Maternal Food Restriction during Pregnancy and Lactation Adversely Affect Hepatic Growth and Lipid Metabolism in Three-Week-Old Rat Offspring
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Male Rat Offspring Aged Three Weeks
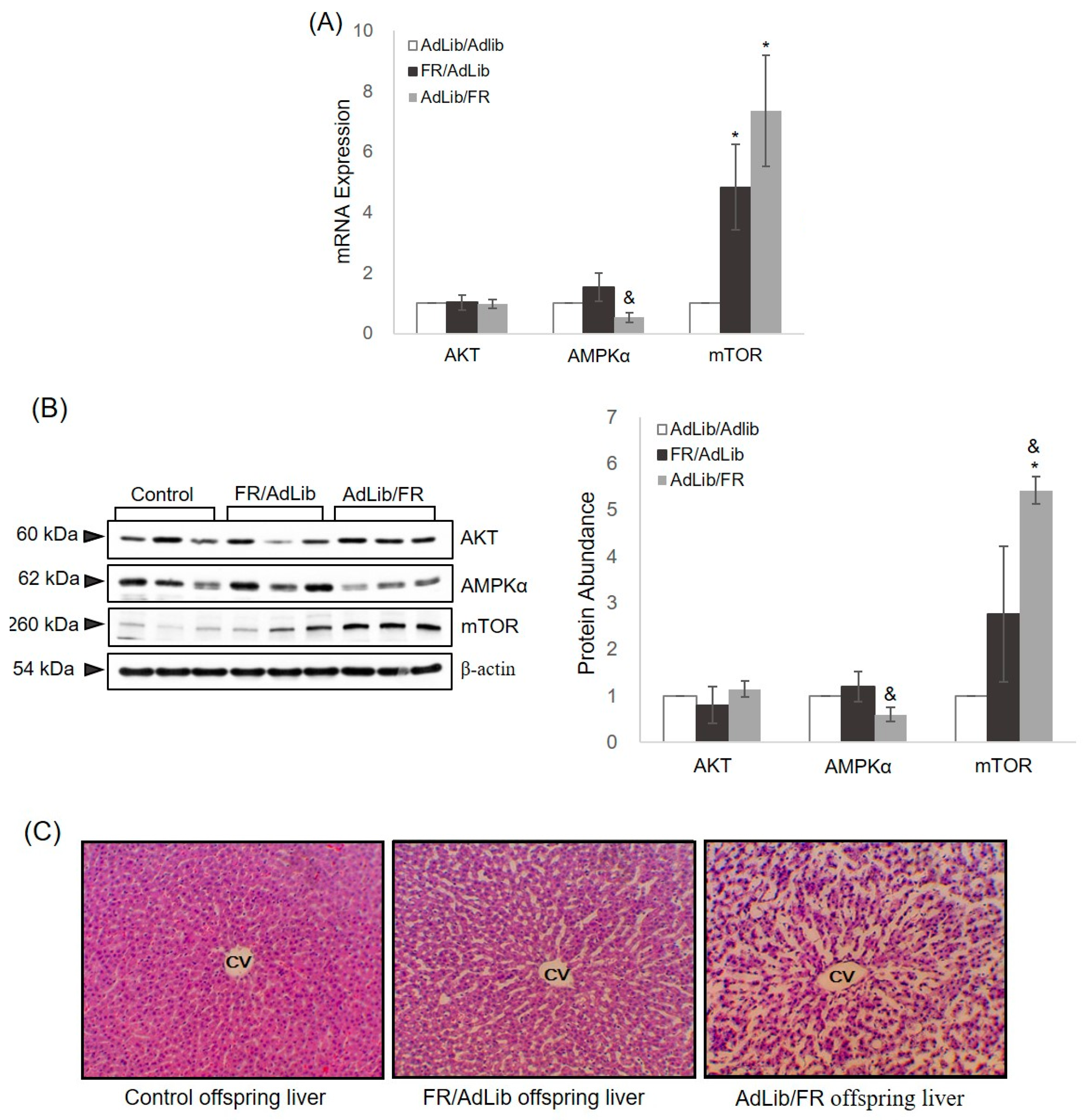
2.2. Hepatic Growth via the AKT/mTOR Pathway
2.3. The Regulation of Hepatic Lipogenesis
3. Discussion
4. Materials and Methods
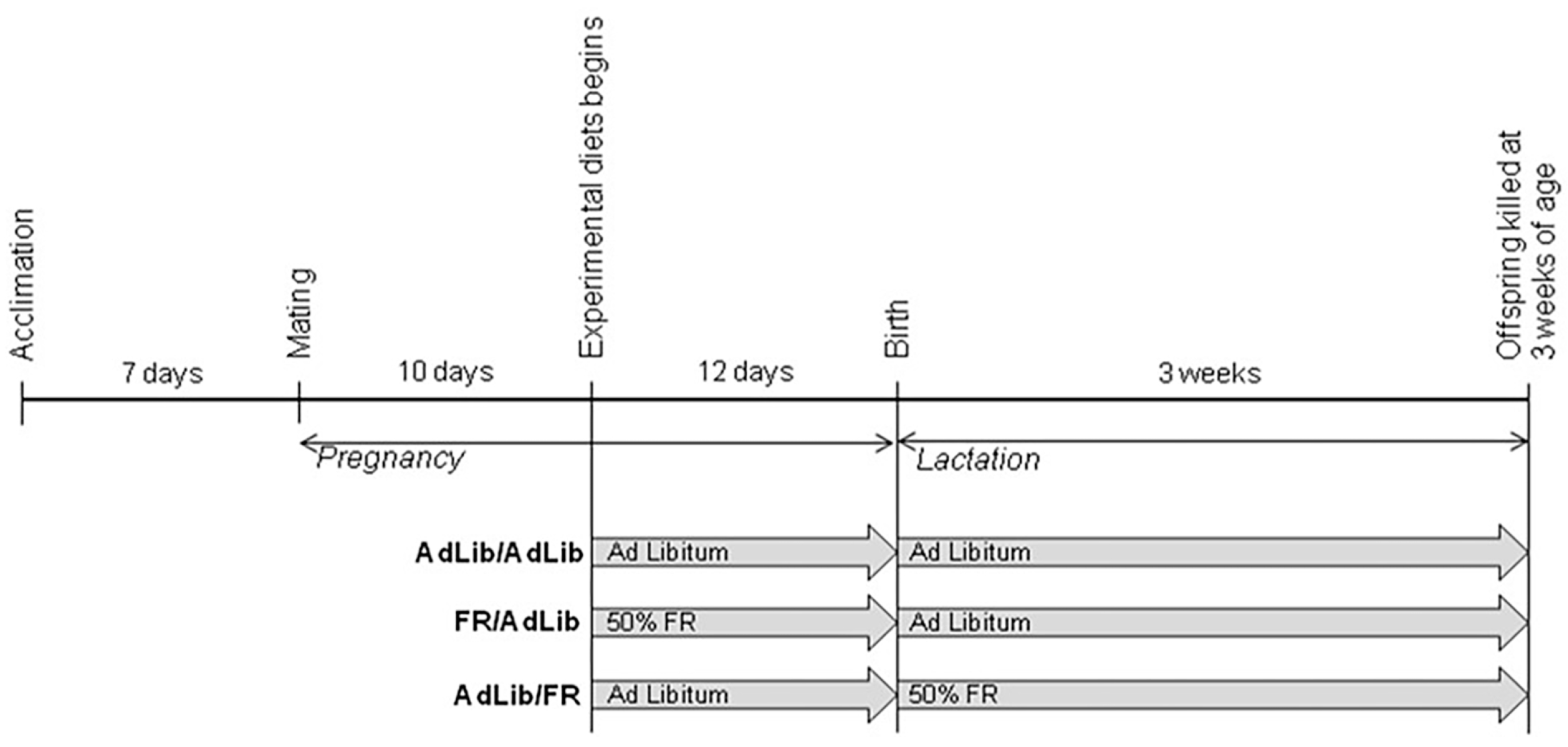
4.1. Animals and Study Design
4.2. Analysis of Lipid Profiles in Plasma
4.3. Quantitative Real-Time Reverse Transcription (RT) Polymerase Chain Reaction (PCR)
4.4. Western Blotting
4.5. Histology
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ergaz, Z.; Avgil, M.; Ornoy, A. Intrauterine growth restriction-aetiology and consequences: What do we know about the human situation and experimental animal models? Reprod. Toxicol. 2005, 20, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Thorn, S.R.; Regnault, T.R.; Brown, L.D.; Rozance, P.J.; Keng, J.; Roper, M.; Wilkening, R.B.; Hay, W.W., Jr.; Friedman, J.E. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 2009, 150, 3021–3030. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Babu, J.; Ross, M.G. Programmed metabolic syndrome: Prenatal undernutrition and postweaning overnutrition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Bieswal, F.; Ahn, M.T.; Reusens, B.; Holvoet, P.; Raes, M.; Rees, W.D.; Remacle, C. The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity 2006, 14, 1330–1343. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.M.; Norman, A.M.; Donkin, S.S.; Shankar, R.R.; Vickers, M.H.; Miles, J.L.; Breier, B.H. Prenatal and postnatal pathways to obesity: Different underlying mechanisms, different metabolic outcomes. Endocrinology 2007, 148, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Magee, T.R.; Han, G.; Cherian, B.; Khorram, O.; Ross, M.G.; Desai, M. Down-regulation of transcription factor peroxisome proliferator-activated receptor in programmed hepatic lipid dysregulation and inflammation in intrauterine growth-restricted offspring. Am. J. Obstet. Gynecol. 2008, 199, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.S.; de Moura, E.G.; Passos, M.C.; Nogueira Neto, F.J.; Reis, A.M.; de Oliveira, E.; Lisboa, P.C. Early weaning causes undernutrition for a short period and programmes some metabolic syndrome components and leptin resistance in adult rat offspring. Br. J. Nutr. 2011, 105, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Hall, B. Changing composition of human milk and early development of an appetite control. Lancet 1975, 1, 779–781. [Google Scholar] [CrossRef]
- Passos, M.; Ramos, C.; Moura, E. Short and long term effects of malnutrition in rats during lactation on the body weight of offspring. Nutr. Res. 2000, 20, 1603–1612. [Google Scholar] [CrossRef]
- Kwon, D.H.; Kang, W.; Nam, Y.S.; Lee, M.S.; Lee, I.Y.; Kim, H.J.; Rajasekar, P.; Lee, J.H.; Baik, M. Dietary protein restriction induces steatohepatitis and alters leptin/signal transducers and activators of transcription 3 signalling in lactating rats. J. Nutr. Biochem. 2012, 23, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Obesity notches up fatty liver. Nat. Med. 2013, 19, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, E.L.; Cho, H.; Birnbaum, M.J. Role of AKT/protein kinase B in metabolism. Trends Endocrinol. Metab. 2002, 13, 444–451. [Google Scholar] [CrossRef]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [PubMed]
- Porstmann, T.; Santos, C.R.; Lewis, C.; Griffiths, B.; Schulze, A. A new player in the orchestra of cell growth: SREBP activity is regulated by mTORC1 and contributes to the regulation of cell and organ size. Biochem. Soc. Trans. 2009, 37, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-activated protein kinase in the liver: A new strategy for the management of metabolic hepatic disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H. Sterol regulatory element-binding proteins (SREBPs): Transcriptional regulators of lipid synthetic genes. Prog. Lipid Res. 2001, 40, 439–452. [Google Scholar] [CrossRef]
- Yokoyama, C.; Wang, X.; Briggs, M.R.; Admon, A.; Wu, J.; Hua, X.; Goldstein, J.L.; Brown, M.S. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell 1993, 75, 187–197. [Google Scholar] [CrossRef]
- Barker, D.J.; Bull, A.R.; Osmond, C.; Simmonds, S.J. Foetal and placental size and risk of hypertension in adult life. BMJ 1990, 301, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S. Early life origins of insulin resistance and type 2 diabetes in India and other Asian countries. J. Nutr. 2004, 134, 205–210. [Google Scholar] [PubMed]
- Kim, H.W.; Lee, S.; Lee, S.H.; Jo, I.; Kim, Y.J. Effect of feed restriction during gestation and lactation period on changes in organ weight in rat offspring. Korean J. Obstet. Gynecol. 2012, 55, 822–829. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.A.; Choi, G.Y.; Desai, M.; Lee, S.H.; Pang, M.G.; Jo, I.; Kim, Y.J. Feed restriction during pregnancy/lactation induces programmed changes in lipid, adiponectin and leptin levels with gender differences in rat offspring. J. Maternal. Foetal. Neonatal. Med. 2013, 26, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Leavens, K.F.; Saleh, D.; Easton, R.M.; Guertin, D.A.; Peterson, T.R.; Kaestner, K.H.; Sabatini, D.M.; Birnbaum, M.J. Postprandial hepatic lipid metabolism requires signaling though AKT2 independent of the transcription factors FoxA2, FoxO1 and SREBP1c. Cell Metab. 2011, 14, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Fernandez, M.L.; Feinman, R.D.; Phinney, S.D. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog. Lipid Res. 2008, 47, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Martin Agnoux, A.; Antignac, J.P.; Boquien, C.Y.; David, A.; Desnots, E.; Ferchaud-Roucher, V.; Darmaun, D.; Parnet, P.; Alexandre-Gouabau, M.C. Perinatal protein restriction affects milk free amino acid and fatty acid profile in lactating rats: Potential role on pup growth and metabolic status. J. Nutr. Biochem. 2015, 26, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Brunn, G.J.; Hudson, C.C.; Sekulić, A.; Williams, J.M.; Hosoi, H.; Houghton, P.J.; Lawrence, J.C., Jr.; Abraham, R.T. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 1997, 277, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Windgassen, A.; Nogueira, V.; Chen, C.C.; Skeen, J.E.; Sonenberg, N.; Hay, N. AKT activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J. Biol. Chem. 2005, 280, 32081–32089. [Google Scholar] [CrossRef] [PubMed]
- Jahan-Mihan, A.; Rodriguez, J.; Christie, C.; Sadeghi, M.; Zerbe, T. The role of maternal dietary proteins in development of metabolic syndrome in offspring. Nutrients 2015, 7, 9185–9217. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Ko, Y.H.; Chin, H.J.; Hsu, C.; Ding, S.T.; Chen, C.Y. The effect of feed restriction on expression of hepatic lipogenic genes in broiler chickens and the function of SREBP1. Comp. Biochem. Physiol. Biochem. Mol. Biol. 2009, 153, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Davis, R.A. Cholesterol and hepatic lipoprotein assembly and secretion. Biochim. Biophys. Acta 2000, 1529, 223–230. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Takayanagi, R.; Nakamuta, M. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Denechaud, P.; Bossard, P.; Lobaccaro, J.; Millatt, L.; Staels, B.; Girard, J.; Postic, C. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver. J. Clin. Investig. 2008, 118, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Brönner, G.; Sattler, A.M.; Hinney, A.; Soufi, M.; Geller, F.; Schäfer, H.; Maisch, B.; Hebebrand, J.; Schaefer, J.R. The 103I variant of the melanocortin 4 receptor is associated with low serum triglyceride levels. J. Clin. Endocrinol. Metab. 2006, 91, 535–538. [Google Scholar] [CrossRef] [PubMed]

| Variables | Control (n = 9) | FR †/AdLib ‡ (n = 9) | AdLib †/FR ‡ (n = 9) |
|---|---|---|---|
| Birth weight (g) | 7.33 ± 0.29 a | 6.67 ± 0.29 b | 7.63 ± 0.32 a |
| Three-week-old | – | – | – |
| Body weight (g) | 56.97 ± 3.97 a | 64.14 ± 4.66 a | 18.88 ± 0.21 b |
| Liver weight (g) | 2.61 ± 0.56 a | 2.38 ± 0.29 a | 0.49 ± 0.02 b |
| Liver weight to body weight ratio | 0.046 ± 0.009 a | 0.037 ± 0.003 a,b | 0.026 ± 0.001 b |
| Variables | Control | FR †/AdLib ‡ | AdLib †/FR ‡ | F-Value | F-Value |
|---|---|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | ANOVA | ANCOVA | |
| Lipid profiles | – | – | – | – | – |
| Triglyceride (mg/dL) | 101.3 ± 15.5 a | 157.3 ± 16.9 b | 50.3 ± 19.5 c | 28.42 * | 37.26 * |
| Total cholesterol (mg/dL) | 104.0 ± 17.0 a | 151.0 ± 9.8 b | 444.0 ± 19.0 c | 408.95 ** | 777.19 ** |
| HDL-cholesterol (mg/dL) | 36.0 ± 6.0 a | 39.7 ± 3.5 a | 50.0 ± 1.0 b | 9.62 * | 25.66 * |
| LDL-cholesterol (mg/dL) | 25.0 ± 3.0 a | 48.0 ± 3.6 b | 196.0 ± 7.0 c | 1091.70 ** | 1226.96 ** |
| Carbohydrate metabolism | – | – | – | – | – |
| Glucose (mmol/L) | 18.9 ± 0.9 a | 21.9 ± 1.9 a | 9.8 ± 0.9 b | 71.86 ** | 73.07 ** |
| Insulin (μU/mL) | 4.5 ± 1.5 a | 10.6 ± 4.3 b | 3.1 ± 0.5 a | 6.65 * | 4.02 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; You, Y.-A.; Kwon, E.J.; Jung, S.-C.; Jo, I.; Kim, Y.J. Maternal Food Restriction during Pregnancy and Lactation Adversely Affect Hepatic Growth and Lipid Metabolism in Three-Week-Old Rat Offspring. Int. J. Mol. Sci. 2016, 17, 2115. https://doi.org/10.3390/ijms17122115
Lee S, You Y-A, Kwon EJ, Jung S-C, Jo I, Kim YJ. Maternal Food Restriction during Pregnancy and Lactation Adversely Affect Hepatic Growth and Lipid Metabolism in Three-Week-Old Rat Offspring. International Journal of Molecular Sciences. 2016; 17(12):2115. https://doi.org/10.3390/ijms17122115
Chicago/Turabian StyleLee, Sangmi, Young-Ah You, Eun Jin Kwon, Sung-Chul Jung, Inho Jo, and Young Ju Kim. 2016. "Maternal Food Restriction during Pregnancy and Lactation Adversely Affect Hepatic Growth and Lipid Metabolism in Three-Week-Old Rat Offspring" International Journal of Molecular Sciences 17, no. 12: 2115. https://doi.org/10.3390/ijms17122115







