The Diagnostic Usefulness of Serum Total Bile Acid Concentrations in the Early Phase of Acute Pancreatitis of Varied Etiologies
Abstract
:1. Introduction
2. Results
2.1. Differences Related to Acute Pancreatitis Etiology
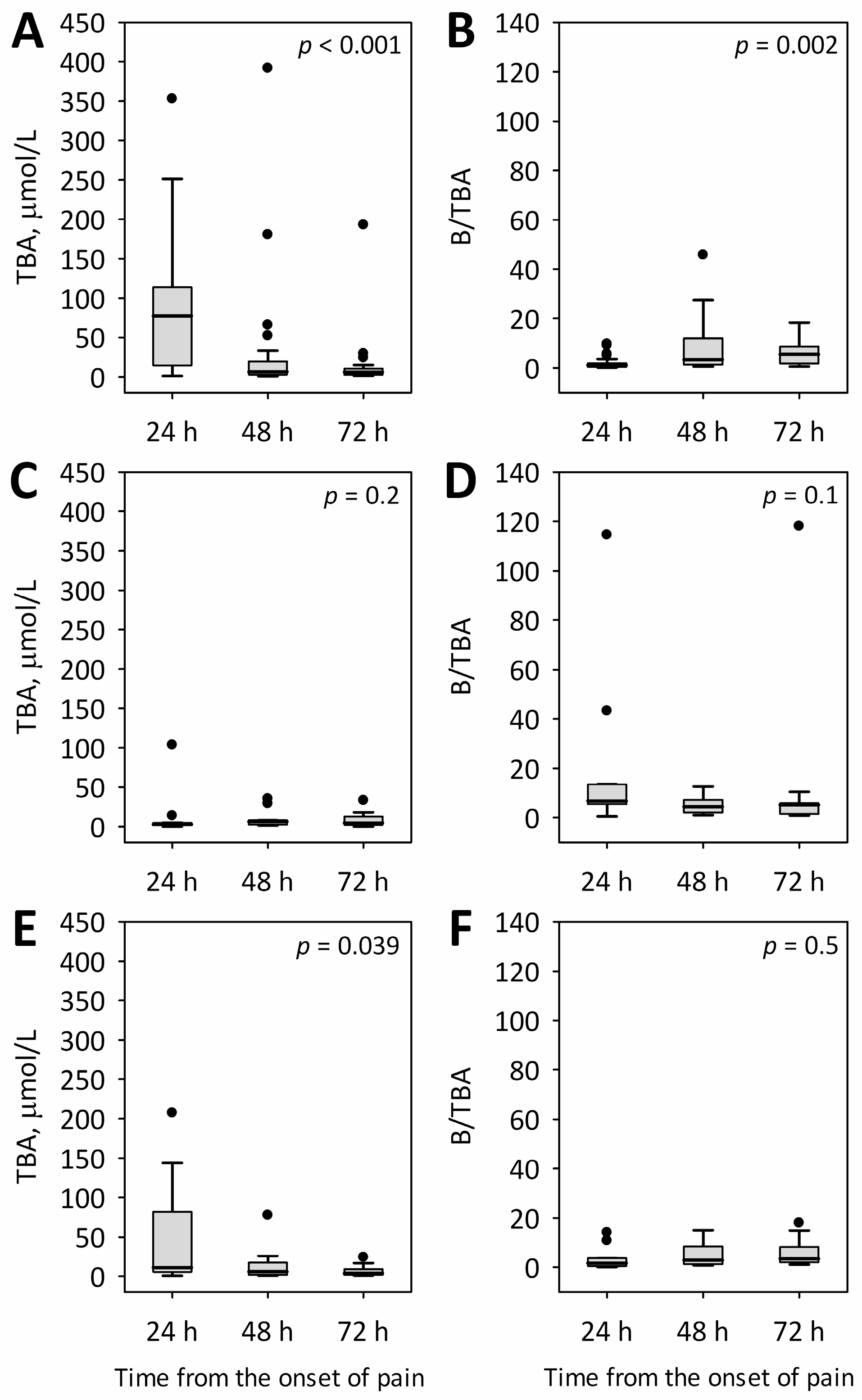
2.2. Changes in Total Bile Acid Concentrations and Serum Bilirubin-to-Total Bile Acids Ratio during the First 72 h of Acute Pancreatitis of Various Etiologies
2.3. The Relationships between Total Bile Acid Concentrations and the Markers Related to Etiology and Severity of Acute Pancreatitis
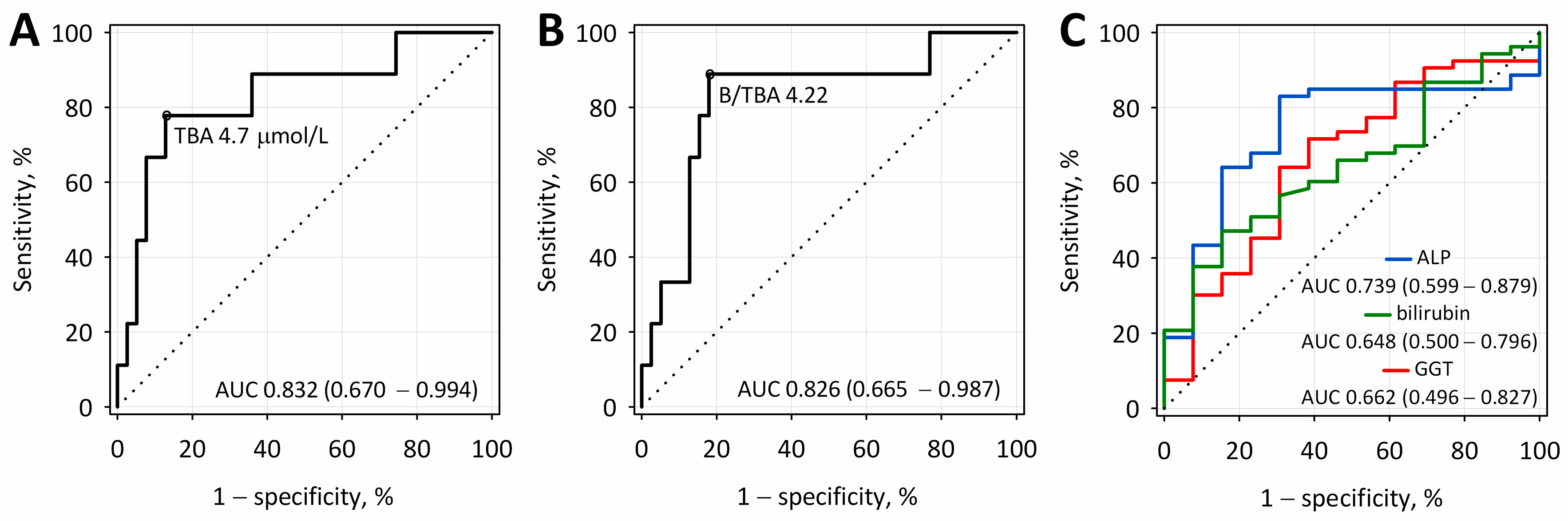
2.4. The Diagnostic Accuracy of Total Bile Acid Concentrations and Bilirubin-to-Total Bile Acids Ratio for Differentiating between Biliary and Other Etiologies of Acute Pancreatitis
3. Discussion
4. Material and Methods
4.1. Patients and Laboratory Tests
4.2. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hammer, H.F. An update on pancreatic pathophysiology (Do we have to rewrite pancreatic pathophysiology?). Wien. Med. Wochenschr. 2014, 164, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Cieszkowski, J.; Warzecha, Z.; Dembiński, A. Experimental models of acute pancreatitis. Postep. Hig. Med. Dosw. 2015, 69, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Tomaszewska, R.; Stachura, J.; Konturek, S.J.; Konturek, P.C. Ghrelin attenuates the development of acute pancreatitis in rats. J. Physiol. Pharmacol. 2003, 54, 561–573. [Google Scholar] [PubMed]
- Wang, G.J.; Gao, C.F.; Wei, D.; Ding, S.Q. Acute pancreatitis: Etiology and common pathogenesis. World J. Gastroenterol. 2009, 15, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Cieszkowski, J.; Warzecha, Z.; Kuśnierz-Cabala, B.; Dembiński, A. The beginnings of pancreatology as a field of experimental and clinical medicine. BioMed Res. Int. 2015, 2015, 128095. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, R.; Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Stachura, J. Morphological changes and morphological-functional correlations in acute experimental ischemia/ reperfusion pancreatitis in rats. Pol. J. Pathol. 2000, 51, 179–184. [Google Scholar] [PubMed]
- Yuksel, M.; Yildar, M.; Bulbul, E. The role of MRCP on management of the acute biliary pancreatitis. MSD 2015, 2, 319–322. [Google Scholar] [CrossRef]
- Takuma, K.; Kamisawa, T.; Hara, S.; Tabata, T.; Kuruma, S.; Chiba, K.; Kuwata, G.; Fujiwara, T.; Egashira, H.; Koizumi, K.; et al. Etiology of recurrent acute pancreatitis, with special emphasis on pancreatobiliary malformation. Adv. Med. Sci. 2012, 57, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.B.; Draganov, P. Is empiric cholecystectomy a reasonable treatment option for idiopathic acute pancreatitis? Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 356–357. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.S.; Bhasin, D.K.; Rao, C.; Singh, K. Role of endoscopic ultrasound in idiopathic acute pancreatitis with negative ultrasound, computed tomography, and magic resonance cholangiopancreatography. Ann. Gastroenterol. 2012, 2, 133–137. [Google Scholar]
- Venneman, N.G.; Brummelen, S.E.; Berge-Henegouwen, G.P.; Erprcum, K.J. Microlithiasis: An impotent cause of “idopathic” acute pancreatitis. Ann. Hepatol. 2003, 2, 30–35. [Google Scholar] [PubMed]
- Ng, W.K.; Tarabain, O. Pancreas divisum: A cause of idiopathic acute pancreatitis. CMAJ 2009, 180, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R. Acute recurrent pancreatitis: An autoimmune disease? World J. Gastroenterol. 2008, 14, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Bohara, T.P.; Parajuli, A.; Joshi, M.R. Role of biochemical investigation in prediction of biliary etiology in acute pancreatitis. J. Nepal. Med. Assoc. 2013, 52, 229–232. [Google Scholar]
- Gungor, B.; Caglayan, K.; Polat, C.; Seren, D.; Erzurumlu, K.; Malazgirt, Z. The predictivity of serum biochemical markers in acute pancreatitis. IRSN Gastroenterol. 2011, 2011, 279607. [Google Scholar] [CrossRef]
- Hebanowska, A. Biosynteza kwasów żółciowych i jej regulacja. Post. Hig. Med. Dosw. 2010, 64, 544–554. [Google Scholar]
- Norlin, M.; Wikvall, K. Enzymes in the conversion of cholesterol into bile AIDS. Curr. Mol. Med. 2007, 7, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Fracchia, M.; Pellegrino, S.; Secreto, P.; Gallo, L.; Masoero, G.; Pera, A.; Galatala, G. Biliary lipid composition in cholesterol microlithiasis. Gut 2001, 48, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Zasada, J.; Panek, J.; Dolecki, M.; Biesiada, Z.; Dembiński, M. Bile infection and bile acids concentration in bile in acute biliary pancreatitis. Pol. Przegl. Chir. 2009, 12, 655–669. [Google Scholar] [CrossRef]
- Venglovecz, V.; Rakoczay, Z., Jr.; Hegyi, P. The effects of bile acids on pancreatic ductal cells. Panceapedia 2012. [Google Scholar] [CrossRef]
- Wu, B.U.; Johannes, R.S.; Sun, X.; Tabak, Y.; Conwell, D.L.; Banks, P. The early prediction of mortality in acute pancreatitis: A large population-based study. Gut 2008, 57, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kuśnierz-Cabala, B.; Tomaszewska, R. Obestatin Accelerates the Recovery in the Course of Ischemia/Reperfusion-Induced Acute Pancreatitis in Rats. PLoS ONE 2015, 10, e0134380. [Google Scholar] [CrossRef] [PubMed]
- Perez-Mateo, M. How we predict the etiology of acute pancreatitis. JOP 2006, 7, 257–261. [Google Scholar] [PubMed]
- Tenner, S.; Baillie, J.; DeWitt, J.; Swaroop, S. American College of Gastroenterology Guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef] [PubMed]
- McMohan, M.J.; Pickford, I.R. Biochemical prediction of gallstones early in an attack of acute pancreatitis. Lancet 1979, 15, 541–543. [Google Scholar] [CrossRef]
- Liu, C.L.; Fan, S.T.; Lo, C.M.; Tso, W.K.; Wong, Y.; Poon, R.T.; Lam, C.M.; Wong, B.C.; Wong, J. Clinico-biochemical prediction of biliary cause of acute pancreatitis in the era of endoscopic ultrasonography. Aliment. Pharmacol. Ther. 2005, 22, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.; Boruchowicz, A.; Hastier, P.; Pariente, A.; Thevenot, T.; Frossard, J.L.; Buscail, L.; Mauvais, F.; Duchmann, J.C.; Courrier, A.; et al. Diagnostic criteria in predicting a biliary origin of acute pancreatitis in the era of endoscopic ultrasound: Multicentre prospective evaluation of 213 patients. Pancreatology 2005, 5, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]

| Biliary AP (N = 38) | Alcoholic AP (N = 13) | Other Etiology of AP (N = 15) | p | Reference Range 1 | |
|---|---|---|---|---|---|
| Age, years | 65 ± 17 | 45 ± 19 | 66 ± 14.5 | 0.007 | - |
| Duration of hospital stay, days | 7 ± 3 | 14 ± 12 | 9 ± 8 | 0.6 | - |
| Male sex, N (%) | 15 (39) | 13 (100) | 6 (40) | 0.005 | - |
| MAP, N (%) MSAP, N (%) SAP, N (%) | 28 (74) 8 (21) 2 (5) | 8 (62) 3 (23) 2 (15) | 10 (67) 4 (27) 1 (7) | 0.8 | - |
| Pre-existing comorbidities, N (%) | 36 (95) | 6 (46) | 9 (60) | <0.001 | - |
| Pre-existing cholelithiasis, N (%) | 34 (89) | 2 (13) | 0 | <0.001 | - |
| Pancreatic necrosis, N (%) | 0 | 2 (15) | 1 (7) | 0.064 | - |
| Peripancreatic fluid collections, N (%) | 0 | 2 (15) | 3 (20) | 0.022 | - |
| Transient organ failure, N (%) | 6 (16) | 0 | 1 (7) | 0.2 | - |
| Persistent organ failure, N (%) | 2 (5) | 2 (15) | 1 (7) | 0.5 | - |
| ERCP, N (%) | 4 (11) | 1 (8) | 1 (7) | 0.9 | - |
| BISAP ≥ 3 | 3 (8) | 2 (15) | 1 (7) | 0.7 | - |
| Death, N (%) | 1 (3) | 1 (8) | 1 (7) | 0.7 | - |
| TBA, µmol/L | 86.6 ± 86.8 | 14.7 ± 33.5 | 47.0 ± 69.1 | 0.003 | 2–10 |
| Bilirubin, µmol/L | 59.2 ± 39.2 | 31.7 ± 19.5 | 33.6 ± 32.1 | 0.004 | 0–21 |
| B/TBA ratio | 5.95 ± 21.18 | 22.48 ± 36.75 | 3.49 ± 4.86 | 0.008 | - |
| CRP, mg/L | 37.0 ± 57.7 | 95.0 ± 108.1 | 56.674.5 | 0.1 | 0–5 |
| Albumin, g/L | 40.0 ± 5.3 | 39.4 ± 5.1 | 39.1 ± 5.0 | 0.8 | 35-52 |
| WBC, ×103/µL | 13.3 ± 7.95 | 13.7 ± 4.37 | 10.8 ± 3.68 | 0.2 | 4–10 |
| HCT, % | 41.9 ± 5.3 | 44.3 ± 4.7 | 42.1 ± 4.3 | 0.2 | F: 37–47 M: 40–54 |
| Amylase, U/L | 1443.1 ± 1005.1 | 911.4 ± 722.0 | 1526.3 ± 896.6 | 0.07 | 28–100 |
| GGT, U/L | 599.5 ± 444.3 | 309.2 ± 337.0 | 332.2 ± 406.3 | 0.01 | F: 5–36 M: 8–61 |
| ALP, U/L | 217.9 ± 151.7 | 100.1 ± 65.6 | 142.7 ± 175.3 | <0.001 | F: 35–104 M: 40–129 |
| ALT, U/L | 314.5 ± 265.3 | 188.3 ± 475.3 | 169.0 ± 213.5 | 0.002 | F: 5–33 M: 5–41 |
| AST, U/L | 257.7 ± 205.3 | 211.4 ± 528.2 | 150.1 ± 194.6 | 0.008 | F: 5–32 M: 5–40 |
| Glucose, mmol/L | 9.0 ± 2.8 | 8.66 ± 3.3 | 7.80 ± 2.9 | 0.3 | 3.3–5.6 |
| Urea, mmol/L | 7.10 ± 3.14 | 5.64 ± 4.39 | 6.49 ± 3.17 | 0.049 | 2.76–8.07 |
| Creatinine µmol/L | 88.7 ± 31.6 | 94.98 ± 54.5 | 89.1 ± 46.3 | 0.5 | F: 44–80 M: 62–106 |
| Total calcium, mmol/L | 2.33 ± 0.19 | 2.19 ± 0.27 | 2.31 ± 0.13 | 0.5 | 2.15–2.55 |
| Variables | Spearman’s Rank Correlation Coefficients; p < 0.05 | |
|---|---|---|
| TBA | B/TBA Ratio | |
| Bilirubin | 0.56 | −0.25 |
| ALT | 0.39 | −0.43 |
| AST | 0.42 | −0.58 |
| ALP | 0.54 | −0.41 |
| GGT | 0.51 | −0.37 |
| Amylase | 0.30 | −0.40 |
| Parameter | Cut-Off Value | Diagnostic Sensitivity, % | Diagnostic Specificity, % | Diagnostic Accuracy, % | PPV, % | NPV, % |
|---|---|---|---|---|---|---|
| TBA, µmol/L | 4.7 | 79 | 87 | 85 | 58 | 94 |
| B/TBA | 4.22 | 89 | 82 | 83 | 53 | 97 |
| ALP, U/L | 79.8 | 83 | 69 | 80 | 92 | 50 |
| Bilirubin, µmol/L | 40 | 47 | 85 | 54 | 92 | 28 |
| GGT, U/L | 282 | 64 | 69 | 65 | 89 | 32 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maleszka, A.; Dumnicka, P.; Matuszyk, A.; Pędziwiatr, M.; Mazur-Laskowska, M.; Sporek, M.; Ceranowicz, P.; Olszanecki, R.; Kuźniewski, M.; Kuśnierz-Cabala, B. The Diagnostic Usefulness of Serum Total Bile Acid Concentrations in the Early Phase of Acute Pancreatitis of Varied Etiologies. Int. J. Mol. Sci. 2017, 18, 106. https://doi.org/10.3390/ijms18010106
Maleszka A, Dumnicka P, Matuszyk A, Pędziwiatr M, Mazur-Laskowska M, Sporek M, Ceranowicz P, Olszanecki R, Kuźniewski M, Kuśnierz-Cabala B. The Diagnostic Usefulness of Serum Total Bile Acid Concentrations in the Early Phase of Acute Pancreatitis of Varied Etiologies. International Journal of Molecular Sciences. 2017; 18(1):106. https://doi.org/10.3390/ijms18010106
Chicago/Turabian StyleMaleszka, Aleksandra, Paulina Dumnicka, Aleksandra Matuszyk, Michał Pędziwiatr, Małgorzata Mazur-Laskowska, Mateusz Sporek, Piotr Ceranowicz, Rafał Olszanecki, Marek Kuźniewski, and Beata Kuśnierz-Cabala. 2017. "The Diagnostic Usefulness of Serum Total Bile Acid Concentrations in the Early Phase of Acute Pancreatitis of Varied Etiologies" International Journal of Molecular Sciences 18, no. 1: 106. https://doi.org/10.3390/ijms18010106







