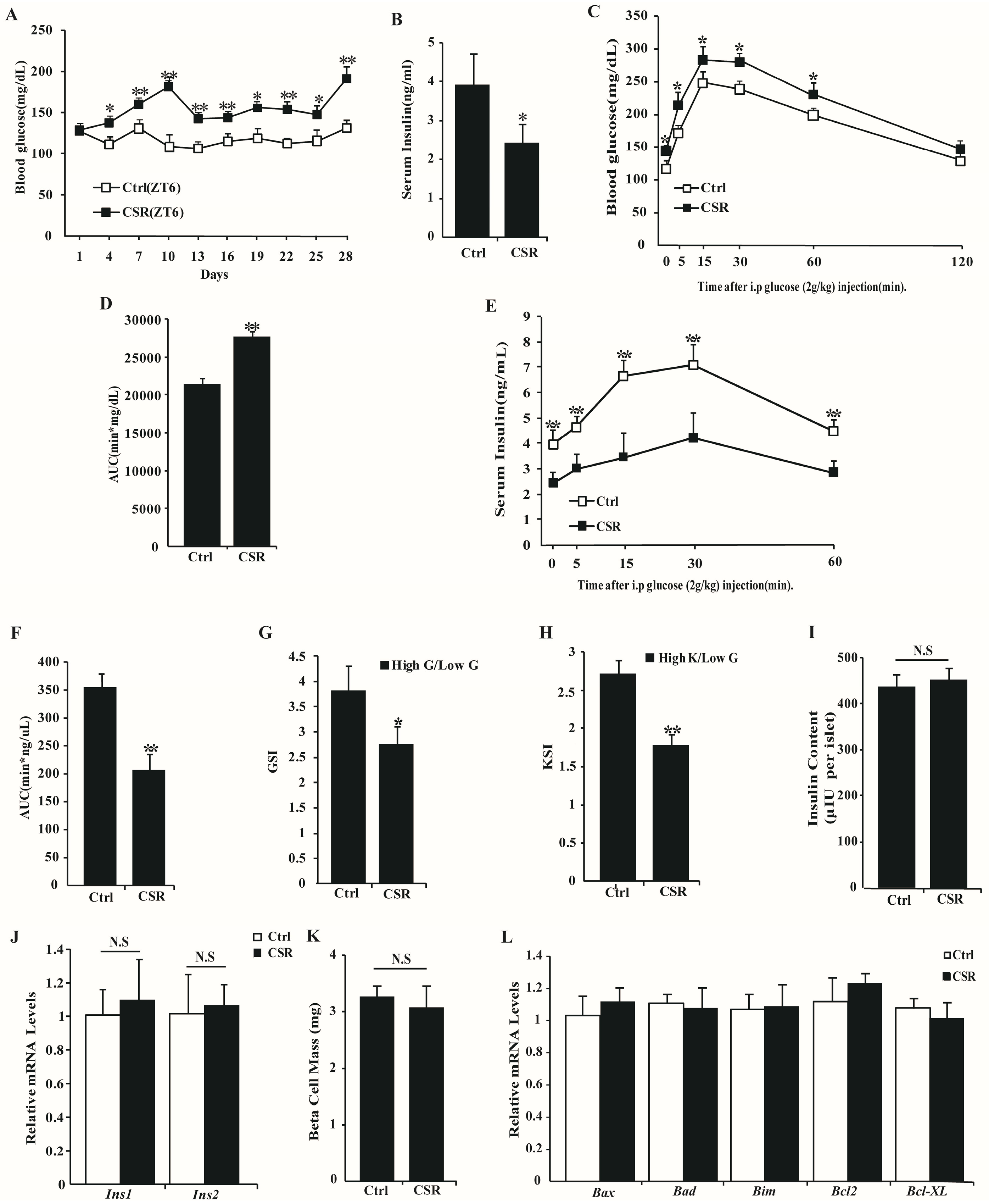
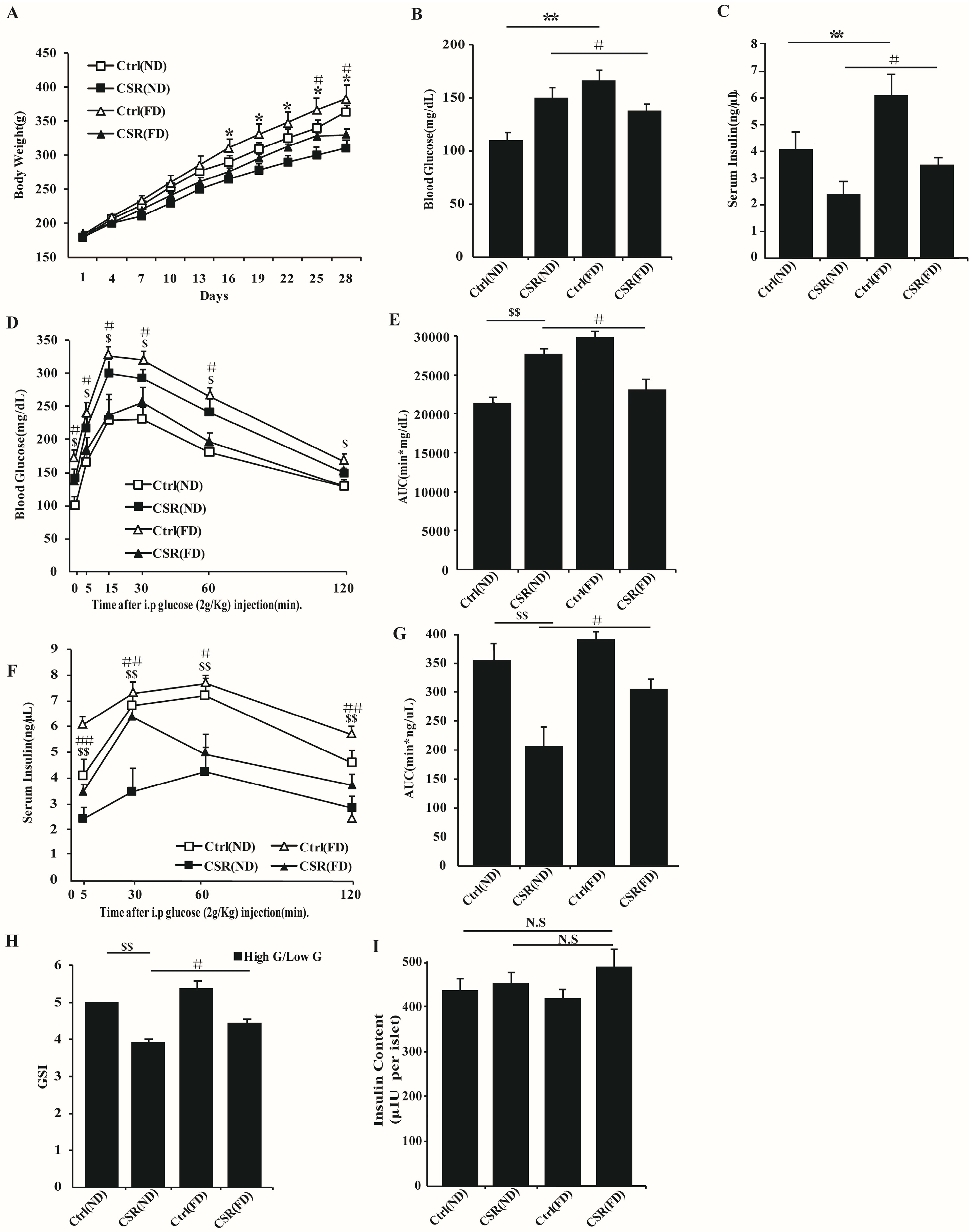
2.1. Metabolic Features of CSR Rat Model
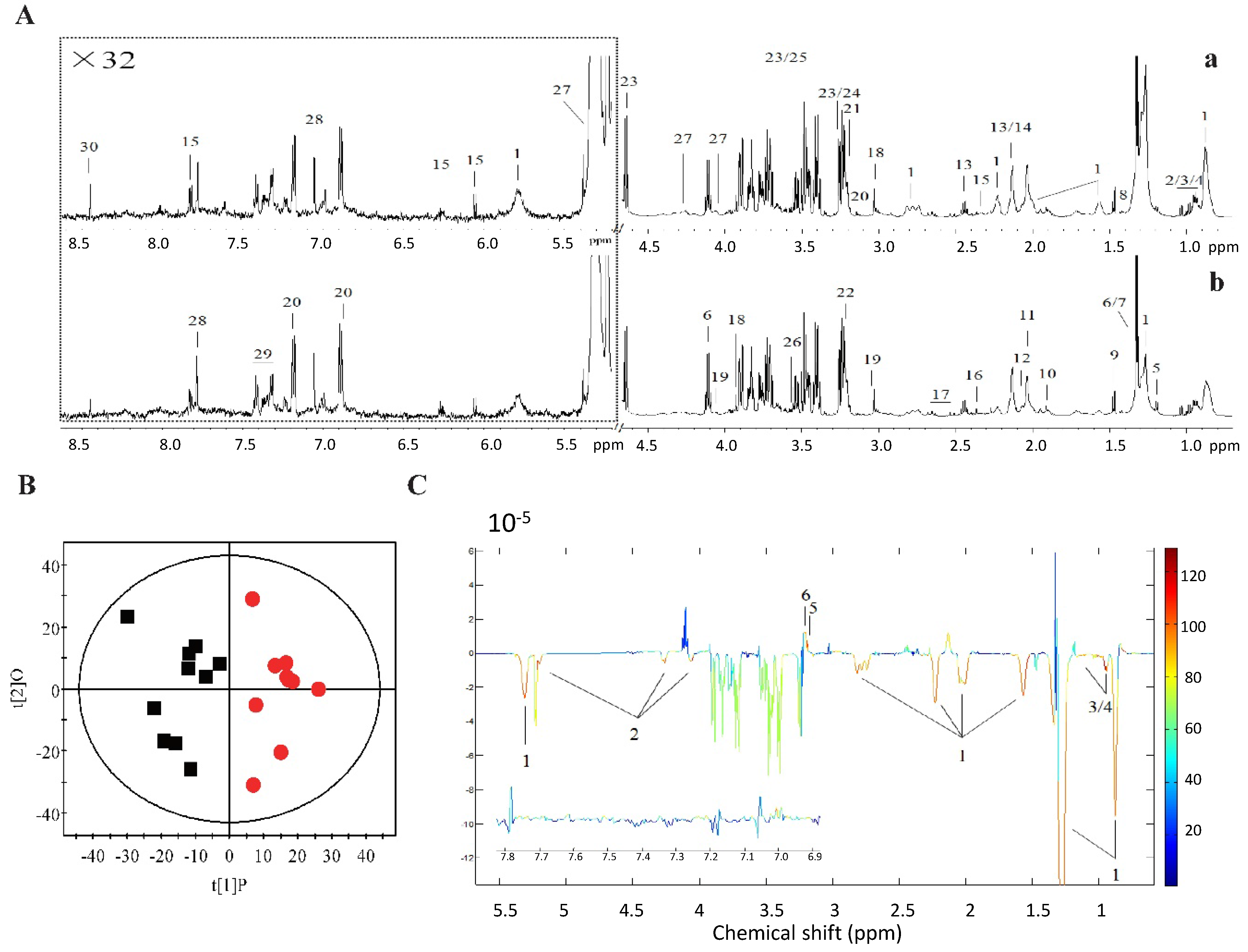
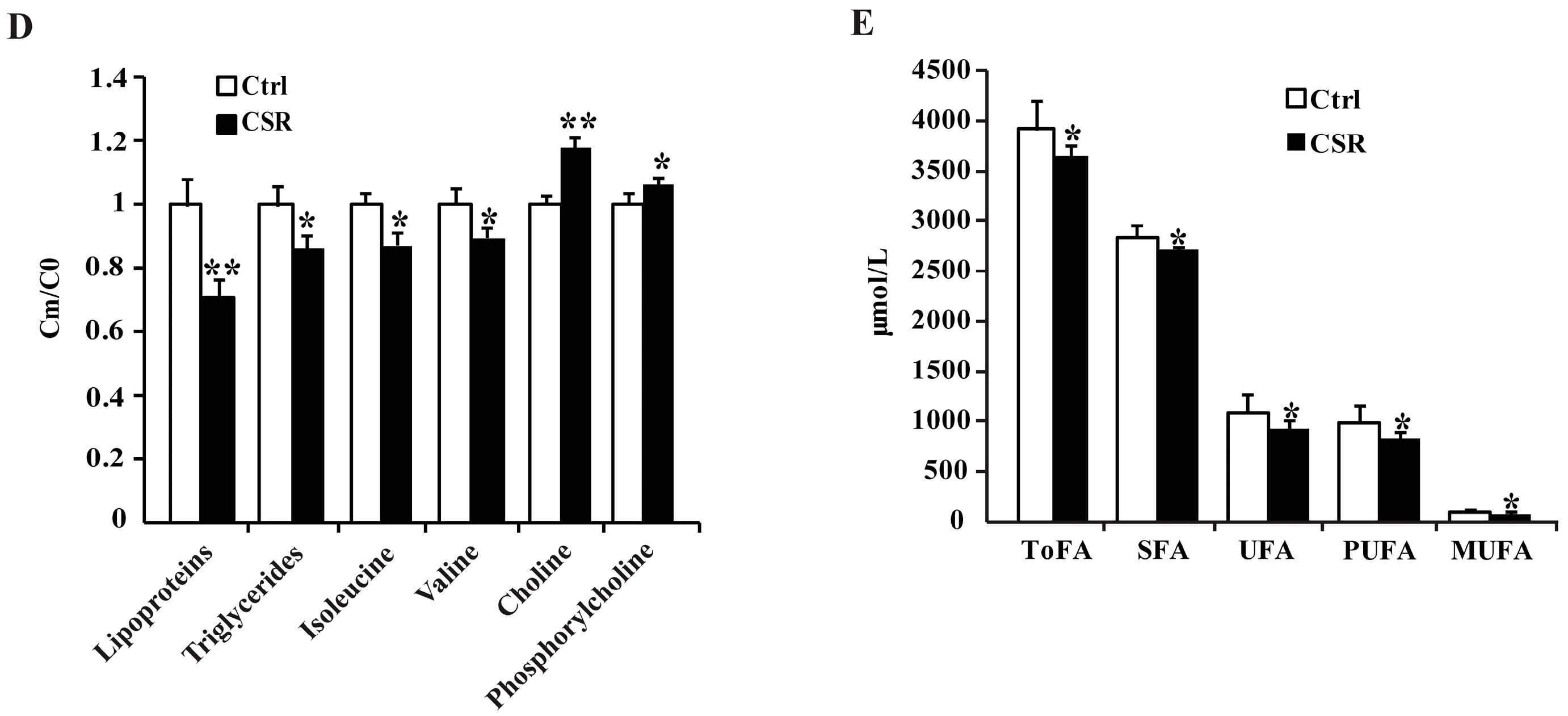
2.2. 1H NMR Metabolomics and GC-FID/MS Analysis
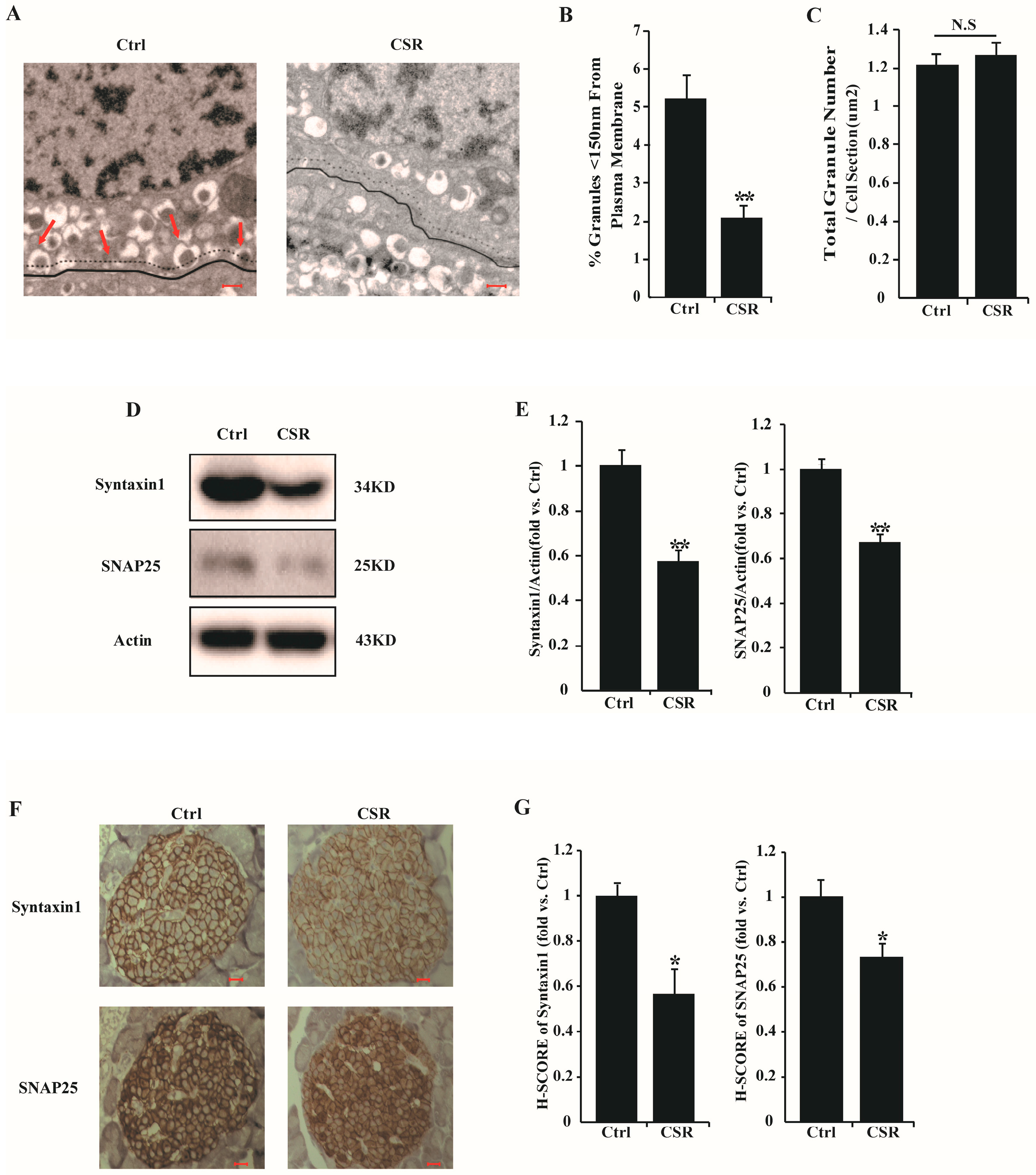
2.3. Dysfunction of CSR Rat Islets
2.4. Increased Dietary Fat Prevented Islets Function of CSR Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Sleep Restriction
4.3. Measurement of Metabolic Factors
4.4. CT Measurements
4.5. 1H NMR Spectroscopy and Fatty Acid Analysis
4.6. Glucose Tolerance Test and Insulin Tolerance Test
4.7. Islet Isolation and Insulin Secretion Assay
4.8. Western Blotting
4.9. RNA Isolation, RT-PCR, Real-Time Quantitative PCR
- Srebp1: F: CGCTACCGTTCCTCTATCA
- R: CTCCTCCACTGCCACAAG
- Scd1: F: GCTAATATCTGGGTGTAATC
- R: GGCTGGGTCATAGTTGTA
- Agpat1: F: CGACCTGCTTGGAATGAT
- R: AGCGTCTCCTGTGCGTTT
- Agpat2: F: GCTGCTGTTGCTGCTTGT
- R: CGTCCACCTCCAGTTTCTT
- Dgat1: F: AGTGGGTTCCCTGTTTGC
- R: TCTCGGTAGGTCAGGTTGT
- Dgat2: F: GGAGATTGGCATCGTGAA
- R: CAGGTGAGGCTTGGTTGG
- Ins1: F: CATAGACCATCAGCAAGCAGG
- R: GAAGAAACCACGTTCCCCAC
- Ins2: F: TGTCAAACAGCACCTTTGTGG
- R: GTGCCAAGGTCTGAAGGTCAC
- Bax: F: GGCGATGAACTGGACAAC
- R: TCCCGAAGTAGGAAAGGAG
- Bad: F: GAGGAAGATGAAGGGATGG
- R: GGACTCGCAACTTAGCACA
- Bim: F: CCTACAGACAGAATCGCAAGA
- R: GACGGAAGATGAATCGTAACAG
- Bcl2: F: CGGGAGAACAGGGTATGA
- R: CAGGCTGGAAGGAGAAGAT
- Bcl-xl: F: GGTATTGGTGAGTCGGATTG
- R: TGGACGGTCAGTGTCTGG
- G6pase: F: TCCACCTTGACACTACACCC
- R: GGGACGGTCGCACTCTT
- Pck1: F: TGTTGGCTGGCTCTCACTG
- R: ACCTTTGGGGATGGGCAC
- Pepck: F: AGTCACCATCACTTCCTGGAAGA
- R: GGTGCAGAATCGCGAGTTG
- Glut2: F: ACACCAGCACATACGACA
- R: CAAAGAACGAGGCGACTA
- β-actin: F: CCATGTTCCAAAACCATTCC
- R: GGGCAACCTTCCCAATAAAT
4.10. Oxygen Consumption Measurements
4.11. ATP Measurements
4.12. Transmission Electron Microscopy
4.13. Morphometric and Histologic Analysis
4.14. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AGPAT | Acylglycerolphosphate acyltransferase |
| BFR | Body fat ratio |
| CSD | Chronic sleep deprivation |
| DGAT | Diacylgycerol acyltransferase |
| FFAs | Free fatty acids |
| GC-FID/MS | Gas chromatography coupled with flame ionization detector/mass spectrometry |
| GPR40 | G-protein-coupled receptor 40 |
| GSIS | Glucose-stimulated insulin secretion |
| HDL-C | High density lipoprotein-cholesterol |
| HFD | High fat diet |
| INS1 | Insulin I |
| INS2 | Insulin II |
| IPGTT | Intraperitoneal glucose tolerance test |
| IPITT | Intraperitoneal insulin tolerance test |
| KSIS | KCl-stimulated insulin secretion |
| MUFA | Monounsaturated fatty acids |
| ND | Normal diet |
| NMR | Nuclear magnetic resonance |
| OPLS-DA | Orthogonal projection to latent structure discriminant analysis |
| OGTT | Oral glucose tolerance test |
| PUFA | polyunsaturated fatty acids |
| qRT-PCR | Quantitative real-time PCR |
| RER | Respiratory exchange ratio |
| SCD1 | Stearoyl CoA desaturease 1 |
| SREBP1 | Sterol-regulatory element binding proteins 1 |
| SFA | Saturated fatty acids |
| SNARE | Soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| T-CHO | Total-cholesterol |
| TG | Triglyceride |
| ToFA | Total Fatty Acids |
| UFA | Unsaturated fatty acids |
References
- Bonnet, M.H.; Arand, D.L. We are chronically sleep deprived. Sleep 1995, 18, 908–911. [Google Scholar] [PubMed]
- Chien, K.L.; Chen, P.C.; Hsu, H.C.; Su, T.C.; Sung, F.C.; Chen, M.F.; Lee, Y.T. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: Report from a community-based cohort. Sleep 2010, 33, 177–184. [Google Scholar] [PubMed]
- Faraut, B.; Boudjeltia, K.Z.; Vanhamme, L.; Kerkhofs, M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med. Rev. 2012, 16, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Leproult, R.; Van Reeth, O.; Byrne, M.M.; Sturis, J.; Van Cauter, E. Sleepiness, performance, and neuroendocrine function during sleep deprivation: Effects of exposure to bright light or exercise. J. Biol. Rhythms 1997, 12, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.R.; Muldoon, M.F.; Hall, M.; Buysse, D.J.; Manuck, S.B. Self-reported sleep quality is associated with the metabolic syndrome. Sleep 2007, 30, 219–223. [Google Scholar] [PubMed]
- Colwell, C.S.; Matveyenko, A.V. Timing is everything: Implications for metabolic consequences of sleep restriction. Diabetes 2014, 63, 1826–1828. [Google Scholar] [CrossRef] [PubMed]
- Del Guerra, S.; Lupi, R.; Marselli, L.; Masini, M.; Bugliani, M.; Sbrana, S.; Torri, S.; Pollera, M.; Boggi, U.; Mosca, F.; et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 2005, 54, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Ruiz, J.; Urbain, J.L.; Chen, X. Evidence for a circadian rhythm of insulin secretion. Am. J. Physiol. 1996, 271, E246–E252. [Google Scholar] [PubMed]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Rechtschaffen, A.; Bergmann, B.M.; Everson, C.A.; Kushida, C.A.; Gilliland, M.A. Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep 1989, 12, 68–87. [Google Scholar] [PubMed]
- Hairston, I.S.; Ruby, N.F.; Brooke, S.; Peyron, C.; Denning, D.P.; Heller, H.C.; Sapolsky, R.M. Sleep deprivation elevates plasma corticosterone levels in neonatal rats. Neurosci. Lett. 2001, 315, 29–32. [Google Scholar] [CrossRef]
- Gaisano, H.Y.; Ostenson, C.G.; Sheu, L.; Wheeler, M.B.; Efendic, S. Abnormal expression of pancreatic islet exocytotic soluble N-ethylmaleimide-sensitive factor attachment protein receptors in Goto-Kakizaki rats is partially restored by phlorizin treatment and accentuated by high glucose treatment. Endocrinology 2002, 143, 4218–4226. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, J.C.; Somashekar, D.; Appugliese, D.; Kaciroti, N.; Corwyn, R.F.; Bradley, R.H. Shorter sleep duration is associated with increased risk for being overweight at ages 9 to 12 years. Pediatrics 2007, 120, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Schioth, H.B.; Benedict, C. Determinants of shortened, disrupted, and mistimed sleep and associated metabolic health consequences in healthy humans. Diabetes 2015, 64, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.C.; D’Almeida, V.; Souza, F.G.; Schoorlemmer, G.H.; Colombari, E.; Tufik, S. Consequences of subchronic and chronic exposure to intermittent hypoxia and sleep deprivation on cardiovascular risk factors in rats. Respir. Physiol. Neurobiol. 2007, 156, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Barf, R.P.; Meerlo, P.; Scheurink, A.J. Chronic sleep disturbance impairs glucose homeostasis in rats. Int. J. Endocrinol. 2010, 2010, 819414. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R.; Joels, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Radahmadi, M.; Shadan, F.; Karimian, S.M.; Sadr, S.S.; Nasimi, A. Effects of stress on exacerbation of diabetes mellitus, serum glucose and cortisol levels and body weight in rats. Pathophysiology 2006, 13, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Retana-Marquez, S.; Bonilla-Jaime, H.; Vazquez-Palacios, G.; Dominguez-Salazar, E.; Martinez-Garcia, R.; Velazquez-Moctezuma, J. Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology 2003, 28, 207–227. [Google Scholar] [CrossRef]
- Barf, R.P.; van Dijk, G.; Scheurink, A.J.; Hoffmann, K.; Novati, A.; Hulshof, H.J.; Fuchs, E.; Meerlo, P. Metabolic consequences of chronic sleep restriction in rats: Changes in body weight regulation and energy expenditure. Physiol. Behav. 2012, 107, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wu, H.W.; Song, G.Q.; Lu, C.; Li, Y.H.; Qu, L.N.; Chen, S.G.; Liu, X.M.; Chang, Q. Chronical sleep interruption-induced cognitive decline assessed by a metabolomics method. Behav. Brain Res. 2016, 302, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.M.; Melanson, E.L.; Frydendall, E.J.; Perreault, L.; Eckel, R.H.; Wright, K.P. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J. Physiol. 2011, 589, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Sgoifo, A.; Buwalda, B.; Roos, M.; Costoli, T.; Merati, G.; Meerlo, P. Effects of sleep deprivation on cardiac autonomic and pituitary-adrenocortical stress reactivity in rats. Psychoneuroendocrinology 2006, 31, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M.; et al. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef] [PubMed]
- Weljie, A.M.; Meerlo, P.; Goel, N.; Sengupta, A.; Kayser, M.S.; Abel, T.; Birnbaum, M.J.; Dinges, D.F.; Sehgal, A. Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc. Natl. Acad. Sci. USA 2015, 112, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yadav, D.; Ahn, S.V.; Koh, S.B.; Park, J.T.; Yoon, J.; Yoo, B.S.; Lee, S.H. A prospective study of total sleep duration and incident metabolic syndrome: The ARIRANG study. Sleep Med. 2015, 16, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B.; Tripathy, S.; Depner, C.M. Fatty acid-regulated transcription factors in the liver. Annu. Rev. Nutr. 2013, 33, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Everson, C.A.; Szabo, A. Repeated exposure to severely limited sleep results in distinctive and persistent physiological imbalances in rats. PLoS ONE 2011, 6, e22987. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.N.; Kilkus, J.M.; Booth, J.N., 3rd; Bromley, L.E.; Imperial, J.G.; Penev, P.D. Effects of sleep restriction on the human plasma metabolome. Physiol. Behav. 2013, 122, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pikovsky, O.; Oron, M.; Shiyovich, A.; Perry, Z.H.; Nesher, L. The impact of sleep deprivation on sleepiness, risk factors and professional performance in medical residents. Isr. Med Assoc. J. 2013, 15, 739–744. [Google Scholar] [PubMed]
- Haber, E.P.; Ximenes, H.M.; Procopio, J.; Carvalho, C.R.; Curi, R.; Carpinelli, A.R. Pleiotropic effects of fatty acids on pancreatic β-cells. J. Cell. Physiol. 2003, 194, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Catalano, K.J.; Hsu, I.R.; Chiu, J.D.; Richey, J.M.; Bergman, R.N. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1590–E1598. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.T.; Esser, V.; Stevenson, B.E.; Lane, K.E.; Whiteside, J.H.; Daniels, M.B.; Chen, S.; McGarry, J.D. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J. Clin. Investig. 1996, 97, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, R.L.; Chester, M.W.; Daniels, M.B.; McGarry, J.D.; Stein, D.T. Circulating fatty acids are essential for efficient glucose-stimulated insulin secretion after prolonged fasting in humans. Diabetes 1998, 47, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Del Guerra, S.; Bugliani, M.; D’Aleo, V.; del Prato, S.; Boggi, U.; Mosca, F.; Filipponi, F.; Lupi, R. G-protein-coupled receptor 40 (GPR40) expression and its regulation in human pancreatic islets: The role of type 2 diabetes and fatty acids. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Chikahisa, S.; Fujiki, N.; Kitaoka, K.; Shimizu, N.; Sei, H. Central AMPK contributes to sleep homeostasis in mice. Neuropharmacology 2009, 57, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Timofeev, I.; Grenier, F.; Steriade, M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: An intracellular study. Proc. Natl. Acad. Sci. USA 2001, 98, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Let there be (NADH) light. Science 2004, 305, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Vacher, P.; Roger, B.; Huyghe, D.; Vandewalle, B.; Kerr-Conte, J.; Pattou, F.; Moustaid-Moussa, N.; Lang, J. Glucotoxicity inhibits late steps of insulin exocytosis. Endocrinology 2007, 148, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.B.; Sheu, L.; Ghai, M.; Bouquillon, A.; Grondin, G.; Weller, U.; Beaudoin, A.R.; Bennett, M.K.; Trimble, W.S.; Gaisano, H.Y. Characterization of SNARE protein expression in β cell lines and pancreatic islets. Endocrinology 1996, 137, 1340–1348. [Google Scholar] [PubMed]
- Zhang, W.; Khan, A.; Ostenson, C.G.; Berggren, P.O.; Efendic, S.; Meister, B. Down-regulated expression of exocytotic proteins in pancreatic islets of diabetic GK rats. Biochem. Biophys. Res. Commun. 2002, 291, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.B.; MacPhail, R.M.; Sheu, L.; Wheeler, M.B.; Gaisano, H.Y. β -cell hypertrophy in FA/FA rats is associated with basal glucose hypersensitivity and reduced SNARE protein expression. Diabetes 1999, 48, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Yam, K.Y.; Naninck, E.F.; Schmidt, M.V.; Lucassen, P.J.; Korosi, A. Early-life adversity programs emotional functions and the neuroendocrine stress system: The contribution of nutrition, metabolic hormones and epigenetic mechanisms. Stress 2015, 18, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Terao, J.; Nakaya, Y.; Miyamoto, K.; Baba, Y.; Chuman, H.; Kaji, R.; Ohmori, T.; Rokutan, K. Stress control and human nutrition. J. Med. Investig. 2004, 51, 139–145. [Google Scholar] [CrossRef]
- Horohov, D.W.; Pourciau, S.S.; Mistric, L.; Chapman, A.; Ryan, D.H. Increased dietary fat prevents sleep deprivation-induced immune suppression in rats. Comp. Med. 2001, 51, 230–233. [Google Scholar] [PubMed]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Mong, J.A.; Baker, F.C.; Mahoney, M.M.; Paul, K.N.; Schwartz, M.D.; Semba, K.; Silver, R. Sleep, rhythms, and the endocrine brain: Influence of sex and gonadal hormones. J. Neurosci. 2011, 31, 16107–16116. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, L.; An, Y.; Zhang, L.; Song, Y.; Wang, Y.; Tang, H. Antagonist of prostaglandin E2 receptor 4 induces metabolic alterations in liver of mice. J. Proteome Res. 2015, 14, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, S.; Sun, Y.; Nadler, J.L.; Bleich, D. Induction of cyclooxygenase-2 gene in pancreatic β cells by 12-lipoxygenase pathway product 12-hydroxyeicosatetraenoic acid. Mol. Endocrinol. 2002, 16, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.X.; Yin, Y.; Lv, J.H.; Sha, M.; Lin, Y.; Gao, L.; Zhu, Y.X.; Sun, Y.J.; Han, X. Aberrant activation of liver X receptors impairs pancreatic beta cell function through upregulation of sterol regulatory element-binding protein 1C in mouse islets and rodent cell lines. Diabetologia 2012, 55, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, S.; Wu, Y.; Sun, P.; Lin, H.; Zhu, Y.; Han, X. Decrease in Circulating Fatty Acids Is Associated with Islet Dysfunction in Chronically Sleep-Restricted Rats. Int. J. Mol. Sci. 2016, 17, 2102. https://doi.org/10.3390/ijms17122102
Zhan S, Wu Y, Sun P, Lin H, Zhu Y, Han X. Decrease in Circulating Fatty Acids Is Associated with Islet Dysfunction in Chronically Sleep-Restricted Rats. International Journal of Molecular Sciences. 2016; 17(12):2102. https://doi.org/10.3390/ijms17122102
Chicago/Turabian StyleZhan, Shanshan, Yangyang Wu, Peng Sun, Haiyan Lin, Yunxia Zhu, and Xiao Han. 2016. "Decrease in Circulating Fatty Acids Is Associated with Islet Dysfunction in Chronically Sleep-Restricted Rats" International Journal of Molecular Sciences 17, no. 12: 2102. https://doi.org/10.3390/ijms17122102