Influence of Anode Potentials on Current Generation and Extracellular Electron Transfer Paths of Geobacter Species
Abstract
:1. Introduction
2. Results and Discussions
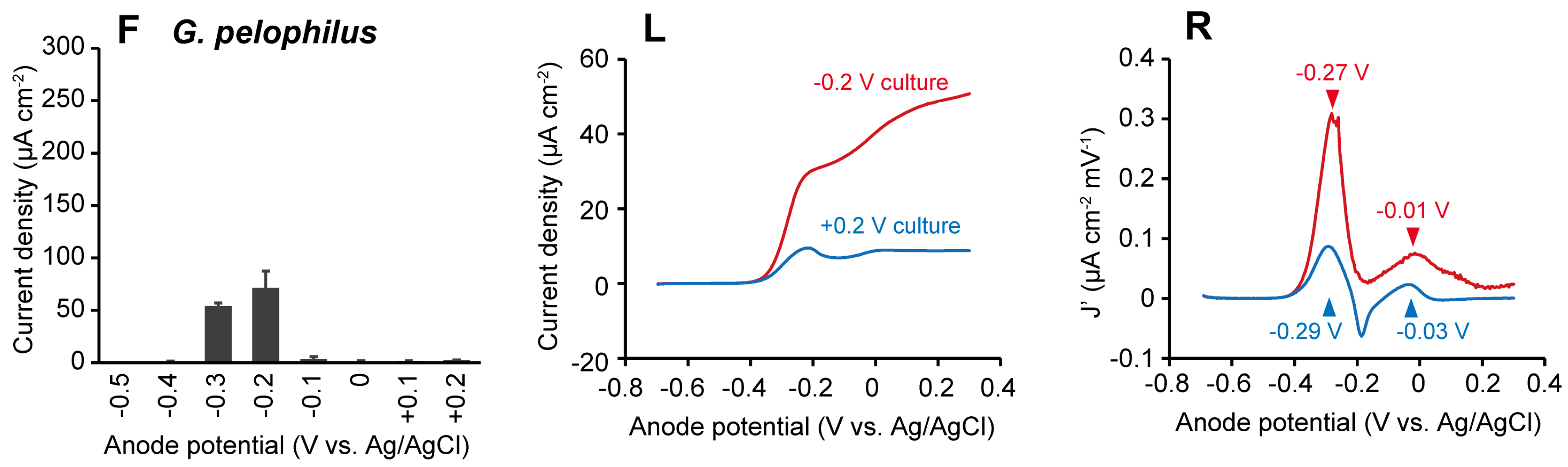
2.1. The Effects of Anode Potentials on Current Generation by Geobacter Species
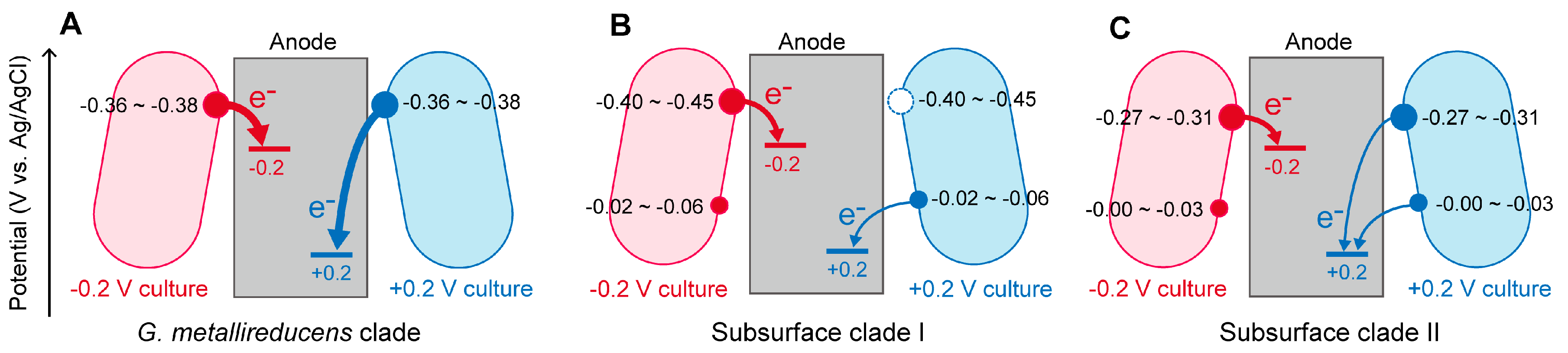
2.2. The Effects of Anode Potentials on EET Paths of Geobacter Species
2.3. Implications
3. Materials and Methods
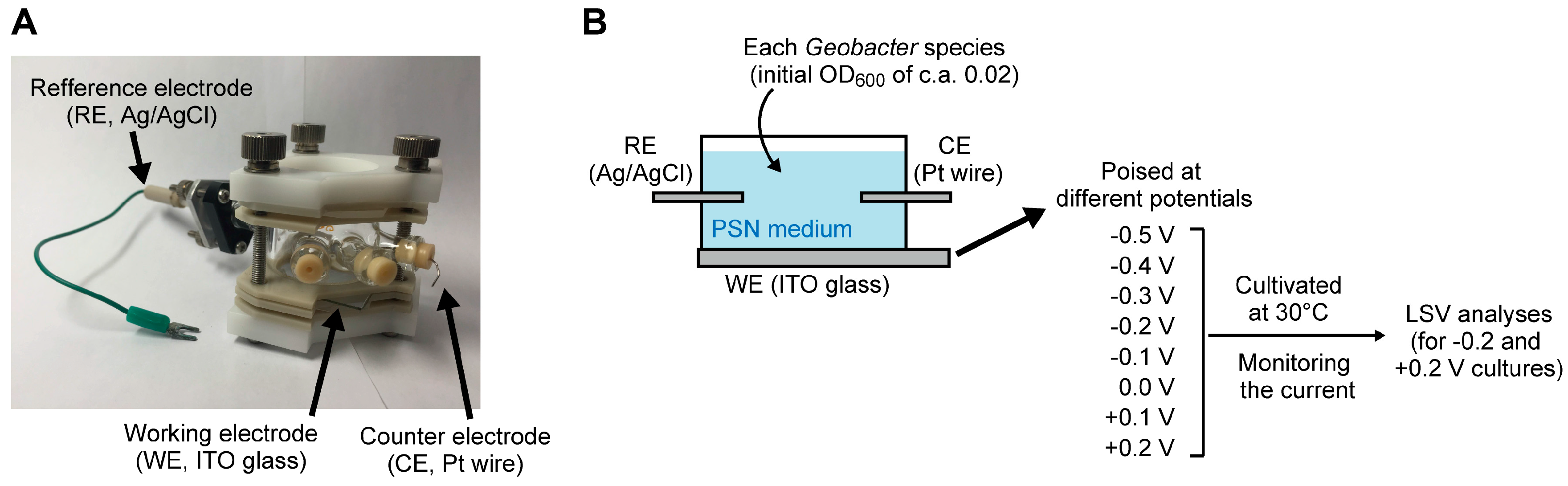
3.1. Bacterial Strains and Culture Conditions
3.2. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
Abbreviations
| EET | extracellular electron transfer |
| OMC | outer membrane c-type cytochrome |
| MFC | microbial fuel cell |
| BES | bioelectrochemical system |
| ITO | tin-doped indium oxide |
| LSV | linear sweep voltammetry |
References
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microbial. Physiol. 2004, 49, 219–286. [Google Scholar]
- Gralnick, J.A.; Newman, D.K. Extracellular respiration. Mol. Microbiol. 2007, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Squier, T.C.; Zachara, J.M.; Fredrickson, J.K. Respiration of metal (hydr)oxides by Shewanella and Geobacter: A key role for multihaem c-type cytochromes. Mol. Microbiol. 2007, 65, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. Biotechnological aspects of microbial extracellular electron transfer. Microbes Environ. 2015, 30, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, P.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Ren, N.; Wang, H.; Lee, H.; Li, N.; Zhao, Q. Accelerated start-up of two-chambered microbial fuel cells: Effect of anodic positive poised potential. Electrochim. Acta 2009, 54, 1109–1114. [Google Scholar] [CrossRef]
- Kumar, A.; Katuri, K.; Lens, P.; Leech, D. Does bioelectrochemical cell configuration and anode potential affect biofilm response? Biochem. Soc. Trans. 2012, 40, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Freguia, S.; Keller, J.; Verstraete, W.; Rabaey, K. The anode potential regulates bacterial activity in microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I.; Krajmalnik-Brown, R.; Parameswaran, P.; Marcus, A.K.; Wanger, G.; Gorby, Y.A.; Rittmann, B.E. Selecting anode-respiring bacteria based on anode potential: Phylogenetic, electrochemical, and microscopic characterization. Environ. Sci. Technol. 2009, 43, 9519–9524. [Google Scholar] [CrossRef] [PubMed]
- Rismani-Yazdi, H.; Christy, A.D.; Carver, S.M.; Yu, Z.; Dehority, B.A.; Tuovinen, O.H. Effect of external resistance on bacterial diversity and metabolism in cellulose-fed microbial fuel cells. Bioresour. Technol. 2011, 102, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Regan, J.M. Influence of external resistance on electrogenesis, methanogenesis, and anode prokaryotic communities in microbial fuel cells. Appl. Environ. Microbiol. 2011, 77, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yates, M.D.; Hatzell, M.C.; Ananda Rao, H.; Saikaly, P.E.; Logan, B.E. Microbial community composition is unaffected by anode potential. Environ. Sci. Technol. 2014, 48, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Commault, A.S.; Lear, G.; Packer, M.A.; Weld, R.J. Influence of anode potentials on selection of Geobacter strains in microbial electrolysis cells. Bioresour. Technol. 2013, 139, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Suzuki, S.; Norden-Krichmar, T.M.; Phan, T.; Wanger, G.; Nealson, K.H.; Sekiguchi, Y.; Gorby, Y.A.; Bretschger, O. Microbial population and functional dynamics associated with surface potential and carbon metabolism. ISME J. 2014, 8, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Suzuki, S.; Tenney, A.; Norden-Krichmar, T.M.; Nealson, K.H.; Bretschger, O. Microbial metabolic networks in a complex electrogenic biofilm recovered from a stimulus-induced metatranscriptomics approach. Sci. Rep. 2015, 5, 14840. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Marsili, E.; Flickinger, M.C.; Bond, D.R. Electrochemical characterization of Geobacter sulfurreducens cells immobilized on graphite paper electrodes. Biotechnol. Bioeng. 2008, 99, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Lu, H.; Hashimoto, K.; Nakanishi, S. Potential and cell density dependence of extracellular electron transfer of anode-respiring Geobacter sulfurreducens cells. Electrochemistry 2012, 80, 330–333. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Cao, X.; Huang, X. A new insight into potential regulation on growth and power generation of Geobacter sulfurreducens in microbial fuel cells based on energy viewpoint. Environ. Sci. Technol. 2010, 44, 3187–3191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yates, M.D.; Logan, B.E. Set potential regulation reveals additional oxidation peaks of Geobacter sulfurreducens anodic biofilms. Electrochem. Commun. 2012, 22, 116–119. [Google Scholar] [CrossRef]
- Liu, H.; Matsuda, S.; Kato, S.; Hashimoto, K.; Nakanishi, S. Redox-responsive switching in bacterial respiratory pathways involving extracellular electron transfer. ChemSusChem 2010, 3, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Martínez, A.; Harnisch, F.; Kuhlicke, U.; Neu, T.R.; Schröder, U. Electron transfer and biofilm formation of Shewanella putrefaciens as function of anode potential. Bioelectrochemistry 2013, 93, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.N.; Garcia, K.E.; Luckarift, H.R.; Falase, A.; Cornejo, J.; Babanova, S.; Schuler, A.J.; Johnson, G.R.; Atanassov, P.B. Applied electrode potential leads to Shewanella oneidensis MR-1 biofilms engaged in direct electron transfer. J. Electrochem. Soc. 2013, 160, H866–H871. [Google Scholar] [CrossRef]
- Matsuda, S.; Liu, H.; Kato, S.; Hashimoto, K.; Nakanishi, S. Negative faradaic resistance in extracellular electron transfer by anode-respiring Geobacter sulfurreducens cells. Environ. Sci. Technol. 2011, 45, 10163–10169. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Liu, H.; Kouzuma, A.; Watanabe, K.; Hashimoto, K.; Nakanishi, S. Electrochemical gating of tricarboxylic acid cycle in electricity-producing bacterial cells of Shewanella. PLoS ONE. 2013, 8, e72901. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.D.; Regan, J.M. Changes in phosphorylation of adenosine phosphate and redox state of nicotinamide-adenine dinucleotide (phosphate) in Geobacter sulfurreducens in response to electron acceptor and anode potential variation. Bioelectrochemistry 2015, 106, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sasaki, D.; Sasaki, K.; Kato, S.; Kondo, A.; Hashimoto, K.; Nakanishi, S. Comprehensive metabolomic analyses of anode-respiring Geobacter sulfurreducens cells: The impact of anode-respiration activity on intracellular metabolite levels. Process Biochem. 2016, 51, 34–38. [Google Scholar] [CrossRef]
- Holmes, D.E.; O’Neil, R.A.; Vrionis, H.A.; N’guessan, L.A.; Ortiz-Bernad, I.; Larrahondo, M.J.; Adams, L.A.; Ward, J.A.; Nicoll, J.S.; Nevin, K.P.; et al. Subsurface clade of Geobacteraceae that predominates in a diversity of Fe(III)-reducing subsurface environments. ISME J. 2007, 1, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Nakamura, R.; Kai, F.; Watanabe, K.; Hashimoto, K. Respiratory interactions of soil bacteria with (semi)conductive iron-oxide minerals. Environ. Microbiol. 2010, 12, 3114–3123. [Google Scholar] [CrossRef] [PubMed]
- Nercessian, O.; Parot, S.; Delia, M.L.; Bergel, A.; Achouak, W. Harvesting electricity with Geobacter bremensis isolated from compost. PLoS ONE 2012, 7, e34216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevin, K.P.; Kim, B.C.; Glaven, R.H.; Johnson, J.P.; Woodard, T.L.; Methé, B.A.; Didonato, R.J.; Covalla, S.F.; Franks, A.E.; Liu, A.; et al. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS ONE 2009, 4, e5628. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Link between capacity for current production and syntrophic growth in Geobacter species. Front. Microbiol. 2015, 6, 744. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Holmes, D.E.; Woodard, T.L.; Hinlein, E.S.; Ostendorf, D.W.; Lovley, D.R. Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., two novel Fe(III)-reducing subsurface isolates. Int. J. Syst. Evol. Microbiol. 2005, 55, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Iron-oxide minerals affect extracellular electron-transfer paths of Geobacter spp. Microbes Environ. 2013, 28, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Young, N.D.; Lovley, D.R. Evolution of electron transfer out of the cell: Comparative genomics of six Geobacter genomes. BMC Genom. 2010, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Thamdrup, B. Bacterial manganese and iron reduction in aquatic sediments. In Advances in Microbial Ecology; Schink, B., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2000; Volume 16, pp. 41–84. [Google Scholar]
- Hori, T.; Müller, A.; Igarashi, Y.; Conrad, R.; Friedrich, M.W. Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. ISME J. 2010, 4, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Hori, T.; Aoyagi, T.; Itoh, H.; Narihiro, T.; Oikawa, A.; Suzuki, K.; Ogata, A.; Friedrich, M.W.; Conrad, R.; Kamagata, Y. Isolation of microorganisms involved in reduction of crystalline iron(III) oxides in natural environments. Front. Microbiol. 2015, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. USA 2012, 109, 10042–10046. [Google Scholar] [CrossRef]

© 2017 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, S. Influence of Anode Potentials on Current Generation and Extracellular Electron Transfer Paths of Geobacter Species. Int. J. Mol. Sci. 2017, 18, 108. https://doi.org/10.3390/ijms18010108
Kato S. Influence of Anode Potentials on Current Generation and Extracellular Electron Transfer Paths of Geobacter Species. International Journal of Molecular Sciences. 2017; 18(1):108. https://doi.org/10.3390/ijms18010108
Chicago/Turabian StyleKato, Souichiro. 2017. "Influence of Anode Potentials on Current Generation and Extracellular Electron Transfer Paths of Geobacter Species" International Journal of Molecular Sciences 18, no. 1: 108. https://doi.org/10.3390/ijms18010108




