Relevance of Endoplasmic Reticulum Stress Cell Signaling in Liver Cold Ischemia Reperfusion Injury
Abstract
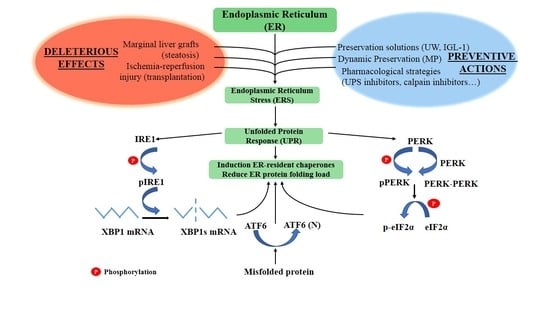
:1. Introduction
2. Key Players in the Endoplasmic Reticulum Stress (ERS) Response
2.1. Inositol-Requiring Enzyme 1 (IRE1)
2.2. PKR-Like ER Kinase (PERK)
2.3. Activating Transcription Factor 6 (ATF6)
3. Unfolded Protein Response (UPR)/ERS in Liver Graft Preservation: The Importance of Preventing ATP Breakdown
3.1. UPR/ERS in Liver Graft Cold Storage
3.2. UPR/ERS in Liver Graft Machine Perfusion
4. UPR/ERS in Liver Transplantation
5. Proteasome Inhibitors as Therapeutic Targets for Liver Preservation and Transplantation
6. Other Therapeutic Targets for Liver Preservation and Transplantation
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ER | endoplasmic reticulum |
| ERS | endoplasmic reticulum stress |
| UPR | unfolded protein response |
| IRI | ischemia-reperfusion injury |
| UPS | ubiquitin proteasome system |
| ATF6 | activated transcription factor 6 |
| IRE1 | Requiring enzyme inositol 1 |
| PERK | RNA protein kinase (PKR) -like ER kinase |
| GRP78 | 78 kDa glucose-regulated protein |
| BiP | immunoglobulin-binding protein |
| AMPK | adenosine mono phosphate protein kinase |
| HIF1 alpha | hypoxia inducible factor 1 alpha |
| XBP-1 | X box binding protein 1 |
| ERAD | Endoplasmic reticulum associated protein degradation |
| eIF2a | elongation initiation factor 2a |
| ATF4 | activating transcription factor 4 |
| UW | University of Wisconsin solution |
| IGL-1 | Institut Georges Lopez-1 |
| PEG-35 | Polyethylenglycol-35 |
| HTK | histidine-tryptophan-ketoglutarate |
| BZ | Bortezomib |
| CHOP | C/EBP homologous protein |
| Belzer-MPS | Belzer gluconate solution |
| MP | Machine perfusion |
| LDLT | Living donor liver transplantation |
| ROLT | reduced-size orthotopic liver transplantation |
| SFSS | Small-for-size liver syndrome |
References
- Diaz-Villanueva, J.F.; Diaz-Molina, R.; Garcia-Gonzalez, V. Protein folding and mechanisms of proteostasis. Int. J. Mol. Sci. 2015, 16, 17193–17230. [Google Scholar] [CrossRef] [PubMed]
- Oslowski, C.M.; Urano, F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011, 490, 71–92. [Google Scholar] [PubMed]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Van Riel, W.G.; van Golen, R.F.; Reiniers, M.J.; Heger, M.; van Gulik, T.M. How much ischemia can the liver tolerate during resection? Hepatobiliary Surg. Nutr. 2016, 5, 58–71. [Google Scholar] [PubMed]
- Zhou, H.; Zhu, J.; Yue, S.; Lu, L.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Wang, X.; Zhai, Y. The dichotomy of endoplasmic reticulum stress response in liver ischemia-reperfusion injury. Transplantation 2016, 100, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Montalvo-Jave, E.E.; Escalante-Tattersfield, T.; Ortega-Salgado, J.A.; Pina, E.; Geller, D.A. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J. Surg. Res. 2008, 147, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.J.; Chehab, M.; Kink, S.; Toledo-Pereyra, L.H. Intracellular calcium signaling pathways during liver ischemia and reperfusion. J. Investig. Surg. 2010, 23, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Braakman, I.; Hebert, D.N. Protein folding in the endoplasmic reticulum. Cold Spring Harbor Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Kaufman, R.J. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007, 18, 716–731. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.; Walter, P. Intracellular signaling from the endoplasmic reticulum to the nucleus: The unfolded protein response in yeast and mammals. Curr. Opin. Cell biol. 2001, 13, 349–355. [Google Scholar] [CrossRef]
- Li, H.; Korennykh, A.V.; Behrman, S.L.; Walter, P. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc. Natl. Acad. Sci. USA 2010, 107, 16113–16118. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.S.; Domingos, P.M. Physiological roles of regulated Ire1 dependent decay. Front. Genet. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Iwakoshi, N.N.; Lee, A.H.; Vallabhajosyula, P.; Otipoby, K.L.; Rajewsky, K.; Glimcher, L.H. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 2003, 4, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Braakman, I.; Bulleid, N.J. Protein folding and modification in the mammalian endoplasmic reticulum. Ann. Rev. Biochem. 2011, 80, 71–99. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Zhang, Y.; Bertolotti, A.; Zeng, H.; Ron, D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 2000, 5, 897–904. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, M.S. Autophagy—A key player in cellular and body metabolism. Nat. Rev. Endocrinol. 2014, 10, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Dave, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ preservation: Current concepts and new strategies for the next decade. Transfus. Med. Hemother. 2011, 38, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Gwak, M.S.; Choi, S.J.; Ko, J.S.; Kim, G.S.; Son, H.J.; Shin, J.C. Risk factors for inadvertent hypothermia during adult living-donor liver transplantation. Transpl. Proc. 2014, 46, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Belzer, F.O.; D’Alessandro, A.M.; Hoffmann, R.M.; Knechtle, S.J.; Reed, A.; Pirsch, J.D.; Kalayoglu, M.; Sollinger, H.W. The use of UW solution in clinical transplantation. A 4-year experience. Ann. Surg. 1992, 215, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Mutter, T.C.; Ruth, C.A.; Dart, A.B. Hydroxyethyl starch (HES) versus other fluid therapies: Effects on kidney function. Cochrane Database Syst. Rev. 2013, 7, CD007594. [Google Scholar] [PubMed]
- Ben Mosbah, I.; Rosello-Catafau, J.; Franco-Gou, R.; Abdennebi, H.B.; Saidane, D.; Ramella-Virieux, S.; Boillot, O.; Peralta, C. Preservation of steatotic livers in IGL-1 solution. Liver Transpl. 2006, 12, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gil, F.A.; Arenas, J.; Guemes, A.; Esteban, E.; Tome-Zelaya, E.; Lamata, F.; Sousa, R.; Jimenez, A.; Barrao, M.E.; Serrano, M.T. Preservation of the liver graft with Celsior solution. Transpl. Proc. 2006, 38, 2385–2388. [Google Scholar] [CrossRef] [PubMed]
- Van Gulik, T.M.; Reinders, M.E.; Nio, R.; Frederiks, W.M.; Bosma, A.; Klopper, P.J. Preservation of canine liver grafts using HTK solution. Transplantation 1994, 57, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Gondo, N.; Arita, M.; Fozzard, H.A.; Hadama, T.; Uchida, Y. University of Wisconsin solution preserves myocardial calcium current response to isoproterenol in isolated canine ventricular myocytes. Circulation 1995, 92 (Suppl. 9), II452–II457. [Google Scholar] [CrossRef] [PubMed]
- Tabka, D.; Bejaoui, M.; Javellaud, J.; Rosello-Catafau, J.; Achard, J.M.; Abdennebi, H.B. Effects of Institut Georges Lopez-1 and Celsior preservation solutions on liver graft injury. World J. Gastroenterol. 2015, 21, 4159–4168. [Google Scholar] [CrossRef] [PubMed]
- Demmy, T.L.; Molina, J.E.; Ward, H.B.; Gorton, M.E.; Kouchoukos, N.T.; Schmaltz, R.A.; Shennib, H. Custodiol versus Plegisol: A phase 3 multicentre myocardial protection study. Int. J. Angiol. 2008, 17, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Salahudeen, A.K.; Huang, H.; Patel, P.; Jenkins, J.K. Mechanism and prevention of cold storage-induced human renal tubular cell injury. Transplantation 2000, 70, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Romero, J.; Saini, V.; Baker, T.A.; Picken, M.M.; Gamelli, R.L.; Majetschak, M. A subset of 26S proteasomes is activated at critically low ATP concentrations and contributes to myocardial injury during cold ischemia. Biochem. Biophys. Res. Commun. 2009, 390, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.A.; Geng, Q.; Romero, J.; Picken, M.M.; Gamelli, R.L.; Majetschak, M. Prolongation of myocardial viability by proteasome inhibition during hypothermic organ preservation. Biochem. Biophys. Res. Commun. 2010, 401, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Grahame Hardie, D. Regulation of AMP-activated protein kinase by natural and synthetic activators. Acta Pharm. Sin. B 2016, 6, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaouali, M.A.; Boncompagni, E.; Reiter, R.J.; Bejaoui, M.; Freitas, I.; Pantazi, E.; Folch-Puy, E.; Abdennebi, H.B.; Garcia-Gil, F.A.; Rosello-Catafau, J. AMPK involvement in endoplasmic reticulum stress and autophagy modulation after fatty liver graft preservation: A role for melatonin and trimetazidine cocktail. J. Pineal Res. 2013, 55, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Delvart, V.; Karam, V.; Ducerf, C.; Navarro, F.; Letoublon, C.; Belghiti, J.; Pezet, D.; Castaing, D.; Le Treut, Y.P.; et al. Compared efficay of preservation solutions in liver transplantation: A long-term graft outcome study from European Liver Transplant Registry. Am. J. Transpl. 2015, 15, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, I.B.; Zaouali, M.A.; Martel, C.; Bjaoui, M.; Abdennebi, H.B.; Hotter, G.; Brenner, C.; Rosello-Catafau, J. IGL-1 solution reduces endoplasmic reticulum stress and apoptosis in rat liver transplantation. Cell Death Dis. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejaoui, M.; Zaouali, M.A.; Folch-Puy, E.; Pantazi, E.; Bardag-Gorce, F.; Carbonell, T.; Oliva, J.; Rimola, A.; Abdennebi, H.B.; Rosello-Catafau, J. Bortezomib enhances fatty liver preservation in Institut George Lopez-1 solution through adenosine monophosphate activated protein kinase and Akt/mTOR pathways. J. Pharm. Pharmacol. 2014, 66, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Calmus, Y.; Cynober, L.; Dousset, B.; Lim, S.K.; Soubrane, O.; Conti, F.; Houssin, D.; Giboudeau, J. Evidence for the detrimental role of proteolysis during liver preservation in humans. Gastroenterology 1995, 108, 1510–1516. [Google Scholar] [CrossRef]
- Majetschak, M.; Patel, M.B.; Sorell, L.T.; Liotta, C.; Li, S.; Pham, S.M. Cardiac proteasome dysfunction during cold ischemic storage and reperfusion in a murine heart transplantation model. Biochem. Biophys. Res. Commun. 2008, 365, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.J.; Zhang, J.; Li, J.H.; Chen, X.D.; Jiang, L.; Zhou, Y.F.; He, N.; Xie, H.Y.; Zhou, L.; Zheng, S.S. Influence of perfusate on liver viability during hypothermic machine perfusion. World J. Gastroenterol. 2015, 21, 8848–8857. [Google Scholar] [CrossRef] [PubMed]
- Manekeller, S.; Schuppius, A.; Stegemann, J.; Hirner, A.; Minor, T. Role of perfusion medium, oxygen and rheology for endoplasmic reticulum stress-induced cell death after hypothermic machine preservation of the liver. Trans. Int. 2008, 21, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Emadali, A.; Nguyen, D.T.; Rochon, C.; Tzimas, G.N.; Metrakos, P.P.; Chevet, E. Distinct endoplasmic reticulum stress responses are triggered during human liver transplantation. J. Pathol. 2005, 207, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.D.; Upadhya, G.; Conzen, K.D.; Jia, J.; Brunt, E.M.; Tiriveedhi, V.; Xie, Y.; Ramachandran, S.; Mohanakumar, T.; Davidson, N.O.; et al. Endoplasmic reticulum stress is a mediator of posttransplant injury in severely steatotic liver allografts. Liver Transpl. 2011, 17, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Eshkenazy, R.; Dreznik, Y.; Lahat, E.; Zakai, B.B.; Zendel, A.; Ariche, A. Small for size liver remnant following resection: Prevention and management. Hepatobiliary Surg. Nutr. 2014, 3, 303–312. [Google Scholar] [PubMed]
- Padrissa-Altes, S.; Zaouali, M.A.; Boncompagni, E.; Bonaccorsi-Riani, E.; Carbonell, T.; Bardag-Gorce, F.; Oliva, J.; French, S.W.; Bartrons, R.; Rosello-Catafau, J. The use of a reversible proteasome inhibitor in a model of Reduced-Size Orthotopic Liver transplantation in rats. Exp. Mol. Pathol. 2012, 93, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Liaw, J.M.; Jia, J.; Glasgow, S.C.; Liu, W.; Csontos, K.; Upadhya, G.A.; Mohanakumar, T.; Chapman, W.C. Ischemia-reperfusion injury in rat steatotic liver is dependent on NFκB P65 activation. Trans. Immunol. 2012, 26, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, A.; Petrov, L.; Georgieva, A.; Kessiova, M.; Tzvetanova, E.; Kirkova, M.; Kukan, M. Effect of MG132 on proteasome activity and prooxidant/antioxidant status of rat liver subjected to ischemia/reperfusion injury. Hepatol. Res. 2008, 38, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Shen, G.; Wang, G.; Zhang, F.; Li, Y.; Luo, F.; Yao, J.; Tian, X.F. MG132 alleviates liver injury induced by intestinal ischemia/reperfusion in rats: Involvement of the AhR and NFκB pathways. J. Surg. Res. 2012, 176, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.H.; Li, Y.H.; Wang, Z.Z.; Zhang, X.S.; Wang, Y.Z.; Yuan, J.C.; Zhou, Q.; Liu, K.X.; Tian, X.F. Proteasome inhibitor lactacystin ablates liver injury induced by intestinal ischaemia-reperfusion. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Fenteany, G.; Schreiber, S.L. Lactacystin, proteasome function, and cell fate. J. Biol. Chem. 1998, 273, 8545–8548. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Mohan, R.; Kwok, B.H.; Elofsson, M.; Sin, N.; Crews, C.M. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc. Natl. Acad. Sci. USA 1999, 96, 10403–10408. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hendershot, L.M. ER chaperone functions during normal and stress conditions. J. Chem. Neuroanat. 2004, 28, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Young, L.H.; Lefer, A.M. Attenuation of neutrophil-mediated myocardial ischemia-reperfusion injury by a calpain inhibitor. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1421–H1426. [Google Scholar] [CrossRef] [PubMed]
- Hernando, V.; Inserte, J.; Sartorio, C.L.; Parra, V.M.; Poncelas-Nozal, M.; Garcia-Dorado, D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J. Mol. Cell. Cardiol. 2010, 49, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, G.; Li, S.; Fan, G.C.; Peng, T. Calpain-1 induces endoplasmic reticulum stress in promoting cardiomyocyte apoptosis following hypoxia/reoxygenation. Biochim. Biophys. Acta 2015, 1852, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Weigand, K.; Brost, S.; Steinebrunner, N.; Buchler, M.; Schemmer, P.; Muller, M. Ischemia/Reperfusion injury in liver surgery and transplantation: Pathophysiology. HPB Surg. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Meissner, G. Regulation of mammalian ryanodine receptors. Front. Biosci. 2002, 7, d2072–d2080. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Neblina, F.; Toledo-Pereyra, L.H.; Toledo, A.H.; Walsh, J. Ryanodine receptor antagonism protects the ischemic liver and modulates TNF-alpha and IL-10. J. Surg. Res. 2007, 140, 121–128. [Google Scholar] [CrossRef] [PubMed]

| Solution Components | University of Wisconsin Solution (UW) | Institute George Lopez 1 (IGL-1) | HTK Custodiol |
|---|---|---|---|
| Electrolytes in mM | |||
| K+ | 125 | 25 | 9 |
| Na+ | 30 | 125 | 15 |
| Mg2+ | 5 | 5 | 4 |
| Ca2+ | 0 | 0 | 0.015 |
| Cl− | 0 | 0 | 32 |
| SO42− | 5 | 5 | 0 |
| Buffers in mM | |||
| Diphosphate | 25 | 25 | 0 |
| Histidine | 0 | 0 | 180 |
| Histidine-HCl | 0 | 0 | 18 |
| Tryptophan | 0 | 0 | 2 |
| Non-Permeants in mM | |||
| Raffinose | 30 | 30 | 0 |
| Lactobionic Acid | 100 | 100 | 0 |
| Mannitol | 0 | 0 | 30 |
| Colloids in g/L | |||
| Hydroxyethyl Starch | 50 | 0 | 0 |
| Polyethylene Glycol-35 | 0 | 1 | 0 |
| Antioxidants in mM | |||
| Glutathione | 3 | 3 | 0 |
| Allopurinol | 1 | 1 | 0 |
| Metabolic Precusors in mM | |||
| Adenosine | 5 | 5 | 0 |
| Ketoglutarate | 0 | 0 | 1 |
| pH | 7.4 | 7.4 | 7.2 |
| Osmolarity in mOsmol/L | 320 | 290 | 310 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Folch-Puy, E.; Panisello, A.; Oliva, J.; Lopez, A.; Castro Benítez, C.; Adam, R.; Roselló-Catafau, J. Relevance of Endoplasmic Reticulum Stress Cell Signaling in Liver Cold Ischemia Reperfusion Injury. Int. J. Mol. Sci. 2016, 17, 807. https://doi.org/10.3390/ijms17060807
Folch-Puy E, Panisello A, Oliva J, Lopez A, Castro Benítez C, Adam R, Roselló-Catafau J. Relevance of Endoplasmic Reticulum Stress Cell Signaling in Liver Cold Ischemia Reperfusion Injury. International Journal of Molecular Sciences. 2016; 17(6):807. https://doi.org/10.3390/ijms17060807
Chicago/Turabian StyleFolch-Puy, Emma, Arnau Panisello, Joan Oliva, Alexandre Lopez, Carlos Castro Benítez, René Adam, and Joan Roselló-Catafau. 2016. "Relevance of Endoplasmic Reticulum Stress Cell Signaling in Liver Cold Ischemia Reperfusion Injury" International Journal of Molecular Sciences 17, no. 6: 807. https://doi.org/10.3390/ijms17060807