Pneumococcal Colonization in the Familial Context and Implications for Anti-Pneumococcal Immunization in Adults: Results from the BINOCOLO Project in Sicily
Abstract
:1. Introduction
2. Results
2.1. Subjects Characteristics
2.2. Vaccination Status
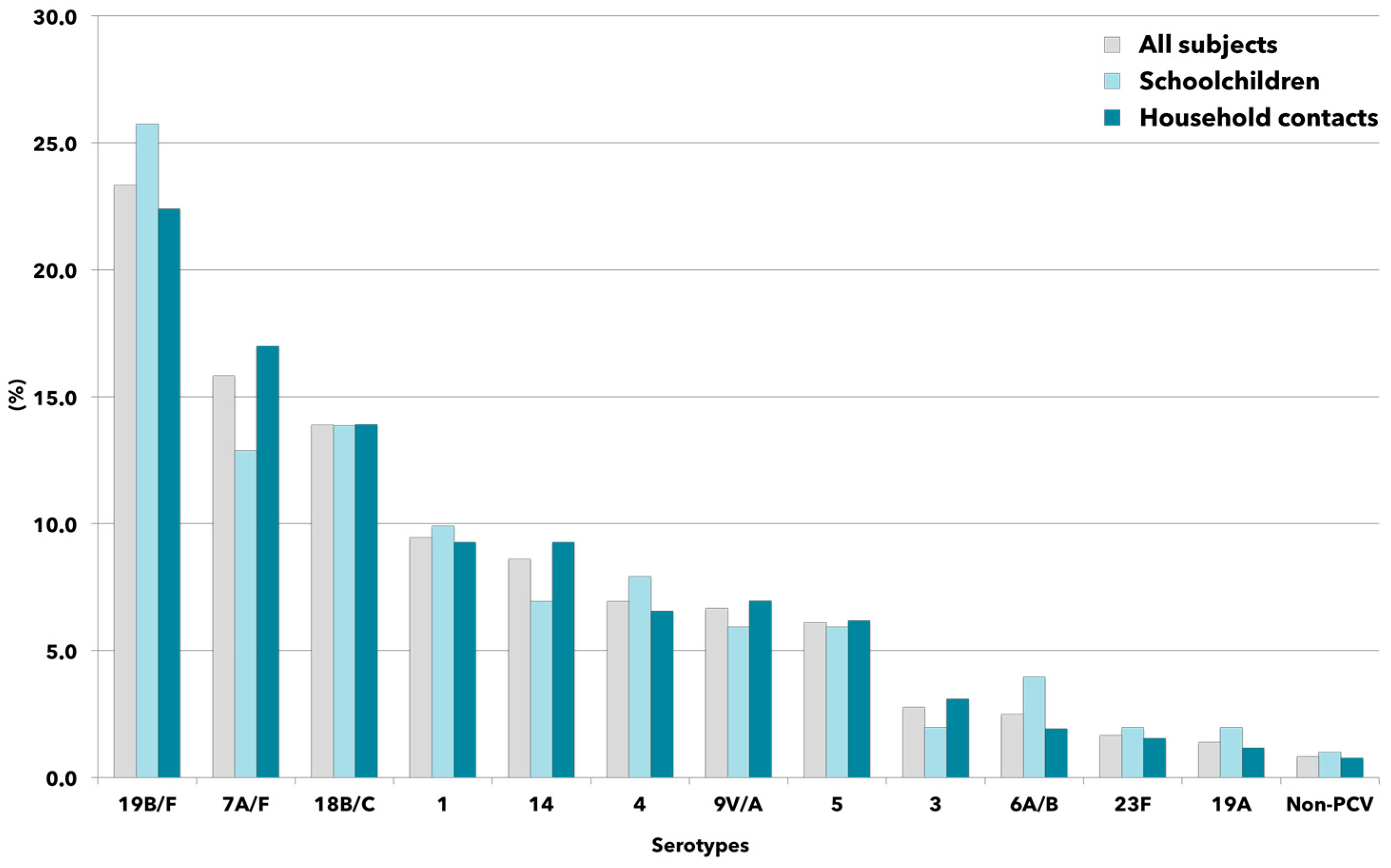
2.3. Pneumococcal Carriage
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Ethics Statement
4.3. Laboratory Methods
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| rt_PCR | real-time PCR |
| OR | odds ratio |
| IQR | interquartile range |
| IPD | invasive pneumococcal disease |
| PCV7 | pneumococcal 7-valent conjugate vaccine |
| PCV13 | pneumococcal 13-valent conjugate vaccine |
| PPV23 | pneumococcal 23-valent polysaccharides vaccine |
References
- Simell, B.; Auranen, K.; Käyhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L. The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T.; et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef]
- Drijkoningen, J.J.; Rohde, G.G. Pneumococcal infection in adults: Burden of disease. Clin. Microbiol. Infect. 2014, 20, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.; Givon-Lavi, N.; Newman, N.; Bar-Ziv, J.; Dagan, R. Nasopharyngeal carriage of individual Streptococcus pneumoniae serotypes during pediatric pneumonia as a means to estimate serotype disease potential. Pediatr. Infect. Dis. J. 2011, 30, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, R.K.; Auranen, K.J.; Leino, T.M.; Kilpi, T.M.; Mäkelä, P.H. Pneumococcal acute otitis media in relation to pneumococcal nasopharyngeal carriage. Pediatr. Infect. Dis. J. 2005, 24, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.C.; Cheung, Y.B.; Akisanya, A.; Sankareh, K.; Lahai, G.; Greenwood, B.M.; Adegbola, R.A. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian infants: A longitudinal study. Clin. Infect. Dis. 2008, 46, 807–814. [Google Scholar] [CrossRef] [PubMed]
- De Le Polain Waroux, O.; Flasche, S.; Prieto-Merino, D.; Edmunds, W.J. Age-dependent prevalence of nasopharyngeal carriage of Streptococcus pneumoniae before conjugate vaccine introduction: A prediction model based on a meta-analysis. PLoS ONE 2014, 9, e86136. [Google Scholar] [CrossRef] [PubMed]
- Sicilian Region Official Gazette (GURS). 2003. Available online: http://www.gurs.regione.sicilia.it/Gazzette/g03-35/g03-35-p15.htm (accessed on 2 November 2016).
- Sicilian Region Official Gazette (GURS). 2004. Available online: http://www.gurs.regione.sicilia.it/Gazzette/g04-35/g04-35-p12.htm (accessed on 2 November 2016).
- Sicilian Region Official Gazette (GURS). 2010. Available online: http://www.gurs.regione.sicilia.it/Gazzette/g10-35/g10-35.pdf (accessed on 2 November 2016).
- Isaacman, D.J.; McIntosh, E.D.; Reinert, R.R. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: Impact of the 7-valent pneu-mococcal conjugate vaccine and considerations for future conjugate vaccines. Int. J. Infect. Dis. 2010, 14, e197–e209. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Zielen, S. Impact of infant immunization programs with pneumococcal conjugate vaccine in Europe. Expert Rev. Vaccines 2009, 8, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.G.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Bennett, N.M.; Lynfield, R.; Reingold, A.; Cieslak, P.R.; Pilishvili, T.; Jackson, D.; et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 2003, 348, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Poehling, K.A.; Talbot, T.R.; Griffin, M.R.; Craig, A.S.; Whitney, C.G.; Zell, E.; Lexau, C.A.; Thomas, A.R.; Harrison, L.H.; Reingold, A.L.; et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 2006, 295, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Amodio, E.; Costantino, C.; Giuffrè, M.; Piccione, M.; Tramuto, F.; Vitale, F. Invasive pneumococcal diseases in children aged 1–59 months in Sicily, Italy: Importance of active family paediatrician surveillance and vaccination coverage. Euromediterranean Biomed. J. 2014, 9, 19–23. [Google Scholar]
- Desai, S.; Policarpio, M.E.; Wong, K.; Gubbay, J.; Fediurek, J.; Deeks, S. The epidemiology of invasive pneumococcal disease in older adults from 2007 to 2014 in Ontario, Canada: A population-based study. CMAJ Open 2016, 29, E545–E550. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Campling, J.; Dicker, A.; Woodhead, M.; Madhava, H. A systematic review of the burden of vaccine preventable pneumococcal disease in UK adults. BMC Pulm. Med. 2016, 11, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasi, F.; Akova, M.; Bonanni, P.; Dartois, N.; Sauty, E.; Webber, C.; Torres, A. Community-acquired pneumonia in adults: Highlighting missed opportunities for vaccination. Eur. J. Intern. Med. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Giese, C.; Mereckiene, J.; Danis, K.; O’Donnell, J.; O’Flanagan, D.; Cotter, S. Low vaccination coverage for seasonal influenza and pneumococcal disease among adults at-risk and health care workers in Ireland, 2013: The key role of GPs in recommending vaccination. Vaccine 2016, 34, 3657–3662. [Google Scholar] [CrossRef] [PubMed]
- Prato, R.; Fortunato, F.; Martinelli, D. Pneumococcal pneumonia prevention among adults: Is the herd effect of pneumococcal conjugate vaccination in children as good a way as the active immunization of the elderly? Curr. Med. Res. Opin. 2016, 32, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Azzari, C.; Cortimiglia, M.; Nieddu, F.; Moriondo, M.; Indolfi, G.; Mattei, R.; Zuliani, M.; Adriani, B.; Degl’Innocenti, R.; Consales, G.; et al. Pneumococcal serotype distribution in adults with invasive disease and in carrier children in Italy: Should we expect herd protection of adults through infants’ vaccination? Hum. Vaccines Immunother. 2016, 12, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Lexau, C.; Bennett, N.M.; Petit, S.; Zansky, S.M.; Harrison, L.H.; Reingold, A.; et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: Analysis of multisite, population-based surveillance. Lancet Infect. Dis. 2015, 15, 301–309. [Google Scholar] [CrossRef]
- Van Hoek, A.J.; Sheppard, C.L.; Andrews, N.J.; Waight, P.A.; Slack, M.P.; Harrison, T.G.; Ladhani, S.N.; Miller, E. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine 2014, 32, 4349–4355. [Google Scholar] [CrossRef] [PubMed]
- Mosser, J.F.; Grant, L.R.; Millar, E.V.; Weatherholtz, R.C.; Jackson, D.M.; Beall, B.; Craig, M.J.; Reid, R.; Santosham, M.; O’Brien, K.L. Nasopharyngeal carriage and transmission of Streptococcus pneumoniae in American Indian households after a decade of pneumococcal conjugate vaccine use. PLoS ONE 2014, 9, e79578. [Google Scholar] [CrossRef] [PubMed]
- Leino, T.; Auranen, K.; Jokinen, J.; Leinonen, M.; Tervonen, P.; Takala, A.K. Pneumococcal carriage in children during their first two years: Important role of family exposure. Pediatr. Infect. Dis. J. 2001, 20, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Dhoubhadel, B.G.; Yasunami, M.; Nguyen, H.A.; Suzuki, M.; Vu, T.H.; Thuy Nguyen, A.T.; Dang, D.A.; Yoshida, L.M.; Ariyoshi, K. Bacterial load of pneumococcal serotypes correlates with their prevalence and multiple serotypes is associated with acute respiratory infections among children less than 5 years of age. PLoS ONE 2014, 9, e110777. [Google Scholar] [CrossRef] [PubMed]
- Ansaldi, F.; de Florentiis, D.; Canepa, P.; Ceravolo, A.; Rappazzo, E.; Iudici, R.; Martini, M.; Botti, G.; Orsi, A.; Icardi, G.; et al. Carriage of Streptoccoccus pneumoniae in healthy adults aged 60 years or over in a population with very high and long-lasting pneumococcal conjugate vaccine coverage in children: Rationale and perspectives for PCV13 implementation. Hum. Vaccines Immunother. 2013, 9, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Camilli, R.; Daprai, L.; Cavrini, F.; Lombardo, D.; D’Ambrosio, F.; del Grosso, M.; Vescio, M.F.; Landini, M.P.; Pascucci, M.G.; Torresani, E.; et al. Pneumococcal carriage in young children one year after introduction of the 13-valent conjugate vaccine in Italy. PLoS ONE 2013, 8, e76309. [Google Scholar] [CrossRef] [PubMed]
- Pasinato, A.; Indolfi, G.; Marchisio, P.; Valleriani, C.; Cortimiglia, M.; Spanevello, V.; Chiamenti, G.; Buzzetti, R.; Resti, M.; Azzari, C.; et al. Pneumococcal serotype distribution in 1315 nasopharyngeal swabs from a highly vaccinated cohort of Italian children as detected by RT-PCR. Vaccine 2014, 32, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Terranova, L.; Ruggiero, L.; Ascolese, B.; Montinaro, V.; Rios, W.P.; Galeone, C.; Principi, N. Streptococcus pneumoniae and Staphylococcus aureus carriage in healthy school-age children and adolescents. J. Med. Microbiol. 2015, 64, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Terranova, L.; Zampiero, A.; Montinaro, V.; Ierardi, V.; Peves Rios, W.; Pelucchi, C.; Esposito, S. Pharyngeal colonization by Streptococcus pneumoniae in older children and adolescents in a geographical area characterized by relatively limited pneumococcal vaccination coverage. Pediatr. Infect. Dis. J. 2015, 34, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.; Mameli, C.; Daprai, L.; Garlaschi, M.L.; Dilillo, D.; Bedogni, G.; Faccini, M.; Gramegna, M.; Torresani, E.; PneuMi Study Group (PMSG). Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine 2014, 32, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.K.; Rifas-Shiman, S.L.; Shea, K.M.; Kleinman, K.P.; Lee, G.M.; Lakoma, M.; Pelton, S.I.; Finkelstein, J.A.; Huang, S.S. Do community-level predictors of pneumococcal carriage continue to play a role in the conjugate vaccine era? Epidemiol. Infect. 2014, 142, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.P.; Azevedo, J.; Leite, M.C.; Campos, L.C.; Cunha, M.; Carvalho Mda, G.; Reis, M.G.; Ko, A.I.; Weinberger, D.M.; Ribeiro, G.; et al. Nasopharyngeal carriage of Streptococcus pneumoniae among children in an urban setting in Brazil prior to PCV10 introduction. Vaccine 2016, 34, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.; Givon-Lavi, N.; Broides, A.; Blancovich, I.; Peled, N.; Dagan, R. The contribution of smoking and exposure to tobacco smoke to Streptococcus pneumoniae and Haemophilus influenzae carriage in children and their mothers. Clin. Infect. Dis. 2006, 42, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Morissette, M.C.; Vanderstocken, G.; Gao, Y.; Hassan, M.; Roos, A.; Thayaparan, D.; Merlano, M.; Dorrington, M.G.; Nikota, J.K.; et al. Cigarette smoke attenuates the nasal host response to Streptococcus pneumoniae and predisposes to invasive pneumococcal disease in mice. Infect. Immun. 2016, 84, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Nuorti, J.P.; Butler, J.C.; Farley, M.M.; Harrison, L.H.; McGeer, A.; Kolczak, M.S.; Breiman, R.F.; Active Bacterial Core Surveillance Team. Cigarette smoking and invasive pneumococcal disease. N. Engl. J. Med. 2000, 342, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Le Rouzic, O.; Koné, B.; Kluza, J.; Marchetti, P.; Hennegrave, F.; Olivier, C.; Kervoaze, G.; Vilain, E.; Mordacq, C.; Just, N.; et al. Cigarette smoke alters the ability of human dendritic cells to promote anti-Streptococcus pneumoniae Th17 response. Respir. Res. 2016, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Fabiano, V.; Daprai, L.; Bedogni, G.; Faccini, M.; Garlaschi, M.L.; Penagini, F.; Dilillo, D.; Torresani, E.; Gramegna, M.; et al. A longitudinal study of Streptococcus pneumoniae carriage in healthy children in the 13-valent pneumococcal conjugate vaccine era. Hum. Vaccines Immunother. 2015, 11, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, R.; Murdoch, C.; Race, G.; Burkinshaw, R.; Shaw, L.; Finn, A. Pneumococcal nasopharyngeal carriage in children following heptavalent pneumococcal conjugate vaccination in infancy. Arch. Dis. Child. 2003, 88, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Myint, T.T.; Madhava, H.; Balmer, P.; Christopoulou, D.; Attal, S.; Menegas, D.; Sprenger, R.; Bonnet, E. The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: A literature review. Adv. Ther. 2013, 30, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Steens, A.; Bergsaker, M.A.; Aaberge, I.S.; Rønning, K.; Vestrheim, D.F. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine 2013, 31, 6232–6238. [Google Scholar] [CrossRef] [PubMed]
- Tocheva, A.S.; Jefferies, J.M.; Rubery, H.; Bennett, J.; Afimeke, G.; Garland, J.; Christodoulides, M.; Faust, S.N.; Clarke, S.C. Declining serotype coverage of new pneumococcal conjugate vaccines relating to the carriage of Streptococcus pneumoniae in young children. Vaccine 2011, 2, 4400–4404. [Google Scholar] [CrossRef] [PubMed]
- Grivea, I.N.; Tsantouli, A.G.; Michoula, A.N.; Syrogiannopoulos, G.A. Dynamics of Streptococcus pneumoniae nasopharyngeal carriage with high heptavalent pneumococcal conjugate vaccine coverage in Central Greece. Vaccine 2011, 29, 8882–8887. [Google Scholar] [CrossRef] [PubMed]
- Kamng’ona, A.W.; Hinds, J.; Bar-Zeev, N.; Gould, K.A.; Chaguza, C.; Msefula, C.; Cornick, J.E.; Kulohoma, B.W.; Gray, K.; Bentley, S.D.; et al. High multiple carriage and emergence of Streptococcus pneumoniae vaccine serotype variants in Malawian children. BMC Infect. Dis. 2015, 15, 234. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, R.; Gurung, M.; Thapa, A.; Ndimah, S.; Adhikari, N.; Murdoch, D.R.; Kelly, D.F.; Waldron, D.E.; Gould, K.A.; Thorson, S.; et al. Multi-serotype pneumococcal nasopharyngeal carriage prevalence in vaccine naïve Nepalese children, assessed using molecular serotyping. PLoS ONE 2015, 10, e0114286. [Google Scholar] [CrossRef] [PubMed]
- Azzari, C.; Moriondo, M.; Indolfi, G.; Cortimiglia, M.; Canessa, C.; Becciolini, L.; Lippi, F.; de Martino, M.; Resti, M. Realtime PCR is more sensitive than multiplex PCR for diagnosis and serotyping in children with culture negative pneumococcal invasive disease. PLoS ONE 2010, 19, e9282. [Google Scholar] [CrossRef] [PubMed]
- Satzke, C.; Dunne, E.M.; Porter, B.D.; Klugman, K.P.; Mulholland, E.K.; PneuCarriage Project Group. The PneuCarriage Project: A Multi-Centre comparative study to identify the best serotyping methods for examining pneumococcal carriage in vaccine evaluation studies. PLoS Med. 2015, 12, e1001903. [Google Scholar] [CrossRef] [PubMed]
- Darboe, M.K.; Fulford, A.J.; Secka, O.; Prentice, A.M. The dynamics of nasopharyngeal Streptococcus pneumoniae carriage among rural Gambian mother-infant pairs. BMC Infect. Dis. 2010, 5, 195. [Google Scholar] [CrossRef] [PubMed]
- Brugger, S.D.; Frey, P.; Aebi, S.; Hinds, J.; Mühlemann, K. Multiple colonization with S. pneumoniae before and after introduction of the seven-valent conjugated pneumococcal polysaccharide vaccine. PLoS ONE 2010, 16, e11638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, P.; Turner, C.; Jankhot, A.; Helen, N.; Lee, S.J.; Day, N.P.; White, N.J.; Nosten, F.; Goldblatt, D. A longitudinal study of Streptococcus pneumoniae carriage in a cohort of infants and their mothers on the Thailand-Myanmar border. PLoS ONE 2012, 7, e38271. [Google Scholar] [CrossRef] [PubMed]
- Brimblecombe, F.S.; Cruickshank, R.; Masters, P.L.; Reid, D.D.; Stewart, G.T. Family studies of respiratory infections. Br. Med. J. 1958, 18, 119–128. [Google Scholar] [CrossRef]
- Masters, P.L.; Brumfitt, W.; Mendez, R.L.; Likar, M. Bacterial flora of the upper respiratory tract in Paddington families, 1952–1954. Br. Med. J. 1958, 1, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.N.; Sheehe, P.R.; Feldman, H.A. Pharyngeal pneumococcal acquisitions in “normal” families: A longitudinal study. J. Infect. Dis. 1971, 124, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gwaltney, J.M., Jr.; Sande, M.A.; Austrian, R.; Hendley, J.O. Spread of Streptococcus pneumoniae in families. II. Relation of transfer of S. pneumoniae to incidence of colds and serum antibody. J. Infect. Dis. 1975, 132, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Satzke, C.; Turner, P.; Virolainen-Julkunen, A.; Adrian, P.V.; Antonio, M.; Hare, K.M.; Henao-Restrepo, A.M.; Leach, A.J.; Klugman, K.P.; Porter, B.D.; et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: Updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2013, 32, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Da Gloria Carvalho, M.; Pimenta, F.C.; Jackson, D.; Roundtree, A.; Ahmad, Y.; Millar, E.V.; O’Brien, K.L.; Whitney, C.G.; Cohen, A.L.; Beall, B.W. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J. Clin. Microbiol. 2010, 48, 1611–1618. [Google Scholar] [CrossRef] [PubMed]

| Study Population | Total | School Children | Family Members | |
|---|---|---|---|---|
| <12 Years | ≥12 Years | |||
| Study participants (n (%)) | 146 | 36 (24.7) | 18 (12.3) | 92 (63.0) |
| Age (years; median (IQR)) | 31.0 (36.0) | 7 (2.0) | 5 (8.0) | 39.0 (23.0) |
| Age-group (years) | ||||
| 5–6 | 22 (15.1) | 12 (33.3) | 10 (55.6) | 0 |
| 7 | 9 (6.2) | 8 (22.2) | 1 (5.5) | 0 |
| 8–11 | 23 (15.7) | 16 (44.5) | 7 (38.9) | 0 |
| 12–25 (teenagers/young adults) | 12 (8.2) | 0 | 0 | 12 (13.1) |
| 26–49 (adults) | 52 (35.6) | 0 | 0 | 52 (56.5) |
| ≥50 (older adults/elderly) | 28 (19.2) | 0 | 0 | 28 (30.4) |
| Sex | ||||
| Male | 64 (43.8) | 23 (63.9) | 10 (55.6) | 31 (33.7) |
| Female | 82 (56.2) | 13 (36.1) | 8 (44.4) | 61 (63.3) |
| Occupation (family members: ≥12 years of age, n = 92) | ||||
| None | 58 (63.0) | 58 (63.0) | ||
| Employed | 34 (37.0) | 34 (37.0) | ||
| Level of education (family members: ≥12 years of age, n = 92) | ||||
| None | 10 (10.9) | 10 (10.9) | ||
| Primary education | 15 (16.3) | 15 (16.3) | ||
| Lower secondary education | 40 (43.5) | 40 (43.5) | ||
| Upper secondary education | 23 (25.0) | 23 (25.0) | ||
| Academic degree | 4 (4.3) | 4 (4.3) | ||
| Smoking habit (family members: ≥12 years of age, n = 92) | ||||
| Non-smokers | 48 (52.2) | 48 (52.2) | ||
| Passive smokers (n = 48) | 16 (33.3) | 16 (33.3) | ||
| Active smokers | 44 (47.8) | 44 (47.8) | ||
| Age of starting smoking (year; median (IQR)) | 15 (4.5) | 15 (4.5) | ||
| Number of cigarettes/cigars (n/day; median (IQR)) | 15 (45) | 15 (45) | ||
| Physical activity | ||||
| Hours/week (n; median (IQR)) | 3 (3) | 2 (1) | 2 (1) | 5 (6) |
| Yes | 23 (15.7) | 8 (22.2) | 4 (22.2) | 11 (12.0) |
| Clinical features | ||||
| Pre-existing diseases | 78 (53.4) | 8 (22.2) | 2 (11.1) | 68 (73.9) |
| Diabetes | 2 (2.6) | 0 | 0 | 2 (2.9) |
| Hypertension | 22 (28.2) | 0 | 0 | 22 (32.4) |
| Heart diseases | 5 (6.4) | 0 | 0 | 5 (7.3) |
| Chronic bronchitis/emphysema | 8 (10.2) | 0 | 0 | 8 (11.8) |
| Other | 41 (52.6) | 8 (100.0) # | 2 (100.0) # | 31 (45.6) |
| Vaccination status | ||||
| Pneumococcal | 46 (31.5) | 31 (86.1) ∆ | 13 (72.2) ∆∆ | 2 (2.2) ∆∆∆ |
| Complete vaccination schedule (n = 46) | 41 (89.1) | 30 (96.8) | 10 (76.9) | 1 (50.0) |
| Hexavalent | 55 (38.7) | 34 (94.4) | 18 (100.0) | 3 (3.3) |
| MMR | 54 (37.0) | 33 (91.7) | 18 (100.0) | 3 (3.3) |
| Varicella | 47 (32.2) | 29 (80.6) | 15 (83.3) | 3 (3.3) |
| Meningococcal C | 13 (8.9) | 5 (13.9) | 7 (38.9) | 1 (1.1) |
| Influenza (last 12 months) | 8 (5.5) | 1 (2.8) | 0 | 7 (7.6) |
| Study Population | Pneumococcal Colonization (lytA-pos) | p-Value | Odds Ratio (95% CI) | |
|---|---|---|---|---|
| No | Yes | |||
| Study participants (n = 146), (n (%)) | 50 (34.2) | 96 (65.8) | ||
| Schoolchildren | 8 (22.2) | 28 (77.8) | 0.084 | 2.161 (0.901–5.185) |
| Family members | 42 (38.2) | 68 (61.8) | (reference) | |
| Age-group (years) | ||||
| 5–6 | 8 (36.4) | 14 (63.6) | 0.833 | 1.132 (0.357–3.587) |
| 7 | 1 (11.1) | 8 (88.9) | 0.145 | 5.176 (0.566–47.323) |
| 8–11 | 5 (21.7) | 18 (78.3) | 0.184 | 2.329 (0.669–8.112) |
| 12–25 | 2 (16.7) | 10 (83.3) | 0.175 | 3.235 (0.593–17.658) |
| 26–49 | 23 (44.2) | 29 (55.8) | 0.670 | 0.816 (0.320–2.079) |
| ≥50 | 11 (39.3) | 17 (60.7) | (reference) | |
| Sex | ||||
| Male | 23 (35.9) | 41 (64.1) | (reference) | |
| Female | 27 (32.9) | 55 (67.1) | 0.704 | 1.143 (0.574–2.273) |
| Crowding index | ||||
| Low | 37 (40.2) | 55 (59.8) | (reference) | |
| High | 13 (24.1) | 41 (75.9) | 0.049 | 2.121 (1.002–4.492) |
| Smoking habit (family members: ≥12 years of age, n = 92) | ||||
| Non-smokers | 23 (46.0) | 27 (54.0) | (reference) | |
| Active smokers | 13 (30.9) | 29 (69.1) | 0.143 | 1.900 (0.805–4.484) |
| Number of cigarettes/cigars per day | ||||
| <5 cigarettes/day | 22 (39.3) | 34 (60.7) | (reference) | |
| ≥5 cigarettes/day | 14 (38.9) | 22 (61.1) | 0.970 | 1.017 (0.431–2.399) |
| <10 cigarettes/day | 28 (44.4) | 35 (55.6) | (reference) | |
| ≥10 cigarettes/day | 8 (27.6) | 21 (72.4) | 0.127 | 2.100 (0.809–5.451) |
| <15 cigarettes/day | 32 (43.8) | 41 (56.2) | (reference) | |
| ≥15 cigarettes/day | 4 (21.0) | 15 (79.0) | 0.078 | 2.927 (0.885–9.678) |
| <20 cigarettes/day | 33 (43.4) | 43 (56.6) | (reference) | |
| ≥20 cigarettes/day | 3 (18.8) | 13 (81.2) | 0.078 | 3.326 (0.875–12.634) |
| Physical activity | ||||
| Yes | 9 (39.1) | 14 (60.9) | (reference) | |
| No | 41 (33.3) | 82 (66.7) | 0.591 | 1.286 (0.514–3.218) |
| Pre-existing respiratory diseases | ||||
| No | 30 (38.5) | 48 (61.5) | (reference) | |
| Yes | 20 (29.4) | 48 (70.6) | 0.251 | 1.500 (0.750–2.999) |
| ID Family Number | Student Age (Reference Case) | Household Size | Intrafamilial Sharing (%) |
|---|---|---|---|
| 14 | 8 | 2 | 100.0 |
| 23 | 9 | 5 | 90.9 |
| 2 | 8 | 5 | 90.5 |
| 11 | 6 | 4 | 84.6 |
| 34 | 7 | 7 | 81.2 |
| 12 | 8 | 3 | 75.0 |
| 25 | 6 | 3 | 75.0 |
| 33 | 9 | 2 | 75.0 |
| 16 | 7 | 7 | 71.4 |
| 3 | 9 | 5 | 70.8 |
| 20 | 8 | 3 | 69.2 |
| 6 | 8 | 4 | 68.7 |
| 28 | 11 | 4 | 68.7 |
| 19 | 8 | 9 | 65.2 |
| 26 | 7 | 3 | 60.0 |
| 27 | 6 | 5 | 50.0 |
| 10 | 7 | 5 | 47.0 |
| 30 | 8 | 4 | 46.7 |
| 5 | 7 | 3 | 44.4 |
| 21 | 6 | 5 | 42.9 |
| 17 | 8 | 4 | 25.0 |
| 31 | 7 | 4 | 18.2 |
| 1 | 8 | 5 | 0 |
| 4 | 6 | 2 | 0 |
| 7 | 6 | 4 | 0 |
| 8 | 6 | 3 | 0 |
| 9 | 7 | 3 | 0 |
| 13 | 7 | 4 | 0 |
| 15 | 6 | 6 | 0 |
| 18 | 6 | 3 | 0 |
| 22 | 8 | 1 | 0 |
| 24 | 8 | 5 | 0 |
| 29 | 6 | 3 | 0 |
| 32 | 8 | 5 | 0 |
| 35 | 6 | 2 | 0 |
| 36 | 6 | 4 | 0 |
| Intrafamilial Sharing | p-Value | Odds Ratio (95% CI) | ||
|---|---|---|---|---|
| Low | High | |||
| Family clusters (n = 36), (n (%)) | 21 (58.3) | 15 (41.7) | ||
| Crowding index | ||||
| Low | 15 (62.5) | 9 (37.5) | (reference) | |
| High | 6 (50.0) | 6 (50.0) | 0.475 | 1.667 (0.410–6.767) |
| Household size | ||||
| ≤2 persons | 3 (60.0) | 2 (40.0) | (reference) | |
| >2 persons | 18 (58.1) | 13 (41.9) | 0.935 | 1.083 (0.158–7.435) |
| ≤3 persons | 9 (60.0) | 6 (40.0) | (reference) | |
| >3 persons | 12 (57.1) | 9 (42.9) | 0.864 | 1.125 (0.292–4.326) |
| ≤4 persons | 15 (65.2) | 8 (34.8) | (reference) | |
| >4 persons | 6 (46.1) | 7 (53.9) | 0.269 | 2.188 (0.546–8.761) |
| ≤5 persons | 20 (62.5) | 12 (37.5) | (reference) | |
| >5 persons | 1 (25.0) | 3 (75.0) | 0.184 | 5.000 (0.466–53.682) |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tramuto, F.; Amodio, E.; Calamusa, G.; Restivo, V.; Costantino, C.; Vitale, F.; On behalf of the BINOCOLO Group. Pneumococcal Colonization in the Familial Context and Implications for Anti-Pneumococcal Immunization in Adults: Results from the BINOCOLO Project in Sicily. Int. J. Mol. Sci. 2017, 18, 105. https://doi.org/10.3390/ijms18010105
Tramuto F, Amodio E, Calamusa G, Restivo V, Costantino C, Vitale F, On behalf of the BINOCOLO Group. Pneumococcal Colonization in the Familial Context and Implications for Anti-Pneumococcal Immunization in Adults: Results from the BINOCOLO Project in Sicily. International Journal of Molecular Sciences. 2017; 18(1):105. https://doi.org/10.3390/ijms18010105
Chicago/Turabian StyleTramuto, Fabio, Emanuele Amodio, Giuseppe Calamusa, Vincenzo Restivo, Claudio Costantino, Francesco Vitale, and On behalf of the BINOCOLO Group. 2017. "Pneumococcal Colonization in the Familial Context and Implications for Anti-Pneumococcal Immunization in Adults: Results from the BINOCOLO Project in Sicily" International Journal of Molecular Sciences 18, no. 1: 105. https://doi.org/10.3390/ijms18010105







