Telocytes and Their Extracellular Vesicles—Evidence and Hypotheses
Abstract
:1. Introduction
2. Telocytes as a Particular Type of Interstitial Cells
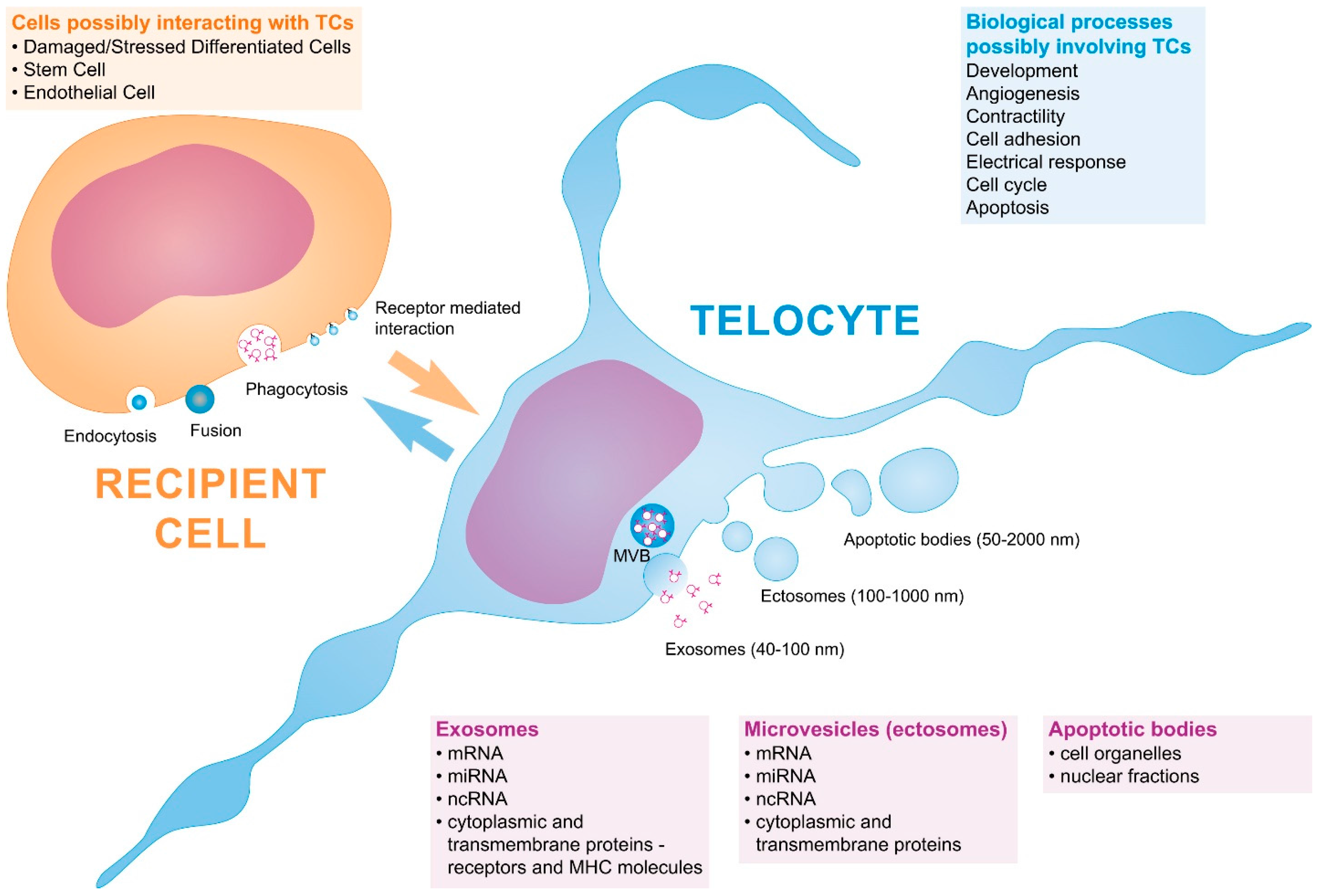
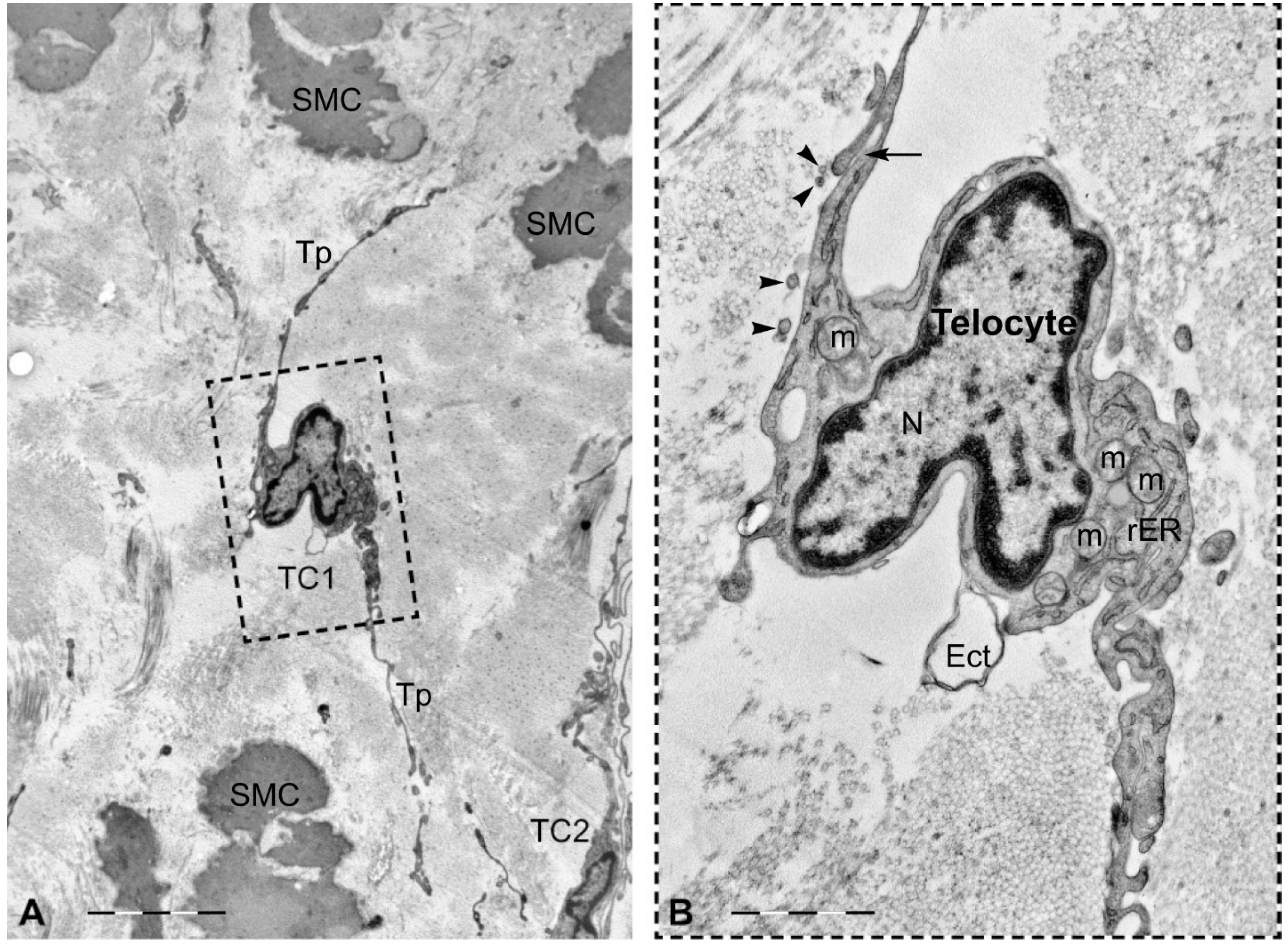
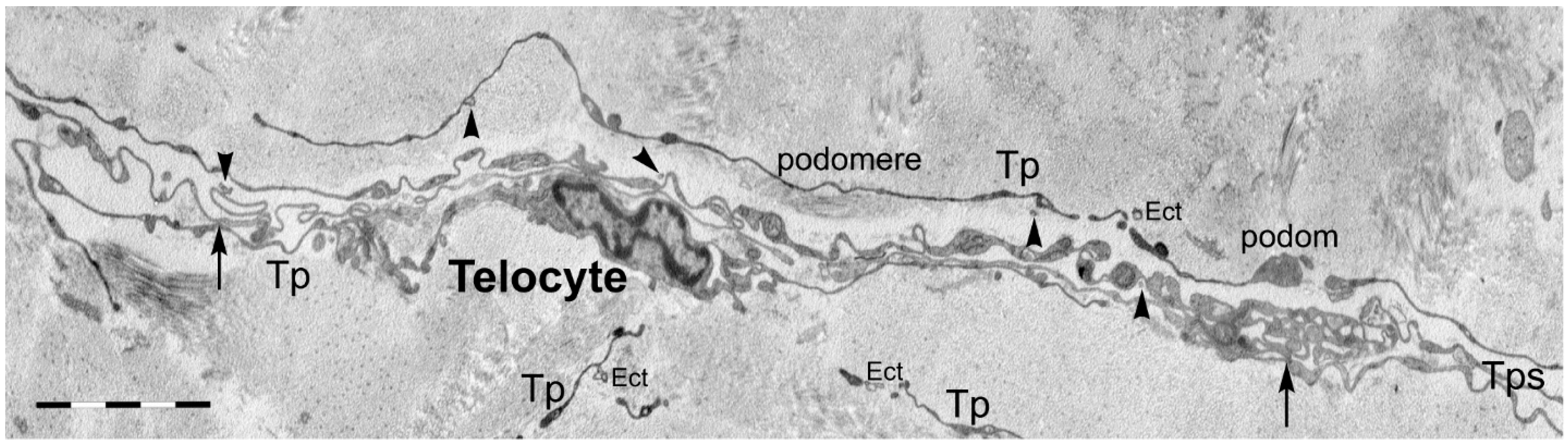
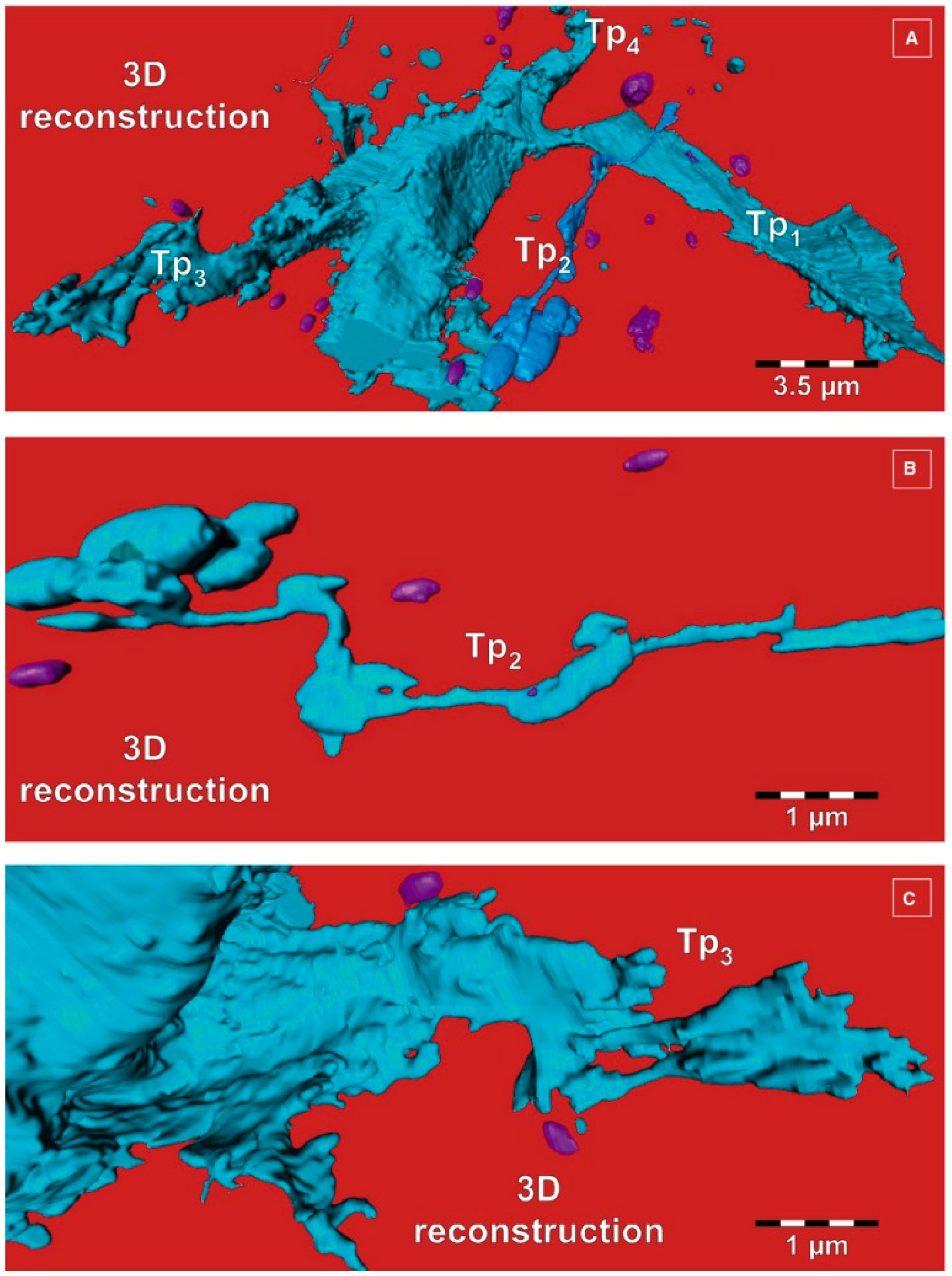
3. Telocytes and the Horizontal Transfer of Information
4. Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [PubMed]
- Andaloussi, S.E.L.; Mager, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, H.F.; Schiel, A.E.; Fijnheer, R.; Geuze, H.J.; Sixma, J.J. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and α-granules. Blood 1999, 94, 3791–3799. [Google Scholar] [PubMed]
- Mora, E.M.; Alvarez-Cubela, S.; Oltra, E. Biobanking of exosomes in the era of precision medicine: Are we there yet? Int. J. Mol. Sci. 2016, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Lasser, C.; Alikhani, V.S.; Ekstrom, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjostrand, M.; Gabrielsson, S.; Lotvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain rna: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, M.K.; Capasso, K.E.; Ajit, S.K. Purification and microrna profiling of exosomes derived from blood and culture media. J. Vis. Exp. 2013, 70, e50294. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Ridinger, J.; Rupp, A.K.; Janssen, J.W.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Tomofuji, T.; Ekuni, D.; Maruyama, T.; Yoneda, T.; Kawabata, Y.; Mizuno, H.; Miyai, H.; Kunitomo, M.; Morita, M. Micrornas in salivary exosome as potential biomarkers of aging. Int. J. Mol. Sci. 2015, 16, 21294–21309. [Google Scholar] [CrossRef] [PubMed]
- Pasalic, L.; Williams, R.; Siupa, A.; Campbell, H.; Henderson, M.J.; Chen, V.M. Enumeration of extracellular vesicles by a new improved flow cytometric method is comparable to fluorescence mode nanoparticle tracking analysis. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Rosas, L.E.; Elgamal, O.A.; Mo, X.; Phelps, M.A.; Schmittgen, T.D.; Papenfuss, T.L. In vitro immunotoxicity assessment of culture-derived extracellular vesicles in human monocytes. J. Immunotoxicol. 2016, 1–14. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling pathways in exosomes biogenesis, secretion and fate. Genes 2013, 4, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Gutierrez-Vazquez, C.; Sanchez-Madrid, F.; Mittelbrunn, M. Sorting it out: Regulation of exosome loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2016, 2016, 7653489. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Kucia, M.; Jadczyk, T.; Greco, N.J.; Wojakowski, W.; Tendera, M.; Ratajczak, J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia 2012, 26, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Lugini, L.; Cecchetti, S.; Huber, V.; Luciani, F.; Macchia, G.; Spadaro, F.; Paris, L.; Abalsamo, L.; Colone, M.; Molinari, A.; et al. Immune surveillance properties of human NK cell-derived exosomes. J. Immunol. 2012, 189, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, R.; Dignat-George, F. Microparticles as a circulating source of procoagulant and fibrinolytic activities in the circulation. Thromb. Res. 2012, 129, S27–S29. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Fernandes, A.R.; Baptista, P.V. Exosome in tumour microenvironment: Overview of the crosstalk between normal and cancer cells. Biomed. Res. Int. 2014, 2014, 179486. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimer’s Dement. 2015, 11, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.A.; Tripodis, Y.; Baugh, C.M.; Fritts, N.G.; Martin, B.M.; Chaisson, C.; Cantu, R.C.; Joyce, J.A.; Shah, S.; Ikezu, T.; et al. Preliminary study of plasma exosomal tau as a potential biomarker for chronic traumatic encephalopathy. J. Alzheimer’s Dis. 2016, 51, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, E. Extracellular vesicles in cardiovascular disease: Focus on vascular calcification. J. Physiol. 2016, 594, 2877–2880. [Google Scholar] [CrossRef] [PubMed]
- Osteikoetxea, X.; Nemeth, A.; Sodar, B.W.; Vukman, K.V.; Buzas, E.I. Extracellular vesicles in cardiovascular disease: Are they Jedi or Sith? J. Physiol. 2016, 594, 2881–2894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yeo, R.W.; Tan, K.H.; Lim, S.K. Focus on extracellular vesicles: Therapeutic potential of stem cell-derived extracellular vesicles. Int. J. Mol. Sci. 2016, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; He, W.; Zheng, L.; Duan, H. From structures to functions: Insights into exosomes as promising drug delivery vehicles. Biomater. Sci. 2016, 4, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Faussone-Pellegrini, M.S. Telocytes—A case of serendipity: The winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to telocytes. J. Cell. Mol. Med. 2010, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, S.M.; Popescu, L.M. Telocytes revisited. Biomol. Concepts 2014, 5, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M. Telocytes—A novel type of interstitial cells. In Recent Researches in Modern Medicine; Braissant, O., Wakamatsu, H., Kuo-Kang, I., Allegaert, K., Lenbury, Y., Wachholtz, A., Eds.; WSEAS Press: Cambridge, UK, 2011; pp. 424–432. [Google Scholar]
- Roatesi, I.; Radu, B.M.; Cretoiu, D.; Cretoiu, S.M. Uterine telocytes: A review of current knowledge. Biol. Reprod. 2015, 93. [Google Scholar] [CrossRef] [PubMed]
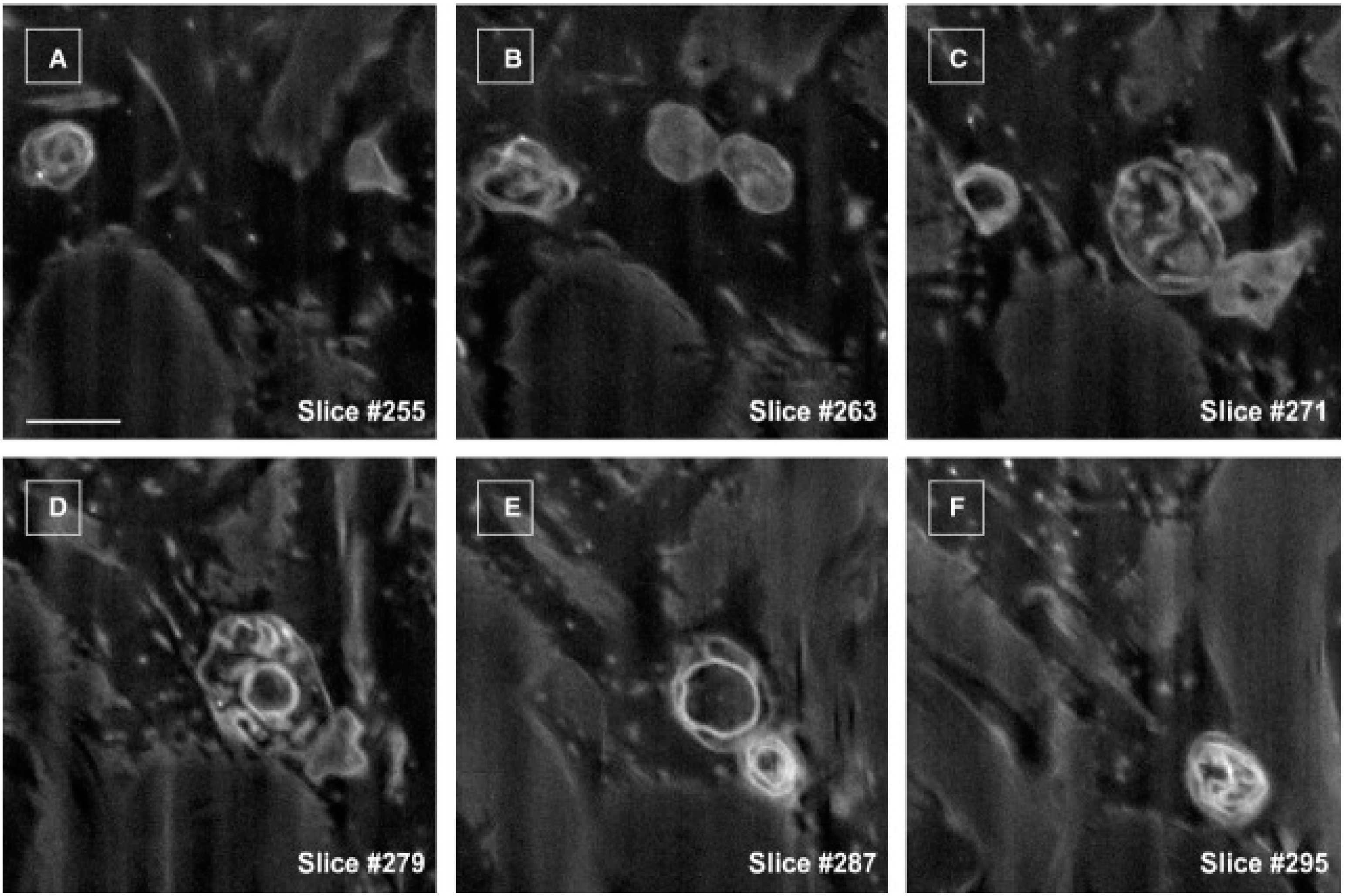
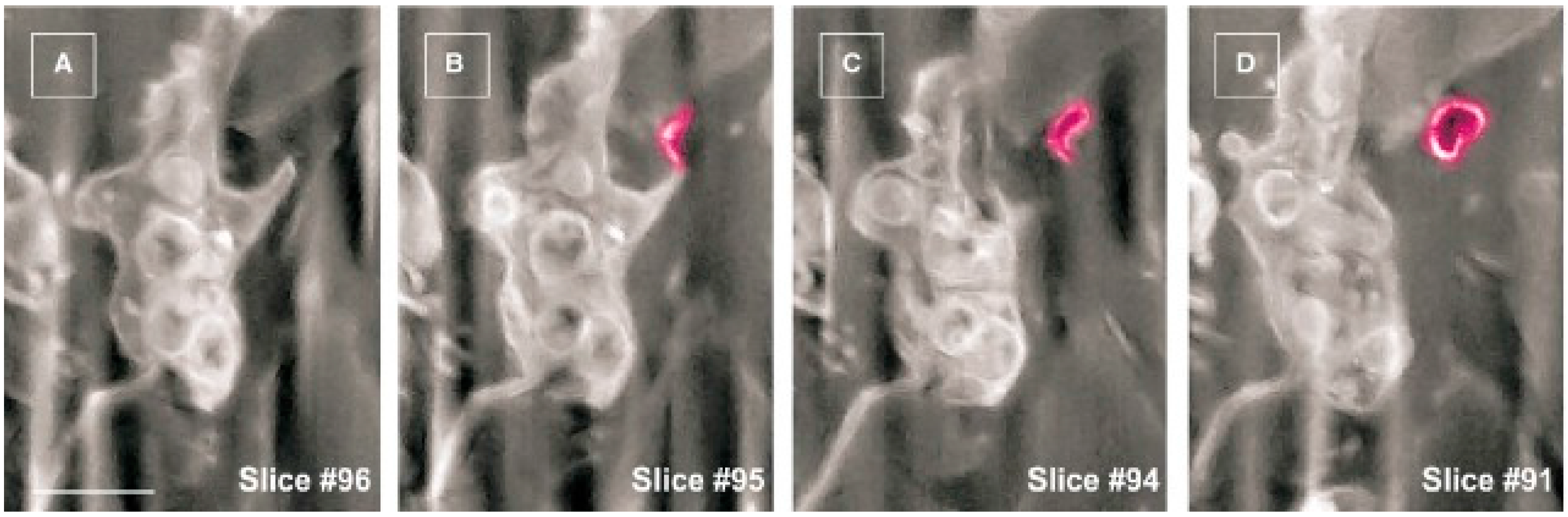
- Cretoiu, D.; Hummel, E.; Zimmermann, H.; Gherghiceanu, M.; Popescu, L.M. Human cardiac telocytes: 3D imaging by fib-sem tomography. J. Cell. Mol. Med. 2014, 18, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, D.; Gherghiceanu, M.; Hummel, E.; Zimmermann, H.; Simionescu, O.; Popescu, L.M. Fib-sem tomography of human skin telocytes and their extracellular vesicles. J. Cell. Mol. Med. 2015, 19, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Faussone-Pellegrini, M.S.; Gherghiceanu, M. Telocyte’s contacts. Semin. Cell Dev. Biol. 2016, 55, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, D.; Cretoiu, S.M.; Simionescu, A.A.; Popescu, L.M. Telocytes, a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol. Histopathol. 2012, 27, 1067–1078. [Google Scholar] [PubMed]
- Cretoiu, S.M.; Cretoiu, D.; Popescu, L.M. Human myometrium—The ultrastructural 3D network of telocytes. J. Cell. Mol. Med. 2012, 16, 2844–2849. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, M.; Qian, M.; Wang, L.; Cismasiu, V.B.; Bai, C.; Popescu, L.M.; Wang, X. Genetic comparison of mouse lung telocytes with mesenchymal stem cells and fibroblasts. J. Cell. Mol. Med. 2013, 17, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zheng, M.; Zhang, M.; Qian, M.; Zheng, Y.; Li, M.; Cretoiu, D.; Chen, C.; Chen, L.; Popescu, L.M.; et al. Differences in the expression of chromosome 1 genes between lung telocytes and other cells: Mesenchymal stem cells, fibroblasts, alveolar type II cells, airway epithelial cells and lymphocytes. J. Cell. Mol. Med. 2014, 18, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Sun, X.; Zhang, M.; Qian, M.; Zheng, Y.; Li, M.; Cretoiu, S.M.; Chen, C.; Chen, L.; Cretoiu, D.; et al. Variations of chromosomes 2 and 3 gene expression profiles among pulmonary telocytes, pneumocytes, airway cells, mesenchymal stem cells and lymphocytes. J. Cell. Mol. Med. 2014, 18, 2044–2060. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Cretoiu, D.; Zheng, M.; Qian, M.; Zhang, M.; Cretoiu, S.M.; Chen, L.; Fang, H.; Popescu, L.M.; Wang, X. Comparison of chromosome 4 gene expression profile between lung telocytes and other local cell types. J. Cell. Mol. Med. 2016, 20, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, L.; Jin, M.; Wang, X. Global analyses of chromosome 17 and 18 genes of lung telocytes compared with mesenchymal stem cells, fibroblasts, alveolar type ii cells, airway epithelial cells, and lymphocytes. Biol. Direct 2015, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, M.; Song, D.; Ye, L.; Wang, X. Global comparison of chromosome x genes of pulmonary telocytes with mesenchymal stem cells, fibroblasts, alveolar type II cells, airway epithelial cells, and lymphocytes. J. Transl. Med. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, R.; Tanase, C.; Codrici, E.; Popescu, D.I.; Cretoiu, S.M.; Popescu, L.M. The secretome of myocardial telocytes modulates the activity of cardiac stem cells. J. Cell. Mol. Med. 2015, 19, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cretoiu, D.; Yan, G.; Cretoiu, S.M.; Popescu, L.M.; Fang, H.; Wang, X. Protein profiling of human lung telocytes and microvascular endothelial cells using iTRAQ quantitative proteomics. J. Cell. Mol. Med. 2014, 18, 1035–1059. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cretoiu, D.; Yan, G.; Cretoiu, S.M.; Popescu, L.M.; Wang, X. Comparative proteomic analysis of human lung telocytes with fibroblasts. J. Cell. Mol. Med. 2014, 18, 568–589. [Google Scholar] [CrossRef] [PubMed]
- Mandache, E.; Popescu, L.M.; Gherghiceanu, M. Myocardial interstitial Cajal-like cells (ICLC) and their nanostructural relationships with intercalated discs: Shed vesicles as intermediates. J. Cell. Mol. Med. 2007, 11, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Manole, E.; Serboiu, C.S.; Manole, C.G.; Suciu, L.C.; Gherghiceanu, M.; Popescu, B.O. Identification of telocytes in skeletal muscle interstitium: Implication for muscle regeneration. J. Cell. Mol. Med. 2011, 15, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Nicolescu, M.I.; Bucur, A.; Dinca, O.; Rusu, M.C.; Popescu, L.M. Telocytes in parotid glands. Anat. Rec. 2012, 295, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Nicolescu, M.I.; Popescu, L.M. Telocytes in the interstitium of human exocrine pancreas: Ultrastructural evidence. Pancreas 2012, 41, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, S.M.; Cretoiu, D.; Marin, A.; Radu, B.M.; Popescu, L.M. Telocytes: Ultrastructural, immunohistochemical and electrophysiological characteristics in human myometrium. Reproduction 2013, 145, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Ibba-Manneschi, L.; Rosa, I.; Manetti, M. Telocyte implications in human pathology: An overview. Semin. Cell Dev. Biol. 2016, 55, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Flores, L.; Gutierrez, R.; Diaz-Flores, L., Jr.; Gomez, M.G.; Saez, F.J.; Madrid, J.F. Behaviour of telocytes during physiopathological activation. Semin. Cell Dev. Biol. 2016, 55, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Li, C.; Lu, Z.; Li, H.; Guo, Z. Multiple immunophenotypes of cardiac telocytes. Exp. Cell Res. 2015, 338, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Fertig, E.T.; Gherghiceanu, M.; Popescu, L.M. Extracellular vesicles release by cardiac telocytes: Electron microscopy and electron tomography. J. Cell. Mol. Med. 2014, 18, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Xiang, J. Mechanisms of cellular communication through intercellular protein transfer. J. Cell. Mol. Med. 2011, 15, 1458–1473. [Google Scholar] [CrossRef] [PubMed]
- Cismasiu, V.B.; Radu, E.; Popescu, L.M. Mir-193 expression differentiates telocytes from other stromal cells. J. Cell. Mol. Med. 2011, 15, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Cismasiu, V.B.; Popescu, L.M. Telocytes transfer extracellular vesicles loaded with micrornas to stem cells. J. Cell. Mol. Med. 2015, 19, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liao, Z.; Chen, S.; Yuan, Z.; Yilin, C.; Lee, K.K.; Qi, X.; Shen, X.; Zheng, X.; Quinn, T.; et al. Intramyocardial transplantation of cardiac telocytes decreases myocardial infarction and improves post-infarcted cardiac function in rats. J. Cell. Mol. Med. 2014, 18, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Ja, K.P.; Miao, Q.; Tee, Z.; Nicole, G.; Lim, S.Y.; Nandihalli, M.; JA Ramachandra, C.; Mehta, A.; Shim, W. Ipsc-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium. J. Cell. Mol. Med. 2016, 20, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, P.; Qu, Y.; Yu, P.; Yao, J.; Wang, H.; Fu, S.; Bei, Y.; Chen, Y.; Che, L.; et al. Telocytes in exercise-induced cardiac growth. J. Cell. Mol. Med. 2016, 20, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, X.; Qian, M.; Zhang, M.; Zhang, D.; Bai, C.; Wang, Q.; Wang, X. Human lung telocytes could promote the proliferation and angiogenesis of human pulmonary microvascular endothelial cells in vitro. Mol. Cell. Ther. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Gao, J.; Xiao, H.; Xu, M. Increased telocytes involved in the proliferation of vascular smooth muscle cells in rat carotid artery balloon injury. Sci. China Life Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Katsman, D.; Stackpole, E.J.; Domin, D.R.; Farber, D.B. Embryonic stem cell-derived microvesicles induce gene expression changes in muller cells of the retina. PLoS ONE 2012, 7, e50417. [Google Scholar] [CrossRef] [PubMed]
- Farber, D.B.; Katsman, D. Embryonic stem cell-derived microvesicles: Could they be used for retinal regeneration? Adv. Exp. Med. Biol. 2016, 854, 563–569. [Google Scholar] [PubMed]
- Cao, Y.; Liu, C.; Gu, Z.; Zhang, Y.; Duan, Y.; Zhang, Y.; Zhang, H.; Tang, K.; Huang, B. Microparticles mediate human papillomavirus type 6 or 11 infection of human macrophages. Cell. Mol. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Woollard, S.M.; Li, H.; Singh, S.; Yu, F.; Kanmogne, G.D. HIV-1 induces cytoskeletal alterations and Rac1 activation during monocyte-blood-brain barrier interactions: Modulatory role of CCR5. Retrovirology 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through met. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Flores, L.; Gutierrez, R.; Garcia, M.P.; Saez, F.J.; Aparicio, F.; Diaz-Flores, L., Jr.; Madrid, J.F. Uptake and intracytoplasmic storage of pigmented particles by human CD34+ stromal cells/telocytes: Endocytic property of telocytes. J. Cell. Mol. Med. 2014, 18, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Flores, L.; Gutierrez, R.; Garcia, M.P.; Gonzalez, M.; Saez, F.J.; Aparicio, F.; Diaz-Flores, L., Jr.; Madrid, J.F. Human resident CD34+ stromal cells/telocytes have progenitor capacity and are a source of αSMA+ cells during repair. Histol. Histopathol. 2015, 30, 615–627. [Google Scholar] [PubMed]
- Diaz-Flores, L.; Gutierrez, R.; Garcia, M.P.; Saez, F.J.; Diaz-Flores, L., Jr.; Valladares, F.; Madrid, J.F. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol. Histopathol. 2014, 29, 831–870. [Google Scholar] [PubMed]
- Cretoiu, D.; Cretoiu, S.M. Telocytes in the reproductive organs: Current understanding and future challenges. Semin. Cell Dev. Biol. 2016, 55, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, S.M. Immunohistochemistry of telocytes in uterus and fallopian tubes. In Telocytes: Connecting Cells; Wang, X., Cretoiu, D., Eds.; Springer: Beijing, China, 2016. [Google Scholar]
- Campeanu, R.A.; Radu, B.M.; Cretoiu, S.M.; Banciu, D.D.; Banciu, A.; Cretoiu, D.; Popescu, L.M. Near-infrared low-level laser stimulation of telocytes from human myometrium. Lasers Med. Sci. 2014, 29, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Mirancea, N.; Morosanu, A.M.; Mirancea, G.V.; Juravle, F.D.; Manoiu, V.S. Infrastructure of the telocytes from tumor stroma in the skin basal and squamous cell carcinomas. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2013, 54, 1025–1037. [Google Scholar]
- Mou, Y.; Wang, Y.; Li, J.; Lu, S.; Duan, C.; Du, Z.; Yang, G.; Chen, W.; Zhao, S.; Zhou, J.; et al. Immunohistochemical characterization and functional identification of mammary gland telocytes in the self-assembly of reconstituted breast cancer tissue in vitro. J. Cell. Mol. Med. 2013, 17, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Horch, R.E.; Kneser, U.; Polykandriotis, E.; Schmidt, V.J.; Sun, J.; Arkudas, A. Tissue engineering and regenerative medicine—Where do we stand? J. Cell. Mol. Med. 2012, 16, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Boos, A.M.; Weigand, A.; Brodbeck, R.; Beier, J.P.; Arkudas, A.; Horch, R.E. The potential role of telocytes in tissue engineering and regenerative medicine. Semin. Cell Dev. Biol. 2016, 55, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Ratajczak, J. Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 Years later. Clin. Transl. Med. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cretoiu, D.; Xu, J.; Xiao, J.; Cretoiu, S.M. Telocytes and Their Extracellular Vesicles—Evidence and Hypotheses. Int. J. Mol. Sci. 2016, 17, 1322. https://doi.org/10.3390/ijms17081322
Cretoiu D, Xu J, Xiao J, Cretoiu SM. Telocytes and Their Extracellular Vesicles—Evidence and Hypotheses. International Journal of Molecular Sciences. 2016; 17(8):1322. https://doi.org/10.3390/ijms17081322
Chicago/Turabian StyleCretoiu, Dragos, Jiahong Xu, Junjie Xiao, and Sanda M. Cretoiu. 2016. "Telocytes and Their Extracellular Vesicles—Evidence and Hypotheses" International Journal of Molecular Sciences 17, no. 8: 1322. https://doi.org/10.3390/ijms17081322







