Perioperative Search for Circulating Tumor Cells in Patients Undergoing Prostate Brachytherapy for Clinically Nonmetastatic Prostate Cancer
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. HDR Brachytherapy and Blood Sample Collection
4.3. LDR Brachytherapy and Blood Sample Collection
4.4. CTC Detection
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LDR | low-dose-rate |
| HDR | high-dose-rate |
| CTC | circulating tumor cell |
| PSA | prostate-specific antigen |
| NCCN | National Comprehensive Cancer Network |
| TURP | transurethral resection of prostate |
| PSMA | prostate-specific membrane antigen |
| ADT | androgen deprivation therapy |
| IBCL | intraoperatively built custom-linked |
| EpCAM | expressing epithelial cell adhesion molecule |
| DAPI | 4′,6-diamidino-2-phenylindole |
References
- Zelefsky, M.J.; Kuban, D.A.; Levy, L.B.; Potters, L.; Beyer, D.C.; Blasko, J.C.; Moran, B.J.; Ciezki, J.P.; Zietman, A.L.; Pisansky, T.M.; et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.N.; Stock, R.G.; Cesaretti, J.A.; Unger, P. Local control following permanent prostate brachytherapy: Effect of high biologically effective dose on biopsy results and oncologic outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Rojas, A.M.; Bownes, P.J.; Lowe, G.J.; Ostler, P.J.; Bryant, L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother. Oncol. 2012, 103, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Prada, P.J.; Gonzalez, H.; Fernandez, J.; Jimenez, I.; Iglesias, A.; Romo, I. Biochemical outcome after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy: 12 Years of experience. BJU Int. 2012, 109, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, H.; Satoh, T.; Kitano, M.; Tabata, K.; Komori, S.; Ikeda, M.; Soda, I.; Kurosaka, S.; Sekiguchi, A.; Kimura, M.; et al. High-dose-rate brachytherapy and hypofractionated external beam radiotherapy combined with long-term hormonal therapy for high-risk and very high-risk prostate cancer: Outcomes after 5-year follow-up. J. Radiat. Res. 2014, 55, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Grimm, P.; Billiet, I.; Bostwick, D.; Dicker, A.P.; Frank, S.; Immerzeel, J.; Keyes, M.; Kupelian, P.; Lee, W.R.; Machtens, S.; et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy: Results from the prostate cancer results study group. BJU Int. 2012, 109, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Stock, R.G.; Stone, N.N.; Wesson, M.F.; DeWyngaert, J.K. A modified technique allowing interactive ultrasound-guided three-dimensional transperineal prostate implantation. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 219–225. [Google Scholar] [CrossRef]
- Ishiyama, H.; Satoh, T.; Kawakami, S.; Tsumura, H.; Komori, S.; Tabata, K.; Sekiguchi, A.; Takahashi, R.; Soda, I.; Takenaka, K.; et al. A prospective quasi-randomized comparison of intraoperatively built custom-linked seeds versus loose seeds for prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Suzuki, O.; Otani, Y.; Yoshida, K.; Nose, T.; Ogawa, K. High-dose-rate brachytherapy as monotherapy for prostate cancer: Technique, rationale and perspective. J. Contemp. Brachytherapy 2014, 6, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.C. High-dose-rate brachytherapy boost for prostate cancer: Rationale and technique. J. Contemp. Brachytherapy 2014, 6, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, A.; Ishiyama, H.; Satoh, T.; Tabata, K.; Komori, S.; Tsumura, H.; Kawakami, S.; Soda, I.; Iwamura, M.; Hayakawa, K. 125Iodine monotherapy for Japanese men with low- and intermediate-risk prostate cancer: Outcomes after 5 years of follow-up. J. Radiat. Res. 2014, 55, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Miki, K.; Kido, M.; Sasaki, H.; Nakamura, W.; Kijima, Y.; Kobayashi, M.; Egawa, S.; Kanehira, C. Analysis of prognostic factors in localized high-risk prostate cancer patients treated with HDR brachytherapy, hypofractionated 3D-CRT and neoadjuvant/adjuvant androgen deprivation therapy (trimodality therapy). J. Radiat. Res. 2014, 55, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.S.; Cisek, V.J.; Mulvihill, M.N.; Cohen, E.L. Role of transurethral resection in dissemination of cancer of prostate. Urology 1986, 28, 179–183. [Google Scholar] [CrossRef]
- Hayashi, N.; Egami, H.; Kai, M.; Kurusu, Y.; Takano, S.; Ogawa, M. No-touch isolation technique reduces intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer. Surgery 1999, 125, 369–374. [Google Scholar] [CrossRef]
- Wiggers, T.; Jeekel, J.; Arends, J.W.; Brinkhorst, A.P.; Kluck, H.M.; Luyk, C.I.; Munting, J.D.; Povel, J.A.; Rutten, A.P.; Volovics, A.; et al. No-touch isolation technique in colon cancer: A controlled prospective trial. Br. J. Surg. 1988, 75, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Fan, S.T.; Lo, C.M.; Tung-Ping Poon, R.; Wong, J. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann. Surg. 2000, 232, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Gall, T.M.; Jacob, J.; Frampton, A.E.; Krell, J.; Kyriakides, C.; Castellano, L.; Stebbing, J.; Jiao, L.R. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg. 2014, 149, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Giesing, M.; Suchy, B.; Driesel, G.; Molitor, D. Clinical utility of antioxidant gene expression levels in circulating cancer cell clusters for the detection of prostate cancer in patients with prostate-specific antigen levels of 4–10 ng/mL and disease prognostication after radical prostatectomy. BJU Int. 2010, 105, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, K.; Burri, R.; Stone, N.; Stock, R.G. Predictors of metastatic disease after prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Eschwege, P.; Dumas, F.; Blanchet, P.; le Maire, V.; Benoit, G.; Jardin, A.; Lacour, B.; Loric, S. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet 1995, 346, 1528–1530. [Google Scholar] [CrossRef]
- Badwe, R.A.; Vaidya, J.S. Haematogenous dissemination of prostate epithelial cells during surgery. Lancet 1996, 347, 325–326. [Google Scholar] [PubMed]
- Davis, J.W.; Nakanishi, H.; Kumar, V.S.; Bhadkamkar, V.A.; McCormack, R.; Fritsche, H.A.; Handy, B.; Gornet, T.; Babaian, R.J. Circulating tumor cells in peripheral blood samples from patients with increased serum prostate specific antigen: Initial results in early prostate cancer. J. Urol. 2008, 179, 2187–2191. [Google Scholar] [CrossRef] [PubMed]
- Eschwege, P.; Moutereau, S.; Droupy, S.; Douard, R.; Gala, J.L.; Benoit, G.; Conti, M.; Manivet, P.; Loric, S. Prognostic value of prostate circulating cells detection in prostate cancer patients: A prospective study. Br. J. Cancer 2009, 100, 608–610. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, E.C.; Lee, M.J.; Alarcon, S.V.; Lee, S.; Hoang, A.N.; Walton Diaz, A.; Chelluri, R.; Vourganti, S.; Trepel, J.B.; Pinto, P.A. Lack of impact of robotic-assisted laparoscopic radical prostatectomy on intraoperative levels of prostate cancer circulating tumor cells. J. Urol. 2015, 195, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Kasahara, T.; Kawasaki, T.; Bilim, V.; Tomita, Y.; Obara, K.; Takahashi, K. Frequency of PSA-mRNA-bearing cells in the peripheral blood of patients after prostate biopsy. Br. J. Cancer 2001, 85, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Selection of successive tumour lines for metastasis. Nat. New Biol. 1973, 242, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Yokota, J. Tumor progression and metastasis. Carcinogenesis 2000, 21, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Mareel, M.M.; van Roy, F.M.; Bracke, M.E. How and when do tumor cells metastasize? Crit. Rev. Oncog. 1993, 4, 559–594. [Google Scholar] [PubMed]
- Meyer, C.P.; Pantel, K.; Tennstedt, P.; Stroelin, P.; Schlomm, T.; Heinzer, H.; Riethdorf, S.; Steuber, T. Limited prognostic value of preoperative circulating tumor cells for early biochemical recurrence in patients with localized prostate cancer. Urol. Oncol. 2016, 34, 211–236. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012, 22, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Macoska, J.; Chen, W.; Sarkar, F.H. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am. J. Transl. Res. 2010, 3, 90–99. [Google Scholar] [PubMed]
- Tsumura, H.; Satoh, T.; Ishiyama, H.; Tabata, K.; Komori, S.; Sekiguchi, A.; Ikeda, M.; Kurosaka, S.; Fujita, T.; Kitano, M.; et al. Prostate-specific antigen nadir after high-dose-rate brachytherapy predicts long-term survival outcomes in high-risk prostate cancer. J. Contemp. Brachytherapy 2016, 8, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Zauls, A.J.; Ashenafi, M.S.; Onicescu, G.; Clarke, H.S.; Marshall, D.T. Comparison of intraoperatively built custom linked seeds versus loose seed gun applicator technique using real-time intraoperative planning for permanent prostate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
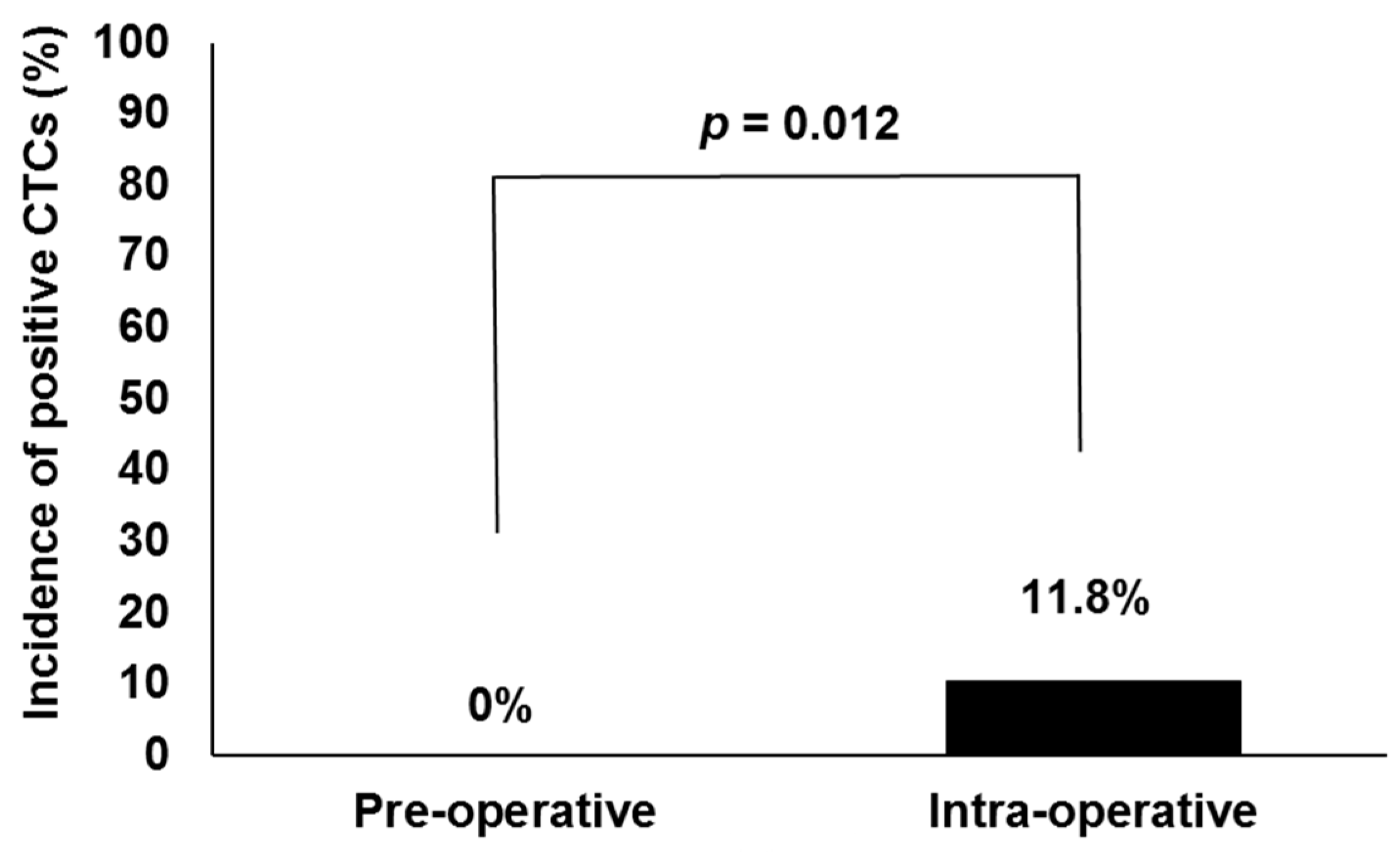
| Factors | HDR (n = 30) | LDR (n = 29) | Total (n = 59) | |||
|---|---|---|---|---|---|---|
| Median | (Range) | Median | (Range) | Median | (Range) | |
| Age (year) | 71.5 | (58–82) | 70 | (51–77) | 71 | (51–82) |
| PSA at diagnosis (ng/mL) | 26.8 | (4.5–396) | 6.5 | (4.2–14.1) | 10.1 | (4.2–396) |
| Prostate volume (cc) * | 14.4 | (4.6–29.7) | 30.7 | (20.3–58.4) | 22.2 | (4.6–58.4) |
| Number of needles | 18 | (18–18) | 21 | (17–29) | – | – |
| Duration of NHT (months) | 16 | (7–25) | 0 | (0) | – | – |
| n | (%) | n | (%) | n | (%) | |
| Gleason Score | ||||||
| ≤6 | 0 | (0) | 8 | (28) | 8 | (14) |
| 7 | 7 | (23) | 19 | (65) | 26 | (44) |
| 8 to 10 | 23 | (77) | 2 | (7) | 25 | (42) |
| Clinical T Stage | ||||||
| 1c–2a | 6 | (20) | 20 | (69) | 26 | (44) |
| 2b–2c | 6 | (20) | 9 | (31) | 15 | (25) |
| 3a | 11 | (37) | 0 | (0) | 11 | (19) |
| 3b | 6 | (20) | 0 | (0) | 6 | (10) |
| 4 | 1 | (3) | 0 | (0) | 1 | (2) |
| n | (%) | n | (%) | n | (%) | |
| Biopsy Positive Core Rate | ||||||
| <34% | 8 | (27) | 21 | (73) | 29 | (49) |
| 34%–67% | 12 | (40) | 7 | (24) | 19 | (32) |
| >67% | 10 | (33) | 1 | (3) | 11 | (19) |
| NCCN Risk Criteria (2015) | ||||||
| Low | 0 | (0) | 6 | (21) | 6 | (10) |
| Intermediate | 0 | (0) | 21 | (72) | 21 | (36) |
| High | 20 | (67) | 2 | (7) | 22 | (37) |
| Very high | 10 | (33) | 0 | (0) | 10 | (17) |
| Type of Brachytherapy | HDR | HDR | HDR | HDR | LDR | LDR | LDR |
|---|---|---|---|---|---|---|---|
| Case number | 9 | 26 | 34 | 36 | 8 | 19 | 43 |
| Number of CTC counts (/7.5 mL) | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Age (years) | 71 | 75 | 65 | 75 | 58 | 65 | 67 |
| Duration of NHT (months) | 17 | 16 | 16 | 17 | 0 | 0 | 0 |
| PSA nadir during NHT (ng/mL) | 0.014 | <0.008 | <0.008 | 0.14 | – | – | – |
| PSA at diagnosis (ng/mL) | 31 | 13.5 | 17.6 | 66.7 | 8.6 | 4.6 | 14.1 |
| Prostate volume (cc) * | 7 | 29.7 | 21.3 | 13.9 | 26.1 | 38.4 | 37 |
| Number of needles | 18 | 18 | 18 | 18 | 24 | 28 | 18 |
| Gleason score | 8 | 8 | 7 | 9 | 6 | 7 | 7 |
| Clinical T stage | 1c | 3a | 3b | 2c | 2a | 2a | 2c |
| Biopsy positive core rate (%) | 75 | 50 | 25 | 100 | 10 | 16.6 | 33.3 |
| NCCN risk criteria 2015 | H | H | VH | H | L | I | I |
| Factors | CTC Positive Rates | (n) | p |
|---|---|---|---|
| Age (>70 vs. ≤70 years) | 8.8% vs. 16.0% | (3/34 vs. 4/25) | 0.442 |
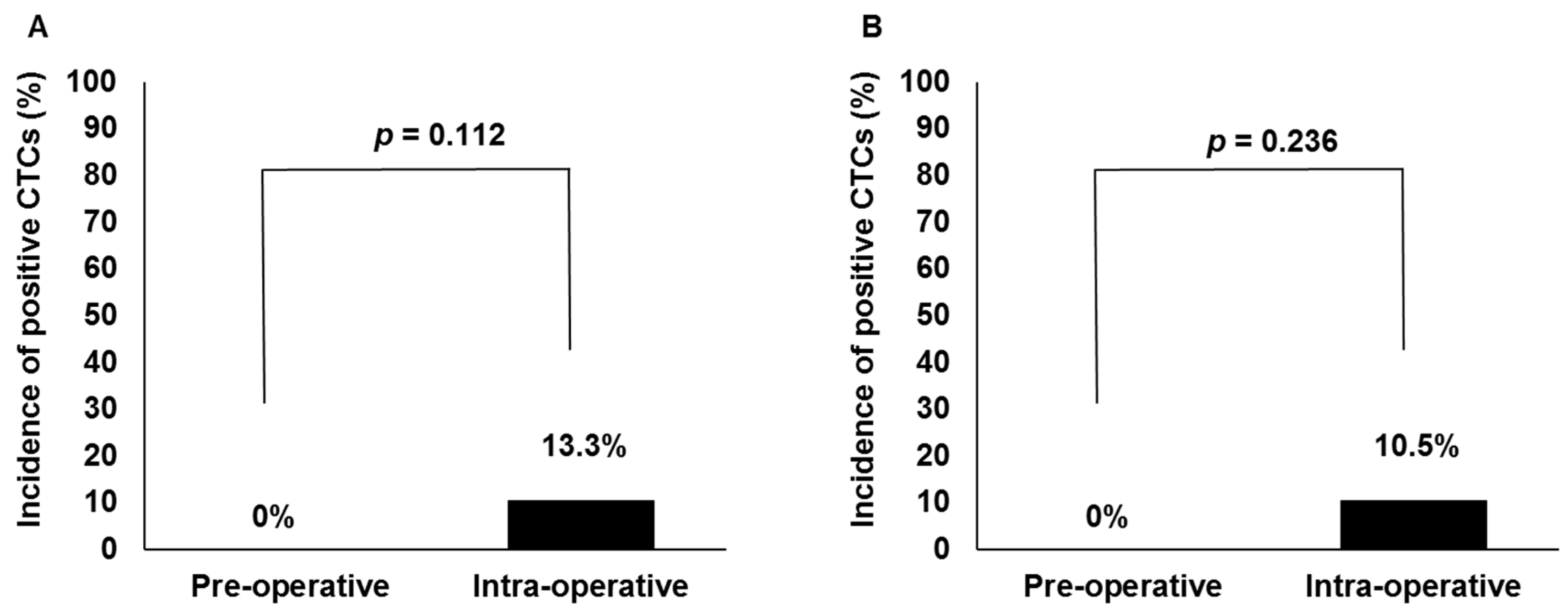
| Type of brachytherapy (HDR vs. LDR) | 13.3% vs. 10.3% | (4/30 vs. 3/29) | >0.999 |
| NHT (yes vs. no) | 13.3% vs. 10.3% | (4/30 vs. 3/29) | >0.999 |
| PSA at diagnosis (≥10 vs. <10 ng/mL) | 16.1% vs. 7.1% | (5/31 vs. 2/28) | 0.424 |
| Prostate volume (cc) | 14.8% vs. 9.3% | (4/27 vs. 3/32) | 0.691 |
| Prostate volume/number of needle (≥1 vs. <1 cc/needle) | 14.2% vs. 8.3% | (5/35 vs. 2/24) | 0.689 |
| Gleason score (≥8 vs. <8) | 12.0% vs. 11.7% | (3/25 vs. 4/34) | >0.999 |
| Clinical T stage (≥3a vs. ≤2c) | 11.7% vs. 11.9% | (2/17 vs. 5/42) | >0.999 |
| Biopsy positive core rate (>34% vs. ≤34%) | 10.0% vs. 13.7% | (3/30 vs. 4/29) | 0.706 |
| NCCN risk criteria 2015 (H or VH vs. I or L) | 12.5% vs. 11.1% | (4/32 vs. 3/27) | >0.999 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsumura, H.; Satoh, T.; Ishiyama, H.; Tabata, K.-i.; Takenaka, K.; Sekiguchi, A.; Nakamura, M.; Kitano, M.; Hayakawa, K.; Iwamura, M. Perioperative Search for Circulating Tumor Cells in Patients Undergoing Prostate Brachytherapy for Clinically Nonmetastatic Prostate Cancer. Int. J. Mol. Sci. 2017, 18, 128. https://doi.org/10.3390/ijms18010128
Tsumura H, Satoh T, Ishiyama H, Tabata K-i, Takenaka K, Sekiguchi A, Nakamura M, Kitano M, Hayakawa K, Iwamura M. Perioperative Search for Circulating Tumor Cells in Patients Undergoing Prostate Brachytherapy for Clinically Nonmetastatic Prostate Cancer. International Journal of Molecular Sciences. 2017; 18(1):128. https://doi.org/10.3390/ijms18010128
Chicago/Turabian StyleTsumura, Hideyasu, Takefumi Satoh, Hiromichi Ishiyama, Ken-ichi Tabata, Kouji Takenaka, Akane Sekiguchi, Masaki Nakamura, Masashi Kitano, Kazushige Hayakawa, and Masatsugu Iwamura. 2017. "Perioperative Search for Circulating Tumor Cells in Patients Undergoing Prostate Brachytherapy for Clinically Nonmetastatic Prostate Cancer" International Journal of Molecular Sciences 18, no. 1: 128. https://doi.org/10.3390/ijms18010128






