Inhibition of REST Suppresses Proliferation and Migration in Glioblastoma Cells
Abstract
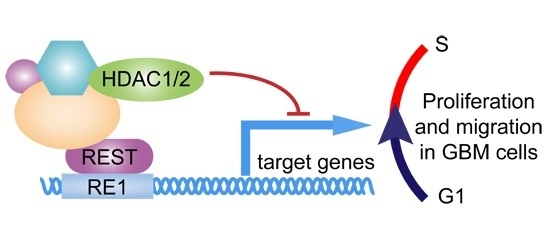
:1. Introduction
2. Results
2.1. Repressor Element 1-Silencing Transcription Factor (REST) Knockdown by siRNA
2.2. REST Knockdown Reduced Cell Proliferation
2.3. G1 Phase Cell Cycle Arrest Induced by REST Silencing
2.4. REST Silencing Does Not Induce Cell Apoptosis
2.5. Cell Migration Is Decreased by REST Silencing
2.6. REST Knockdown Enhanced Genes Involved in Cytostasis and Migration Inhibition
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. siRNA Transfection
4.3. In Vitro Scratch Assay
4.4. Transwell Migration Assay
4.5. Cell Proliferation Assay
4.6. Cell Cycle Analysis
4.7. Cell Apoptosis Assay
4.8. Quantitative Real-Time PCR
4.9. Western Blotting Analysis
4.10. Chromatin Immunoprecipitation (ChIP)
4.11. Protein Interactions Analysis by Search Tool for the Retrieval of Interacting Genes/Proteins (STRING)
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas a clinical review. J. Am. Med. Assoc. 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.A.; Ye, X.; Piantadosi, S.; Desideri, S.; Nabors, L.B.; Rosenfeld, M.; Fisher, J.; Consortium, N.C. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the united states. Clin. Cancer Res. 2010, 16, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Rockowitz, S.; Lien, W.H.; Pedrosa, E.; Wei, G.; Lin, M.; Zhao, K.; Lachman, H.M.; Fuchs, E.; Zheng, D. Comparison of REST cistromes across human cell types reveals common and context-specific functions. PLoS Comput. Biol. 2014, 10, e1003671. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.M.; Hwang, J.Y.; Follenzi, A.; Athanasiadou, R.; Miyawaki, T.; Greally, J.M.; Bennett, M.V.; Zukin, R.S. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc. Natl. Acad. Sci. USA 2012, 109, E962–E971. [Google Scholar] [CrossRef] [PubMed]
- Kellis, M.; Wold, B.; Snyder, M.P.; Bernstein, B.E.; Kundaje, A.; Marinov, G.K.; Ward, L.D.; Birney, E.; Crawford, G.E.; Dekker, J.; et al. Defining functional DNA elements in the human genome. Proc. Natl. Acad. Sci. USA 2014, 111, 6131–6138. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Bao, S. Ubiquitination and deubiquitination of REST and its roles in cancers. FEBS Lett. 2012, 586, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, M.; Yu, Y.; Qiu, L.; Zhang, Y.; He, L.; Zhang, J. Brain REST/NRSF is not only a silent repressor but also an active protector. Mol. Neurobiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Gao, Z.; Wu, N.; Zeng, L.; Tang, X.; Chen, X.; Liu, Z.; Zhang, W.; Wang, L.; Li, Z. Expression of REST4 in human gliomas in vivo and influence of pioglitazone on REST in vitro. Biochem. Biophys. Res. Commun. 2015, 463, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.M.; Sathyan, P.; Singh, S.K.; Zinn, P.O.; Marisetty, A.L.; Liang, S.; Gumin, J.; El-Mesallamy, H.O.; Suki, D.; Colman, H.; et al. Rest regulates oncogenic properties of glioblastoma stem cells. Stem Cells 2012, 30, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Crisafulli, L.; Caldera, V.; Tortoreto, M.; Brilli, E.; Conforti, P.; Zunino, F.; Magrassi, L.; Schiffer, D.; Cattaneo, E. Rest controls self-renewal and tumorigenic competence of human glioblastoma cells. PLoS ONE 2012, 7, e38486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lathia, J.D.; Flavahan, W.A.; Rich, J.N.; Mattson, M.P. Squelching glioblastoma stem cells by targeting REST for proteasomal degradation. Trends Neurosci. 2009, 32, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Tivnan, A.; Zhao, J.; Johns, T.G.; Day, B.W.; Stringer, B.W.; Boyd, A.W.; Tiwari, S.; Giles, K.M.; Teo, C.; McDonald, K.L. The tumor suppressor microRNA, miR-124a, is regulated by epigenetic silencing and by the transcriptional factor, REST in glioblastoma. Tumour Biol. 2014, 35, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, K.R.; Sloan, C.A.; Malladi, V.S.; Dreszer, T.R.; Learned, K.; Kirkup, V.M.; Wong, M.C.; Maddren, M.; Fang, R.; Heitner, S.G.; et al. ENCODE data in the UCSC genome browser: Year 5 update. Nucleic Acids Res. 2013, 41, D56–D63. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. String v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Reimand, J.; Lan, X.; Head, R.; Zhu, X.; Kushida, M.; Bayani, J.; Pressey, J.C.; Lionel, A.C.; Clarke, I.D.; et al. Single cell-derived clonal analysis of human glioblastoma links functional and genomic heterogeneity. Proc. Natl. Acad. Sci. USA 2015, 112, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar]
- Jackson, A.L.; Linsley, P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010, 9, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Das, C.M.; Taylor, P.; Gireud, M.; Singh, A.; Lee, D.; Fuller, G.; Ji, L.; Fangusaro, J.; Rajaram, V.; Goldman, S.; et al. The deubiquitylase USP37 links REST to the control of p27 stability and cell proliferation. Oncogene 2013, 32, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, N.; Lerdrup, M.; Landt, E.; Agrawal-Singh, S.; Bak, M.; Tommerup, N.; Rappsilber, J.; Sodersten, E.; Hansen, K. REST-mediated recruitment of Polycomb repressor complexes in mammalian cells. PLoS Genet. 2012, 8, e1002494. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, U.; Jori, F.P.; Giordano, A. Cell cycle regulation and neural differentiation. Oncogene 2003, 22, 5208–5219. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; McGowan, C.H.; Zhao, M.; He, L.; Downey, J.S.; Fearns, C.; Wang, Y.; Huang, S.; Han, J. Involvement of the MKK6-p38γ cascade in γ-radiation-induced cell cycle arrest. Mol. Cell. Biol. 2000, 20, 4543–4552. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Tian, B.; Gearing, M.; Hunter, S.; Ye, K.; Mao, Z. Cdk5-mediated regulation of the PIKE-A-Akt pathway and glioblastoma cell invasion. Proc. Natl. Acad. Sci. USA 2008, 105, 7570–7575. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.; Lombari, V.; de Gregorio, G.; Porcellini, A.; Ucci, S.; Arcella, A.; Caruso, R.; Gagliardi, F.M.; Gulino, A.; Lanzetta, G.; et al. Inhibition of cell growth by EGR-1 in human primary cultures from malignant glioma. Cancer Cell Int. 2004, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, M.; Lee, S.K.; Lee, B.; Ruiz, E.C.; Pfaff, S.L.; Gill, G.N. Small CTD phosphatases function in silencing neuronal gene expression. Science 2005, 307, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Roopra, A.; Qazi, R.; Schoenike, B.; Daley, T.J.; Morrison, J.F. Localized domains of G9a-mediated histone methylation are required for silencing of neuronal genes. Mol. Cell 2004, 14, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Matson, C.; Lan, F.; Iwase, S.; Baba, T.; Shi, Y. Regulation of LSD1 histone demethylase activity by its associated factors. Mol. Cell 2005, 19, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A. Dynamic epigenetic regulation in neurons: Enzymes, stimuli and signaling pathways. Nat. Neurosci. 2010, 13, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, J.; Wang, Z.; Zhang, T.; Shi, P.; Wang, X.; Zhao, F.; Liu, X.; Lin, X.; Pang, X. miR-136 modulates TGF-β1-induced proliferation arrest by targeting PPP2R2A in keratinocytes. BioMed Res. Int. 2015, 2015, 453518. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, M.; Moreno, J.; Juves, S.; Moreno, D.; Olive, M.; Ferrer, I. Target genes of neuron-restrictive silencer factor are abnormally up-regulated in human myotilinopathy. Am. J. Pathol. 2007, 171, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Aron, L.; Zullo, J.; Pan, Y.; Kim, H.; Chen, Y.; Yang, T.H.; Kim, H.M.; Drake, D.; Liu, X.S.; et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature 2014, 507, 448–454. [Google Scholar] [CrossRef] [PubMed]













| Gene | Primer Sequence | Product Length (bp) |
|---|---|---|
| P39 | forward: 5′-CCTTCATTACGCCTGCAAA-3′ | 144 |
| reverse: 5′-TCTCGTTGCCCATGTAGGA-3′ | ||
| CDK5R1 | forward: 5′-CAAACCAGGAGCATTTTGTGT-3′ | 91 |
| reverse: 5′-ATTCCTGTGGCTTGTTCTGTG-3′ | ||
| BID | forward: 5′-AGTGGGAGGGCTACGATGAG-3′ | 155 |
| reverse: 5′-GATGCTACGGTCCATGCTGT-3′ | ||
| BBC3 | forward: 5′-CCCGTGAAGAGCAAATGAG-3′ | 153 |
| reverse: 5′-ACCCCCTGATGAAGGTGAG-3′ | ||
| AATF | forward: 5′-GCCAGGATCGTCTGATGAGG-3′ | 173 |
| reverse: 5′-CCGGTGTTTTTGCAGAGTGG-3′ | ||
| EGR1 | forward: 5′-GTTACCCCAGCCAAACCAC-3′ | 143 |
| reverse: 5′-TGGGTTGGTCATGCTCACT-3′ | ||
| SLC25A4 | forward: 5′-GGGCTCTACCAGGGTTTCA-3′ | 150 |
| reverse: 5′-CGTCACACTCTGGGCAATC-3′ | ||
| PDCD7 | forward: 5′-GCAGGAGGTGGAGGAGAAG-3′ | 178 |
| reverse: 5′-TGGAGGACAGACCCCTTTC-3′ | ||
| MAPK11 | forward: 5′-TACCGGCAGGAGCTGAAC-3′ | 137 |
| reverse: 5′-TTCTTCACCGCCACCTTC-3′ | ||
| MAPK12 | forward: 5′-CCACCTTCACCTTCCACCT-3′ | 91 |
| reverse: 5′-GCGTCTGCTCTGATGGATG-3′ | ||
| FADD | forward: 5′-CTGGGGAAGAAGACCTGTG-3′ | 150 |
| reverse: 5′-GCACACGCTCTGTCAGGTT-3′ | ||
| BCL2 | forward: 5′-GGAGGATTGTGGCCTTCTTT-3′ | 176 |
| reverse: 5′-GCCGTACAGTTCCACAAAGG-3′ | ||
| CASP2 | forward: 5′-TTGCCGAAGATGAGACTGC-3′ | 179 |
| reverse: 5′-GCGTTCACCTTAACCAGCA-3′ | ||
| DAXX | forward: 5′-AAGCCTCCTTGGATTCTGGT-3′ | 203 |
| reverse: 5′-ATCATCCTCCTGACCCTCCT-3′ | ||
| FAS | forward: 5′-AGTTGGGGAAGCTCTTTCACTT-3′ | 163 |
| reverse: 5′-CAGTCTTCCTCAATTCCAATCC-3′ | ||
| REST | forward: 5′-CGCCCATATAAATGTGAACTTTGTC-3′ | 145 |
| reverse: 5′-GGCGGGTTACTTCATGTTGATTAG-3′ | ||
| GAPDH | forward: 5′-GCACCGTCAAGGCTGAGAAC-3′ | 138 |
| reverse: 5′-TGGTGAAGACGCCAGTGGA-3′ | ||
| BBC3 (ChIP) | forward: 5′-TTTCCGTCTGGGTGTGTGT-3′ | 153 |
| reverse: 5′-TCCAGGGTCCACAAAGTCA-3′ | ||
| DAXX (ChIP) | forward: 5′-CGCGTTGTGCTCATTTGT-3′ | 146 |
| reverse: 5′-TCCCATTTCCACGGCTTA-3′ | ||
| SYN-1 (ChIP) | forward: 5′-GGTGCTGAAGCTGGCAGT-3′ | 169 |
| reverse: 5′-TGGGTTTTAGGACCAGGATG-3′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Li, Y.; Wang, R.; Li, Y.; Shi, P.; Kan, Z.; Pang, X. Inhibition of REST Suppresses Proliferation and Migration in Glioblastoma Cells. Int. J. Mol. Sci. 2016, 17, 664. https://doi.org/10.3390/ijms17050664
Zhang D, Li Y, Wang R, Li Y, Shi P, Kan Z, Pang X. Inhibition of REST Suppresses Proliferation and Migration in Glioblastoma Cells. International Journal of Molecular Sciences. 2016; 17(5):664. https://doi.org/10.3390/ijms17050664
Chicago/Turabian StyleZhang, Dianbao, Ying Li, Rui Wang, Yunna Li, Ping Shi, Zhoumi Kan, and Xining Pang. 2016. "Inhibition of REST Suppresses Proliferation and Migration in Glioblastoma Cells" International Journal of Molecular Sciences 17, no. 5: 664. https://doi.org/10.3390/ijms17050664