1. Introduction
Diabetes mellitus is a multiple etiology metabolic disorder with abnormalities in the metabolism of carbohydrate, fat and protein, being characterized by chronic hyperglycemia and defects in insulin secretion, insulin action, or both [
1]. It is projected that diabetes will affect over 300 million people by the year 2030 [
2,
3,
4]. The relevance of this disorder, namely its social impact, is clearly highlighted by the World Health Organization (WHO) which proposes that further research is urgently needed to evaluate the effectiveness of interventions to prevent it, including behavioral changes, favoring a diet with increased fruit and vegetable consumption and, thus, improving dietary patterns [
2]. Diets or food supplements that contribute to control and/or prevent hyperglycemia might be crucial to reducing diabetes incidence [
5], namely through the development of alternative sources of antidiabetic agents.
European elderberry (
S. nigra L.) is a deciduous shrub that produces violet-black drupes which grow in clusters, holding hundreds of berries [
6]. This plant is considered from the days of Hippocrates as the “medicine chest” [
7], and has been used in the formulation of diverse medicinal preparations to prevent and/or control different diseases [
8]. Several bioactive compounds are reported on elderberries, namely phenolic compounds as anthocyanin derivatives, including cyanidin 3-glucoside, cyanidin 3-sambubioside, cyanidin 3-sambubioside-5-glucoside and cyanidin 3,5-diglucoside [
9,
10,
11]; as well as triterpenic compounds such as ursolic and oleanolic acids, and sterols, as β-sitosterol were reported as elderberry bioactive components [
12,
13].
The huge importance of searching for alternative sources of antidiabetic agents and the limited number of studies dealing with elderberry extract supplementation to reduce diabetes complications, highlights the need to conduct more detailed studies on this topic. Previous studies revealed the potential of elderberry extracts in diabetes status management [
5,
14,
15,
16,
17,
18,
19]. The effects of acidified methanol elderberry extracts dietary supplementation (28–70 mg of extract/kg body weight (b.w.) streptozotocin (STZ)-induced diabetic Wistar rats, during 12 to 16 weeks) were evaluated showing a reduction in serum glycemic and lipidic levels (cholesterol and triacylglycerol); reduction in the levels of oxidative markers (as superoxide dismutase and glutathione peroxidase activities) and inflammatory markers (as interleukin-6); and an increase in immunological parameters from T lymphocytes populations [
5,
14,
15,
16,
17,
18,
19]. The metabolic effects of elderberry extract supplementation in an obese mouse model C57BL/6J were also evaluated (20–200 mg of extract/kg b.w., fed either with a low-fat diet and high-fat diet, for 16 weeks), and decreased serum triacylglycerol, inflammatory markers (as TNF-α) and insulin resistance were reported [
20]. Despite the claim that these effects were attributable to phenolic components [
5,
14,
15,
16,
17,
18,
19], structure–activity relationship studies were not conducted. Furthermore, only a few in vivo studies were performed, in which diets were supplemented with bioactive components present in elderberries, such as cyanidin 3-glucoside, quercetin 3-rutinoside and ursolic acid. Cyanidin 3-glucoside dietary supplementation (0.2% of the diet during 5 weeks) promoted a reduction in the blood glucose levels and an enhancement of insulin sensitivity in type 2 diabetic KK-A
y mice [
21]; quercetin 3-rutinoside supplementation (25–100 mg/kg during 45 days) revealed antihyperglycemic and antioxidant activity on STZ-injected Wistar rats [
22]; while ursolic acid supplementation lowered the urine excretion and renal oxidative stress levels in STZ-injected Wistar rats (0.2% of the diet during 16 weeks) [
23]. The fact that elderberry extracts and their major components are linked with different health benefits including preventing diabetes complications warrants considering the use of elderberry enriched extracts to access their potential antidiabetic effect.
It is worth noting that any compound that interferes with a biological system might raise toxicity concerns, thus a screening of plant extract toxicity before in vivo assays is of major importance. Different models are currently used for this purpose; namely based on the use of microorganisms.
Aliivibrio fischeri bioluminescence method is widely used to evaluate the toxicity response as it correlates bioluminescence signal and viable counts, where light output reflects the cells’ metabolic rate being therefore a rapid, sensitive and cost-effective option [
24,
25]. Disturbances in the bacterial metabolism implies alterations to light production, as the
A. fischeri cellular respiration and light emission metabolic pathway are intrinsically linked [
26].
From this perspective, this study aims to evaluate the
S. nigra L. lipophilic (dichloromethane) and polar (acidified methanol) extract dietary supplementation effects on an animal model of diabetes, in order to obtain insights on their effects on diabetes and related complications. Wistar streptozotocin (STZ)-induced diabetic rats fed with a high-fat diet were enrolled in this study, and non-diabetic and diabetic rats without supplementation were also used as controls. Hematological and biochemical blood indices, as well as blood and tissular trace elements were assessed. A specific set of biochemical parameters were analyzed, namely fasting blood glucose and insulin, as there have been increasing efforts in search of bioactive compounds or extracts that can improve insulin action and lower blood glucose levels [
27]. Furthermore, type 2 diabetes allied with a high fat diet regimen might induce changes in lipidic patterns, as well as hepatic dysfunctions, highlighting the need to understand whether elderberry extract supplementation might improve these conditions. In order to obtain fractions enriched in lipophilic and polar bioactive components (e.g., in triterpenic acids and in phenolic compounds), dietary elderberry supplementations were performed using extracts instead of whole elderberries. Thus, firstly the polar extract was characterized by ultra-high-pressure liquid chromatography-tandem mass spectrometry (UHPLC-MS
n) analysis, while lipophilic fraction characterization by gas chromatography-mass spectrometry (GC-MS) was reported elsewhere [
13]. Secondly, elderberry extract toxicity was evaluated using the bioluminescence
A. fischeri assay. Diets were then prepared and administrated to rats using doses selected based on the preliminary toxicity assays.
3. Materials and Methods
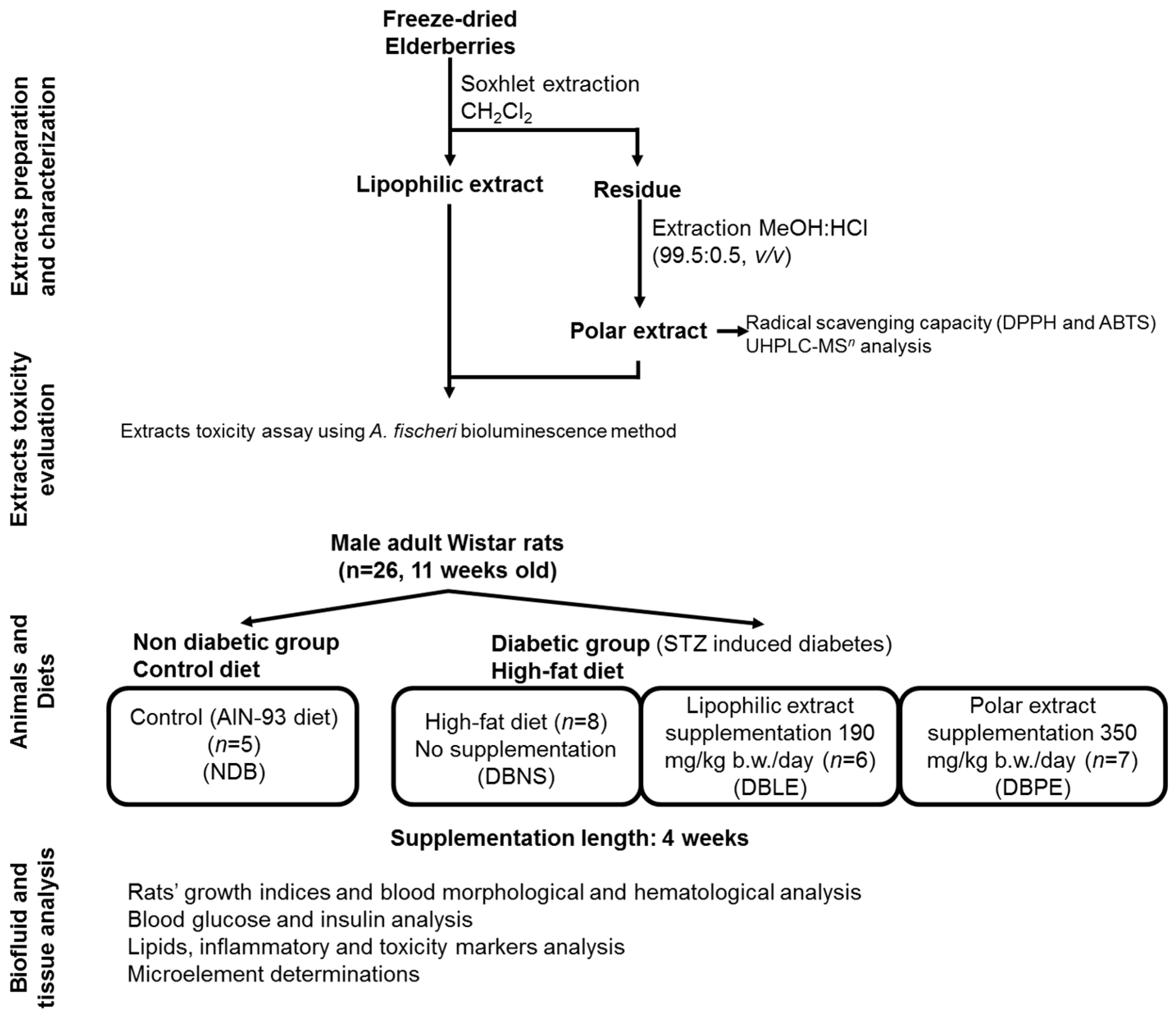
The experimental setup of this study (
Figure 1) includes extracts preparation and partial chemical characterization (phenolic composition by UHPLC-MS
n analysis), evaluation of extract toxicity; animals (Local Animal Bioethics Committee in Poznan, Poland (No 3/2015)—16 January 2015), diet and supplementation; and finally, the biofluid and tissue analysis, as described in detail in the following sections.
3.1. Reagents
Methanol (≥99.9%) was purchased from Panreac (Barcelona, Spain). Dichloromethane (≥99.9%) was supplied by Sigma Chemical Co. (Madrid, Spain). Hydrochloric acid (37%, w/w) was purchased from Riedel-De Haёn, Sigma (Seelze, Germany). 2,2-Diphenyl-1-picrylhydrazyl (DPPH·), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, ≥97%), 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS, >98%), cyanidin 3-glucoside chloride (≥95%) and quercetin-3-glucoside (≥98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Maltodextrin was purchased from Nowamyl (Łobez, Poland). Nitric acid (65%, w/w) was purchased from Merck (Darmstadt, Germany). Formic acid (≥98%) was purchased from Fluka Chemie (Madrid, Spain). HPLC-grade methanol and water were supplied from Fisher Scientific Chemicals (Loures, Portugal) and further filtered using a Solvent Filtration Apparatus 58,061 from Supelco (Bellefonte, PA, USA). Reference Bovine Liver, NIST-1577C was purchased from LGC standards (Dziekanów Leśny, Poland).
3.2. Elderberry Samples
Elderberries (S. nigra L.) were supplied by the Adega Cooperativa do Vale Varosa—RégieFrutas (Tarouca, Portugal). The samples were collected on an experimental field (41.043233° N, 7.728820° W) with 0.5 ha, from 13/14-years old plants, where each plant produces approximately 15 kg of elderberries per year.
Samples were harvested on the same day between 9 and 12 a.m., in which several bunches from diverse shrubs were randomly harvested and mixed together. Samples were immediately transported under refrigeration (ca. 2–4 °C) to the laboratory and then stored at −20 °C. Prior to extraction, elderberries were freeze-dried using a VirTis BenchTop K (SP Industries, Stone Ridge, NY, USA).
3.3. Extract Preparation
The lipophilic extract (LE) was obtained as previously described [
13]. Briefly, freeze-dried elderberries (approximately 850 g) were Soxhlet extracted using dichloromethane for 8 h. The solvent was evaporated to dryness in a rotary evaporator and the extracts weighed. This extract was previously chemically characterized by gas chromatography-mass spectrometry analysis [
13]. The resulting lipophilic-free solid residue was then extracted (
m/
v 1:5) with acidified methanol (0.5% HCl) for one hour under constant stirring, based on previous publications [
14,
28]. The suspension was then filtered and the extraction process repeated 5 times. The extracts were combined and then evaporated to dryness by low-pressure evaporation. The extract was freeze-dried to ensure the absence of water. As this extract is highly hygroscopic, it was mixed with maltodextrin at a ratio of 1:0.7 (
m/
m, extract/maltodextrin).
3.4. Phenolic Compound Analysis
3.4.1. UHPLC-MSn Analysis
UHPLC-MS
n analysis was conducted based on previous methodologies developed in our lab [
31], in which the UHPLC system consisted of a variable loop Accela autosampler (200 vial capacity set at 16 °C), an Accela 600 LC pump and an Accela 80 Hz PDA detector (Thermo Fisher Scientific, San Jose, CA, USA). Before UHPLC injection, each extract was dissolved in methanol (HPLC grade), with a concentration of 15 mg/mL, being subsequently filtered with a 0.2 μm PTFE syringe filter. A gradient elution program was carried out for the separation of the analytes, using a Kinetex C
18 (100 mm × 2.1 mm × 1.7 μm) column supplied by Phenomenex (Torrance, CA, USA), at 45 °C and a flow rate of 0.39 μL/min. The injection volume was 20 μL and the mobile phase consisted of methanol (A) and water:formic acid (95:5,
v/
v) (B). It was applied at a linear gradient that consisted of: 0–3 min: 1% A, 3–8 min: 1%–10% A, 8–21 min: 10%–28% A, 21–28 min: 28%–65% A, 28–31 min: 65% A, 31–35 min: 65%–1% A, followed by 4 min of column re-equilibration before the next run. Detection was carried out in the diode array detector (DAD) at 280, 340 and 520 nm, and UV spectra in a range of 210–600 nm were also recorded. A LCQ Fleet ion trap mass spectrometer (Thermo Finnigan, San Jose, CA, USA), equipped with an electrospray ionization source and operating in negative and positive modes was used to perform tandem mass spectrometry analysis. The nitrogen sheath and auxiliary gas were 40 and 10 (arbitrary units), respectively. The capillary temperature was 330 °C and the spray voltage was 5 kV. The capillary and tune lens voltages were set at 41 V and 110 V for positive mode and for negative mode at −36 V and −120 V. CID-MS
n experiments were performed on mass-selected precursor ions in the range of
m/
z 100–1500. The isolation width of precursor ions was 1.0 mass units. Collision energy was optimized between 20 and 35 (arbitrary units), using helium as collision gas and scan time was equal to 100 ms. Xcalibur
® data system (Thermo Finnigan, San Jose, CA, USA) was used for data acquisition.
Cyanidin 3-glucoside and quercetin 3-glucoside standard solutions (in methanol, with five different concentrations each, between 0.1 and 20 μg/mL), were used for quantification using UHPLC-DAD system. Limits of detection (LOD) and quantification (LOQ) were also estimated using the S/N approach (n = 5). Individual compound quantification was accomplished with calibration data for the most similar standards in terms of maximum wavelength absorption, when no pure reference compounds were available. The concentration of each compound was expressed as the mean value (n = 3).
3.4.2. Radical Scavenging Capacity
DPPH and ABTS radical scavenging capacities were determined using Lambda 35 spectrophotometer (Perkin-Elmer, Waltham, MA, USA) following previously described procedures [
44,
45]. The samples were appropriately diluted in methanol. Calibration curves were performed using Trolox as standard, with concentrations between 0.10 and 0.40 mg/mL (
r2 = 0.9946) for ABTS assay and 0.02 and 0.20 mg/mL (
r2 = 0.9955) for DPPH assay. The results are expressed in mmol Trolox equivalents. All determinations were performed in triplicate.
3.5. Sugar Content of the Polar Extract
Phenol-sulfuric acid colorimetric method was used to determine the sugar content of the polar extract [
46]. It was added to the extract 1 mL of H
2SO
4 72% (
w/
w) and 160 μL of phenol 5% (
w/
w). The tubes were heated at 100 °C during 5 min, cooled to room temperature and stirred. Absorbance was measured at 490 nm (Lambda 35, Perkin-Elmer) and a calibration curve was prepared using glucose as standard (0–1 mg/mL). The determinations were performed in triplicate.
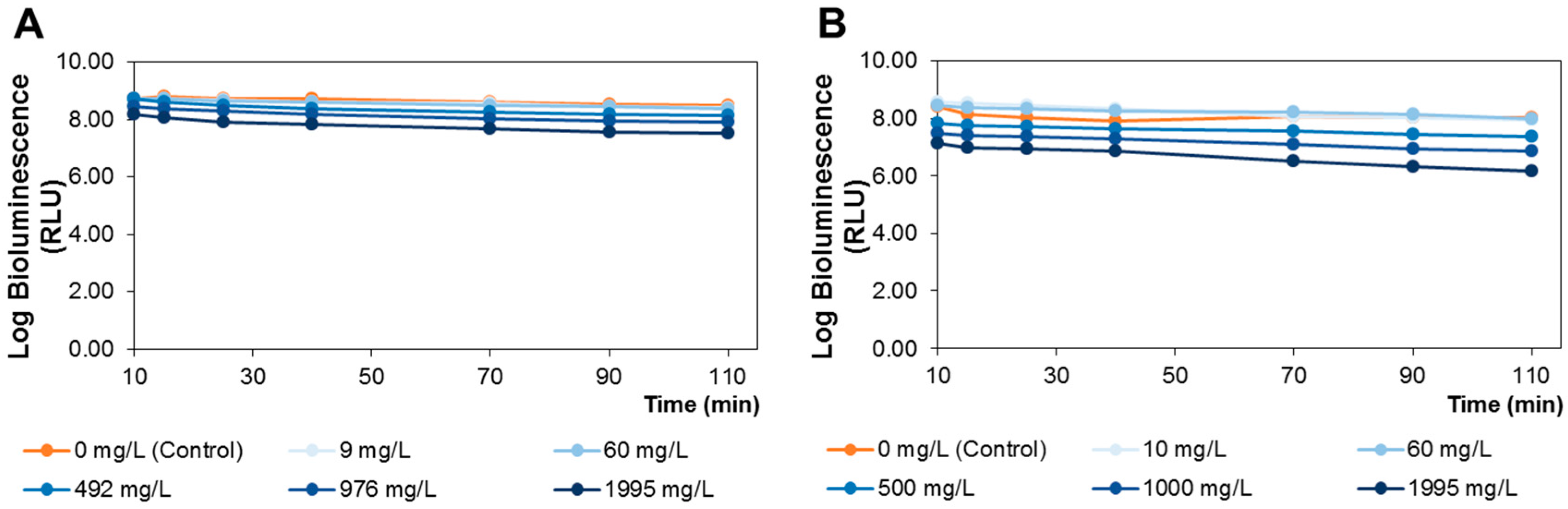
3.6. A. fischeri Bioluminescence Assay
Toxicity was evaluated through the bioluminescence assay using the bacteria
A. fischeri, based on a previously established methodology [
25]. A bioluminescent marine bacterium
A. fischeri ATCC 49387 (USA) bacterial strain was used (stored at −80 °C in 10% glycerol). The bioluminescent
A. fischeri fresh plate cultures were maintained at 4 °C in solid BOSS medium (1% peptone, 0.3% beef extract, 0.1% glycerol, 3% NaCl, 1.5% agar, pH 7.3). One isolated colony was aseptically inoculated in liquid BOSS medium (30 mL), and kept at 26 °C under constant stirring (170 rpm) during 18 h. Then, an aliquot (200 μL) was sub-cultured in BOSS medium (30 mL), and grew at 26 °C under stirring (170 rpm) overnight. The colony-forming units (CFU) and the bioluminescent signal (in relative light units, RLU) correlation of
A. fischeri was also assessed [
25].
For bioassay purposes, an overnight culture of
A. fischeri was used after a ten-fold dilution in phosphate buffered saline (PBS: 30 g NaCl, 0.2 g KCl, 1.44 g Na
2HPO
4 and 0.24 g KH
2PO
4 per liter; pH 7.4). For each, 15 mL of bacterial suspension were aseptically distributed in 100 mL acid-washed and sterilized glass beakers containing appropriate amounts of
S. nigra extract to achieve a final concentration between 0 (control) and 1995 mg of extract/L, respectively. For the lipophilic (dichloromethane) extract, 2% (
v/
v) of dimethyl sulfoxide (DMSO) was added in order to dissolve this extract. Previously to the development of toxicity tests, solutions of 2% DMSO were analyzed to check the absence of toxic effects for
A. fischeri, revealing no toxic effects for up to 110 min. All beakers were wrapped with aluminum foil to protect from light exposure and incubated under 120 rpm stirring at 25 °C. Aliquots, 500 μL, of treated and control samples were collected at time 10, 15, 25, 40, 70, 90 and 110 min and the bioluminescence signal was measured in a luminometer (peak wavelength detected at 420 nm, standard range: 300–650 nm) (Promega Glomax 20/20 luminometer, Turner Designs, Inc., Madison, WI, USA). The tested extract concentrations were selected to have a wide range of concentrations (from 0 to 1995 mg of extract/L), in order to establish the non-toxic doses to be administrated to rats. These doses were calculated based on previously established correlations between the
A. fischeri bioluminescent model and rat toxicity assays [
34,
47]. Three independent experiments for each tested condition were done.
3.7. Animals, Diets and Elderberry Extract Supplementation
All animal procedures and the protocol were conducted according to EU Directive 2010/63/EU for animal experiments and approved by the Local Animal Bioethics Committee in Poznan, Poland (No. 3/2015). All necessary efforts were made to minimize the number of animals used and their suffering.
Male adult Wistar rats (
n = 26, 11 weeks old) were purchased from the Licensed Laboratory of the Animal Breeding Center (Poznan, Poland). After arrival at the animal care facility, rats were kept under controlled temperature (21 ± 2 °C) and humidity (55%–60%) with a 12 h/12 h day/night cycle throughout the experiment. After a 5-day adaptation period, animals were divided into 4 groups (initial mean body weight = 330 g), and kept in metal-free individual cages: NDB (non-diabetic group,
n = 5), DBNS (diabetic group/not supplemented,
n = 8), DBLE (diabetic group/supplemented with lipophilic extract,
n = 6) and DBPE (diabetic group/supplemented with polar extract,
n = 7). Animals were fed ad libitum for 2 weeks: (i) non-diabetic group was fed with semi-synthetic standard composed by casein (14%), sunflower oil (10%), wheat starch (56.5%), sucrose (10%), potato starch (5%), vitamin mix AIN-93M (1%) and mineral mix AIN-93M (3.5%); while (ii) the three diabetic groups (DBNS, DBLE and DBPE) received high fat (HF) diet (40% calories from fat), which were obtained from the basal AIN-93M diet [
48], by replacement of wheat starch with fat, being thereby composed by casein (14%), sunflower oil (10%), wheat starch (46.5%), lard (10%), sucrose (10%), potato starch (5%), vitamin mix AIN-93M (1%) and mineral mix AIN-93M (3.5%). Polar extract was incorporated on wheat starch, while lipophilic extract was mixed with sunflower oil. An excessive amount of fat in the diet is one of the factors contributing to insulin resistance in animal models, and thus, the group of rats fed with a high-fat diet was formed to elucidate changes associated with this syndrome [
27].
After 2 weeks of controlled diet, the three diabetic groups (DBNS, DBLE and DBPE) were subjected to multiple intraperitoneal injection of STZ freshly dissolved in 0.1 M-citrate buffer (pH 4.4), given in 3 subsequent doses: 20, 10 and 25 mg/kg body weight, in weekly intervals, while NDB group were injected in the same manner, but with the carrier alone (citrate buffer). The approach with multiple doses of STZ combined with a high-fat diet has been shown to be more efficient and stable animal model of diabetes type-2 [
49]. The presence of diabetes in rats was confirmed by measuring fasting blood glucose concentration in blood samples (>11 mmol/L) withdrawn from the tail tip after 48 h using a glucometer iXell
®, Genexo (Warsaw, Poland). After that, dietary supplementation was performed using 500 mg of polar extract/100 g HF diet for DBPE group and 190 mg of lipophilic extract/100 g HF diet for DBLE group.
All diets were prepared weekly and stored in sealed containers at 4 ± 1 °C. Food intake was measured daily and body mass every 7 days.
After 4 weeks of feeding and overnight fasting, the animals were anesthetized with CO2 inhalation and dissected to collect blood and the internal organs. Blood samples were drawn from the heart aorta into Vacutest tubes with plasma coagulant Medlab-Products (Raszyn, Poland), coagulated at room temperature for 20 min, and centrifuged at 4000 rpm. Inner organs (liver, kidneys, heart, spleen, pancreas and testes) and femoral bones were also removed, being washed in a saline solution (0.9% NaCl), weighed and stored at −70 °C. Serum samples were separated and kept in aliquots at −70 °C for biochemical assays.
3.8. Biochemical Analyses
Blood morphology and biochemical analyses were conducted in a certified laboratory (Laboratorium Medyczne Synevo, Poznan, Poland).
3.8.1. Blood Morphology
The Drabkin cyanmethemoglobin method was employed to determine blood hemoglobin (HGB) concentration [
50]. The remaining parameters were obtained using the CELLDYN-1700 analytical hematology system [
51], analyzing the following parameters: red blood cell count (RBC), hematocrit, mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width based on standard deviation (RDW-SD), white blood cell count (WBC), monocytes (MONO), lymphocytes (LYMPH), platelets (PLT), mean platelet volume (MPV), platelet distribution width (PDW) and platelet large-cell ratio (p-LCR). For each analyzed parameter, three replicates were performed for each animal.
3.8.2. Blood Biochemical Indices
The serum glucose concentration was determined by the hexokinase method [
52], while the total cholesterol, LDL-c and HDL-c levels and triacylglycerol levels were all determined using Olympus AU 560 equipment by the colorimetric methods [
53,
54,
55]. The colorimetric method using Biuret method [
56], was used to measure total protein concentration, while the Jaffe kinetic method with picric acid was employed to analyze the creatinine levels [
57]. The kinetic method using urease and glutamine dehydrogenase was used to determine urea concentration [
57]. Enzyme activities of alanine aminotransaminase (ALT), alkaline phosphatase (ALP) and aspartate aminotransferase (AST) were measured by kinetic methods [
58]. Plasma insulin concentration was measured by the RIA method using kits specific for rats, Linco Research (St. Charles, MO, USA) [
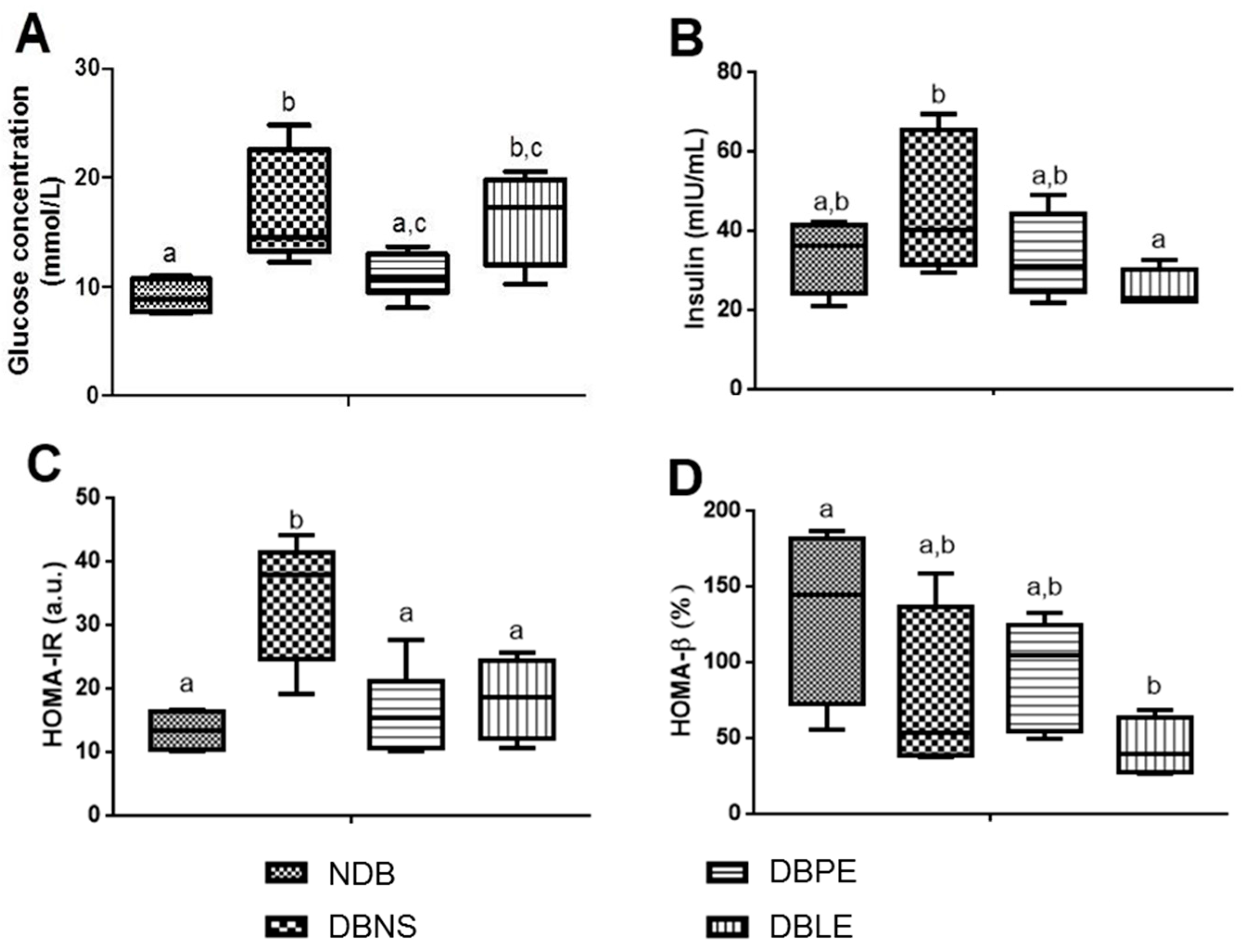
59]. The efficacy of glucose utilization, insulin resistance and β-cell function was characterized by the homeostasis model assessment (HOMA) indices [
60]. For each analyzed parameter, three replicates were performed for each animal.
3.8.3. Trace Element (Fe, Cu and Zn) Status in Blood Sera and Organs
Trace element analysis were based on previous established methodologies [
42], in which the rat tissues were digested in spectra pure HNO
3 (65%,
w/
w) in the Microwave Digestion System (MARS 5, CEM). Flame atomic-absorption spectrometry F-AAS method (AAS-3 spectrometer, Zeiss, with BC, Jena, Germany), was used to measure Fe, Zn and Cu concentrations in the mineral solutions. Simultaneous analyses of certified reference material (Bovine Liver, NIST-1577C for tissues (Gaithersburg, MD, USA), HUMASY CONTROL 2 for serum (Randox, London, UK)) were performed to assure the accuracy of quantitative determinations of Fe, Zn and Cu. Water content of the tested organs was determined for the expression of the results on dry basis. Ca. 1 g of each sample was weighed and kept overnight at 105 °C. Zn and Cu concentrations in sera samples were determined by F-AAS after diluting these samples with 0.01% Triton-X100 solution (Merck). The serum Fe concentration was determined by the Guanidine/Iron-Zine method [
61,
62]. Zn, Cu and Fe were selected as their metabolism might be disturbed in insulin resistance and in diabetic states. Particularly, Fe overload may affect glucose homeostasis, while alterations in Zn and Cu metabolism may increase oxidative damage of cells and exacerbate complications in diabetes [
27]. For each analyzed parameter, three replicates were performed for each animal.
3.9. Statistical Analysis
All the results presented in the tables are expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) followed by a Fisher’s Least Significant Difference (LSD) test using the GraphPad Prism version 7 for Windows (trial version, GraphPad Software, San Diego, CA, USA), was applied for the obtained results. It was considered statistically significant when p < 0.05.
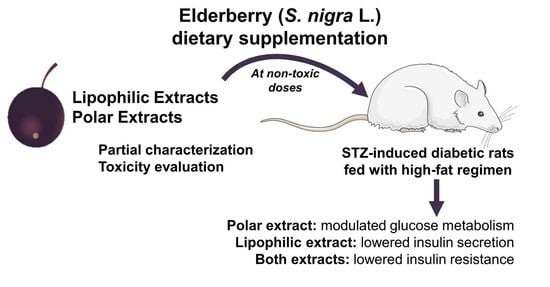
4. Conclusions
The lipophilic and polar elderberry extract dietary supplementation effects on STZ-induced diabetic Wistar rats fed with a high-fat regimen were evaluated. Extracts toxicity was first assessed using an
A. fischeri model, revealing that neither extract significantly altered the viable bacterial metabolic activity at concentrations of up to 60 mg/L. Therefore, by applying the equation of interspecies correlation between
A. fischeri and rats (oral administration), the resultant daily dietary intake of the lipophilic and polar extracts was 190 and 350 mg/kg b.w., respectively. Elderberry polar extract led to a reduction in fasting blood glucose, while lipophilic extract decreased insulin levels. Furthermore, both extracts lowered insulin resistance, without remarkable alterations in the hematological indices, sera lipidic pattern and the homeostasis of trace elements (Zn, Fe, Cu) from sera and tissues (kidney and liver). The highlighted results are the result of an elderberry polar extract dietary supplementation during a shorter period and at higher doses compared to literature (four-fold shorter length and five-fold higher doses) [
5,
14,
15,
16,
17,
18,
19]. Thus, considering this dietary supplementation length, these findings illustrate that a subacute elderberry dietary regimen ameliorated diabetic complications. Regarding elderberry lipophilic extract supplementation, it was tested for the first time, as far as we know. Additionally, the dietary
S. nigra extract supplementation effects in STZ-diabetic Wistar rats fed with HF diet on trace elements as well as on the insulin status have not been studied previously, as far as we know.
The observed improvement of the studied diabetic indices caused by elderberry extract supplementation demonstrates their potential as suitable substrates for the development of new dietary adjuncts that could help alleviate metabolic disorders in diabetes type 2. To go further with the valorization of these extracts, it is crucial to establish in detail their chemical profile, aiming at extract standardization and by relating the elderberry biological effects with their molecular structures.