Metal Complexes of New Bioactive Pyrazolone Phenylhydrazones; Crystal Structure of 4-Acetyl-3-methyl-1-phenyl-2-pyrazoline-5-one phenylhydrazone Ampp-Ph
Abstract
:1. Introduction
2. Results
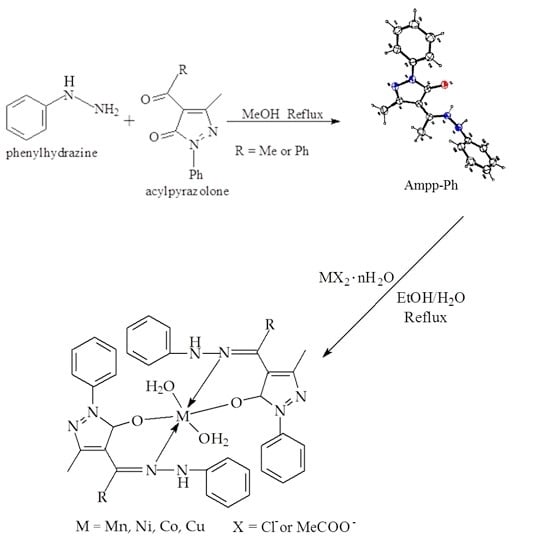
2.1. Synthesis
2.2. 1H and 13C NMR Spectroscopy
2.3. Mass Spectroscopy
2.4. X-ray Crystallography
2.5. Infrared Spectroscopy
2.6. UV-VIS Spectroscopy and Magnetic Moments
2.7. Thermogravimetric Studies
2.8. Biological Studies
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Synthesis of Phenylhydrazones
3.2.1. 4-Acetyl-3-methyl-1-phenyl-2-pyrazolin-5-one dinitrophenylhydrazone Ampp-Dh
3.2.2. 4-Benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one dinitrophenylhydrazone Bmpp-Dp
3.2.3. 4-Benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one phenylhydrazone Bmpp-Ph
3.3. Synthesis of Phenylhydrazones Metal Complexes
3.3.1. Bis(4-acetyl-3-methyl-1-phenyl-2-pyrazolin-5-one dinitrophenylhydrazone) Diaquamanganesse (Ii) Monohydrate Mn(Ampp-Dh)2(H2O)2∙H2O
3.3.2. Bis(4-acetyl-3-methyl-1-phenyl-2-pyrazolin-5-one dinitrophenylhydrazone) Diaquacobalt(Ii) Co(Ampp-Dh)2(H2O)2
3.3.3. Bis(4-acetyl-3-methyl-1-phenyl-2-pyrazolin-5-one dinitrophenylhydrazone) Diaquanickel(Ii) Ni(Ampp-Dh)2(H2O)2
3.3.4. Bis(4-acetyl-3-methyl-1-phenyl-2-pyrazolin-5-one dinitrophenylhydrazone) Diaquacopper(Ii) Cu(Ampp-Dh)2(H2O)2
3.3.5. Bis(4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one dinitrophenylhydrazone) Diaquamanganese (II) Mn(Bmpp-Dh)2(H2O)2
3.3.6. Bis(4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one dinitrophenylhydrazone) Diaquacobalt(Ii) Monohydrate Co(Bmpp-Dh)2(H2O)2∙H2O
3.3.7. Bis(4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one dinitrophenylhydrazone) Diaquanickel(Ii) Monohydrate Ni(Bmpp-Dh)2(H2O)2∙H2O
3.3.8. Bis(4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one dinitrophenylhydrazone) Diaquacopper(Ii) Dihydrate Cu(Bmpp-Dh)2(H2O)2∙2H2O
3.3.9. Bis(4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one phenylhydrazone) Diaquamanganese (Ii) Dihydrate Mn(Bmpp-Ph)2(H2O)2∙2H2O
3.3.10. Bis(4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one phenylhydrazone) Diaquacobalt(Ii) Monohydrate Co(Bmpp-Ph)2(H2O)2∙H2O
3.3.11. Bis(4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one phenylhydrazone) Diaquanickel(Ii) Dihydrate Ni(Bmpp-Ph)2(H2O)2∙2H2O
3.3.12. Bis(4-benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one phenylhydrazone) Diaquacopper(Ii) Dihydrate Cu(Bmpp-Ph)2(H2O)2∙H2O
3.3.13. Bis(4-acetyl-3-methyl-1-phenyl-2-pyrazolin-5-one phenylhydrazone) Diaquacobalt(Ii) Dihydrate Co(Ampp-Ph)2(H2O)2∙2H2O
3.3.14. Bis(4-acetyl-3-methyl-1-phenyl-2-pyrazolin-5-one phenylhydrazone) Diaquacopper(Ii) Cu(Ampp-Ph)2(H2O)2
3.4. X-ray Diffraction Study of Ampp-Ph
3.5. Antibacterial Studies
3.6. Antioxidant (Free Radical Scavenging) Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DMF | Dimethylformamide |
| DTG | Thermogravimetric derivative |
| DPPH | 1,1-diphenyl-2-picryl-hydrazil |
| DMSO | Dimethylsulfoxide |
| TLC | Thin Layer Chromatography |
References
- Cozzi, P.G. Metal–Salen Schiff base complexes in catalysis: Practical aspects. Chem. Soc. Rev. 2004, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P.; Bhatnagar, J.M. Copper(II) selective electrochemical sensor based on Schiff base complexes. Talanta 2004, 64, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.; Lee, H.K.; Jeong, D.C.; Jeon, S. A lead (II)-selective PVC membrane based on a Schiff base complex of N,N-bis(salicylidene)-2,6-pyridinediamine. Talanta 2005, 65, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Avaji, P.G.; Kumar, C.H.V.; Patil, S.A.; Shivananda, K.N.; Nagaraju, C. Synthesis, spectral characterization, in vitro microbiological evaluation and cytotoxic activities of novel macrocyclic bis hydrazone. Eur. J. Med. Chem. 2009, 44, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Puthilibai, G.; Vasudevan, S.; Rani, S.K.; Rajagopal, G. Synthesis, spectroscopic characterization, electrochemical behaviour and antibacterial activity of Ru(III) complexes of 2-[(4-N,N-dimethylaminophenylimino)-methyl]-4-halophenol. Spectrochim. Acta Part A: Mol. Biomol. Spect. 2009, 72, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Avaji, P.G.; Bagihalli, G.B.; Patil, S.A.; Badami, P.S. Synthesis, spectral, electrochemical and biological studies of Co(II), Ni(II) and Cu(II) complexes with Schiff bases of 8-formyl-7-hydroxy-4-methyl coumarin. J. Coord. Chem. 2009, 62, 481–492. [Google Scholar] [CrossRef]
- Shiri-Yekta, Z.; Yaftian, M.R. Anion control selectivity of neutral N4-type schiff base extractants towards transition metal ions. Iran. J. Chem. Chem. Eng. 2010, 29, 11–17. [Google Scholar]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Kumar, R.; Mani, G. Exhibition of the Brønsted acid–base character of a Schiff base in palladium(II) complex formation: Lithium complexation, fluxional properties and catalysis of Suzuki reactions in water. Dalton Trans. 2015. [Google Scholar] [CrossRef] [PubMed]
- Nejati, K.; Rezvani, Z.; Massoumi, B. Syntheses and investigation of thermal properties of copper complexes with azo-containing Schiff-base dyes. Dyes Pigments 2007, 75, 653–657. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Isabirye, D.A.; Eddy, N.O. Adsorption and quantum chemical studies on the inhibition potentials of some thiosemicarbazides for the corrosion of mild steel in acidic medium. Int. J. Mol. Sci. 2010, 11, 2473–2498. [Google Scholar] [CrossRef] [PubMed]
- Gomez, B.; Likhanova, N.V.; Aguilar, M.A.D.; Palou, R.M.; Vela, A.; Gasquez, J.L. Quantum chemical study of the inhibitive properties of 2-pyridyl-azoles. J. Phys. Chem. 2006, B110, 8928–8934. [Google Scholar] [CrossRef] [PubMed]
- Finsgar, M.; Lesar, A.; Kokaij, A.; Milosev, I. A comparative electrochemical and quantum chemical calculation study of BTAH and BTAOH as copper corrosion inhibitors in near neutral chloride solution. Electrochim. Acta 2008, 53, 8287–8297. [Google Scholar] [CrossRef]
- Cinarli, A.; Gürbüz, D.; Tavman, A.; Birteksöz, A.S. Synthesis, spectra characterization and antimicrobial activity of some Schiff bases of 4-chloro-2-aminophenol. Bull. Chem. Soc. Ethiop. 2011, 25, 407–417. [Google Scholar] [CrossRef]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff bases: A versatile pharmacophore. J. Catal. 2013. [Google Scholar] [CrossRef]
- Vashi, K.; Naik, H.B. Synthesis of novel Schiff base and azetidinone derivatives and their antibacterial activity. Eur. J. Chem. 2004, 1, 272–276. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Pettinari, R. Acylpyrazolone ligands: Synthesis, structures, metal coordination chemistry and applications. Coord. Chem. Rev. 2005, 249, 2909–2945. [Google Scholar] [CrossRef]
- Mariappan, G.; Saha, B.P.; Sutharson, L.; Ankits, G.; Pandey, L.; Kumar, D. The diverse pharmacological importance of pyrazolone derivatives: A Review. J. Pharm. Res. 2010, 3, 2856–2859. [Google Scholar]
- Abdel-Aziz, H.A.; Mekawey, A.A.; Dawood, K.M. Convenient synthesis and antimicrobial evaluation of some novel 2-substituted-3-methylbenzofuran derivatives. Eur. J. Med. Chem. 2009, 44, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, P.; Huczynski, A.; Pyta, K.; Brzezinski, B.; Bartl, F. Biological properties of schiff bases and azo derivatives of phenols. Curr. Org. Chem. 2009, 13, 124–148. [Google Scholar] [CrossRef]
- Idemudia, O.G.; Sadimenko, A.P.; Afolayan, A.J.; Hosten, E.C. 3-Methyl-1-phenyl-4-[(phenyl)(2-phenylhydrazin-1-yl)methylidene]-1H-pyrazol-5(4H)-one. Acta Cryst. 2012, E68, o1280–o1281. [Google Scholar] [CrossRef] [PubMed]
- Idemudia, O.G.; Sadimenko, A.P.; Hosten, E.C. Crystal structure of 4-propyl-3-methyl-1-phenyl-2-pyrazolin-5-one thiosemicarbazone C15H19N5OS. Z. Kristallogr. NCS 2015, 230, 81–82. [Google Scholar] [CrossRef]
- Brady, O.L.; Elsmie, G.V. The use of 2,4-dinitrophenylhydrazine as a reagent for aldehydes and ketones. Analyst 1926, 51, 77–78. [Google Scholar] [CrossRef]
- Idemudia, O.G.; Sadimenko, A.P.; Hosten, E.C. 4-{[2-(2,4-Dinitrophenyl) hydrazinylidene] (phenyl)methyl}-5-methyl-2-phenyl-1H-pyrazol-3(2H)-one ethanol monosolvate. Acta Cryst. 2012, E68. [Google Scholar] [CrossRef] [PubMed]
- Idemudia, O.G.; Sadimenko, A.P.; Afolayan, A.J. Potential therapeutic Mn2+ and Ni2+ complexes of new 4-acetyl-3-methyl-1-phenyl-2-pyrazoline-5-one phenylhydrazone Ampp-Ph. Macromol. Symp. 2015, 351, 61–68. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Parihar, S.; Vyas, K.; Gupta, V.K. Synthesis and crystal structure of a series of pyrazolone based Schiff base ligands and DNA binding studies of their copper complexes. J. Mol. Struct. 2012, 1013, 86–94. [Google Scholar] [CrossRef]
- Idemudia, O.G.; Sadimenko, A.P.; Afolayan, A.J.; Hosten, E.C. Synthesis and characterization of bioactive acylpyrazolone sulfanilamides and their transition metal complexes: Single crystal structure of 4-Benzoyl-3-methyl-1-phenyl-2-pyrazolin-5-one Sulfanilamide. Bioinorg. Chem. Appl. 2015. [Google Scholar] [CrossRef] [PubMed]
- Dolaz, M.; McKee, V.; Golcu, A.; Tumer, M. Synthesis, structural characterization, spectroscopic and electrochemical studies of N,N-bis[(2,4-dimethoxyphenyl) methylidene]butane-1,4-diamine. Curr. Org. Chem. 2010, 14, 281–288. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. 1990, B46, 256–262. [Google Scholar] [CrossRef]
- Maurya, R.C.; Pandey, A.; Chaurasia, J.; Martin, H. Metal nitrosyl complexes of bioinorganic, catalytic, and environmental relevance: A novel single-step synthesis of dinitrosylmolybdenum(0) complexes of {Mo(NO)2}6 electron configuration involving Schiff bases derived from 4-acyl-3-methyl-1-phenyl-2-pyrazolin-5-one and 4-aminoantipyrine, directly from molybdate(VI) and their characterization. J. Mol. Struct. 2006, 798, 89–101. [Google Scholar]
- Nakamato, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; Wiley-Interscience: New York, NY, USA, 1979. [Google Scholar]
- Yang, Z.-Y.; Yang, R.-D.; Li, F.-S.; Yu, K.-B. Crystal structure and antitumor activity of some rare earth metal complexes with Schiff base. Polyhedron 2000, 19, 2599–2604. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Bowan, W.U.; Zhang, K.; Shenli, H.U. The direct electrochemical synthesis of Ti(II), Fe(II), Cd(II), Sn(II), and Pb(II) complexes with N,N-bis(Salicylidene)-ophenylenediamine. Turkish J. Chem. 2007, 31, 623–629. [Google Scholar]
- Naeimi, H.; Moradian, M. Synthesis and characterization of nitro-Schiff bases derived from 5-nitro-salicylaldehyde and various diamines and their complexes of Co(II). J. Coord. Chem. 2010, 63, 156–162. [Google Scholar] [CrossRef]
- West, D.X.; Lockwood, M.A.; Liberta, A.E.; Chen, X.; Willett, R.D. Spectral nature, antifungal activity and molecular structure of metal complexes of acetylpyrazine N4-substituted thiosemicarbazones. Transit. Met. Chem. 1993, 18, 221–227. [Google Scholar] [CrossRef]
- Inada, Y.; Sugimoto, K.I.; Ozutsumi, K.; Funahashi, S. Solvation structures of manganese(II), iron(II), cobalt(II), nickel(II), copper(II), zinc(II), cadmium(II), and indium(III) ions in 1,1,3,3-tetramethylurea as studied by EXAFS and electronic spectroscopy. Variation of coordination number. Inorg. Chem. 1994, 33, 1875–1880. [Google Scholar] [CrossRef]
- Bayri, A.; Karakaplan, M. Theoretical approach to the magnetic properties of Mn(II), Cr(III), and Cu(II) complexes in the newly reported 12- and 15-membered macrocyclic ligands. Pramana J. Phys. 2007, 69, 301–306. [Google Scholar] [CrossRef]
- Ferenc, W.; Czapla, K.; Sarzy, J. Magnetic, thermal and spectral characterization of 2,4-dimethoxybenzoates of Mn(II), Co(II) and Cu(II). Ecletica Quımica 2007, 32, 7–12. [Google Scholar] [CrossRef]
- Dholakiya, P.P.; Patel, M.N. Preparation, magnetic, spectral, and biocidal studies of some transition metal complexes with 3,5-dibromosalicylideneaniline and neutral bidentate ligands. Synth. React. Inorg. Met.-Org. Chem. 2002, 32, 819–829. [Google Scholar] [CrossRef]
- Sallam, S.A. Synthesis, characterization and thermal decomposition of copper(II), nickel(II) and cobalt(II) complexes of 3-amino-5-methylpyrazole Schiff bases. Trans. Met. Chem. 2005, 30, 341–351. [Google Scholar] [CrossRef]
- Soares, R.O.A.; Echevarria, A.; Bellieny, M.S.S.; Pinho, R.T.; de Leo, R.M.M.; Seguins, W.S.; Machado, G.M.; Canto-Cavalheiro, M.M.; Leon, L.L. Evaluation of thiosemicarbazones and semicarbazones as potential agents anti-Trypanosoma cruzi. Exp. Parasit. 2011, 129, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Pawar, V.; Uma, V. Antibacterial and antioxidant properties of Mn(II), Co(II), Ni(II) and Zn(II) complex of Schiff base derived from cephalexin. Res. J. Pharm. Biol. Chem. Sci. 2001, 2, 61–70. [Google Scholar]
- Parihar, S.; Pathan, S.; Jadeja, R.N.; Patel, A.; Gupta, V.K. Synthesis and crystal structure of an oxovanadium(IV) complex with a pyrazolone ligand and its use as a heterogeneous catalyst for the oxidation of styrene under mild conditions. Inorg. Chem. 2012, 51, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- APEX2; SADABS; SAINT; Bruker AXS Inc.: Madison, WI, USA, 2010.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Hubschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin Pathol. 1996, 45, 493–496. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
















| Compound | Ampp-Ph |
|---|---|
| Formula | C18H18N4O |
| Crystal colour and form | Golden yellow/Block |
| Formula weight | 306.36 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a | 8.7006(5) (Å) |
| b | 9.6088(5) (Å) |
| c | 9.9124(5) (Å) |
| α | 104.740(2)° |
| β | 96.360(2)° |
| γ | 103.196(2)° |
| V | 767.62(7) (Å3) |
| Z | 2 |
| D(calc) | 1.326 (Mg cm−1) |
| F(000) | 324 |
| θ range | 2.2–28.3 (°) |
| Crystal size | 0.35 × 0.47 × 0.53 (mm) |
| Total Reflection measured | 20,159 |
| R | 0.0378 |
| wR2 | 0.1078 |
| S | 1.02 |
| Independent/observed | 3801/3374 |
| Mu(MoKa) | 0.71073 (/mm) |
| Temperature | 200 (K) |
| Parameters | 218 |
| Ligand and Complexes | Staphylococus aureus | Bacillus pumillus | Proteus vulgaris | Aeromonas hydrophillia |
|---|---|---|---|---|
| Bmpp-Dh | 4.0 | 8.3 | NI | NI |
| Ampp-Dh | 24.0 | 9.5 | NI | 4.5 |
| Bmpp-Ph | 12.0 | 10.0 | 8.0 | NI |
| Mn(Bmpp-Ph)2(H2O)2∙2H2O | 10.0 | NI | 8.0 | NI |
| Co(Bmpp-Ph)2(H2O)2∙H2O | 8.0 | 12.0 | NI | NI |
| Ni(Bmpp-Ph)2(H2O)2∙2H2O | NI | 8.0 | 4.0 | 10.0 |
| Cu(Bmpp-Ph)2(H2O)2∙H2O | 12.0 | 6.0 | 8.0 | NI |
| Mn(Bmpp-Dh)2(H2O)2 | 8.0 | 7.0 | NI | NI |
| Co(Bmpp-Dh)2(H2O)2∙H2O | 8.3 | 7.0 | NI | NI |
| Ni(Bmpp-Dh)2(H2O)2∙H2O | 10.5 | 6.0 | 12.0 | 12.0 |
| Cu(Bmpp-Dh)2(H2O)2∙2H2O | NI | 8.5 | NI | 12.0 |
| Co(Ampp-Ph)2(H2O)2∙2H2O | 8.0 | 12.0 | NI | 6.0 |
| Cu(Ampp-Ph)2(H2O)2 | 4.0 | 4.0 | NI | NI |
| Mn(Ampp-Dh)2(H2O)2∙H2O | 8.0 | 8.5 | 9.5 | 5.0 |
| Co(Ampp-Dh)2(H2O)2 | 15.5 | 4.0 | NI | 20.0 |
| Ni(Ampp-Dh)2(H2O)2 | 8.0 | 12.5 | NI | 8.3 |
| Cu(Ampp-Dh)2(H2O)2 | 13.0 | 12.3 | NI | 20 |
| Chloramphenicol | 30.0 | 20.0 | 42.0 | 40.0 |
| DMSO | NI | NI | NI | NI |
| Ligand and Complexes | Percentage Antioxidant Activity | ||
|---|---|---|---|
| 0.50 mg/mL | 0.25 mg/mL | 0.13 mg/mL | |
| Bmpp-Dh 1 | ‒ | 31.05 | 30.70 |
| Ampp-Dh 2 | ‒ | 63.27 | 76.33 |
| Bmpp-Ph 3 | 62.48 | 89.11 | 87.79 |
| Mn(Bmpp-Ph)2(H2O)2∙2H2O 3a | 52.42 | 42.76 | 64.61 |
| Co(Bmpp-Ph)2(H2O)2∙H2O 3b | 17.22 | 3.13 | 32.32 |
| Ni(Bmpp-Ph)2(H2O)2∙2H2O 3c | 37.33 | 4.17 | 40.94 |
| Cu(Bmpp-Ph)2(H2O)2∙H2O 3d | ‒ | 12.98 | 1.73 |
| Mn(Bmpp-Dh)2(H2O)2 1a | ‒ | 68.83 | 52.28 |
| Co(Bmpp-Dh)2(H2O)2∙H2O 1b | 8.58 | 59.56 | 47.11 |
| Ni(Bmpp-Dh)2(H2O)2∙H2O 1c | 87.92 | 62.80 | 61.28 |
| Cu(Bmpp-Dh)2(H2O)2∙2H2O 1d | 2.28 | 89.90 | 53.76 |
| Co(Ampp-Ph)2(H2O)2∙2H2O 4a | 60.07 | 87.25 | 80.64 |
| Cu(Ampp-Ph)2(H2O)2 4b | 38.35 | 57.36 | 44.76 |
| Mn(Ampp-Dh)2(H2O)2∙H2O 2a | 68.48 | 85.86 | 87.42 |
| Co(Ampp-Dh)2(H2O)2 2b | ‒ | 55.50 | 55.98 |
| Ni(Ampp-Dh)2(H2O)2 2c | 65.32 | 84.01 | 84.83 |
| Cu(Ampp-Dh)2(H2O)2 2d | 35.73 | 85.40 | 82.98 |
| Ascorbic acid std | 87.62 | 93.63 | 91.99 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idemudia, O.G.; Sadimenko, A.P.; Hosten, E.C. Metal Complexes of New Bioactive Pyrazolone Phenylhydrazones; Crystal Structure of 4-Acetyl-3-methyl-1-phenyl-2-pyrazoline-5-one phenylhydrazone Ampp-Ph. Int. J. Mol. Sci. 2016, 17, 687. https://doi.org/10.3390/ijms17050687
Idemudia OG, Sadimenko AP, Hosten EC. Metal Complexes of New Bioactive Pyrazolone Phenylhydrazones; Crystal Structure of 4-Acetyl-3-methyl-1-phenyl-2-pyrazoline-5-one phenylhydrazone Ampp-Ph. International Journal of Molecular Sciences. 2016; 17(5):687. https://doi.org/10.3390/ijms17050687
Chicago/Turabian StyleIdemudia, Omoruyi G., Alexander P. Sadimenko, and Eric C. Hosten. 2016. "Metal Complexes of New Bioactive Pyrazolone Phenylhydrazones; Crystal Structure of 4-Acetyl-3-methyl-1-phenyl-2-pyrazoline-5-one phenylhydrazone Ampp-Ph" International Journal of Molecular Sciences 17, no. 5: 687. https://doi.org/10.3390/ijms17050687