Retinoic Acid Protects and Rescues the Development of Zebrafish Embryonic Retinal Photoreceptor Cells from Exposure to Paclobutrazol
Abstract
:1. Introduction
2. Results
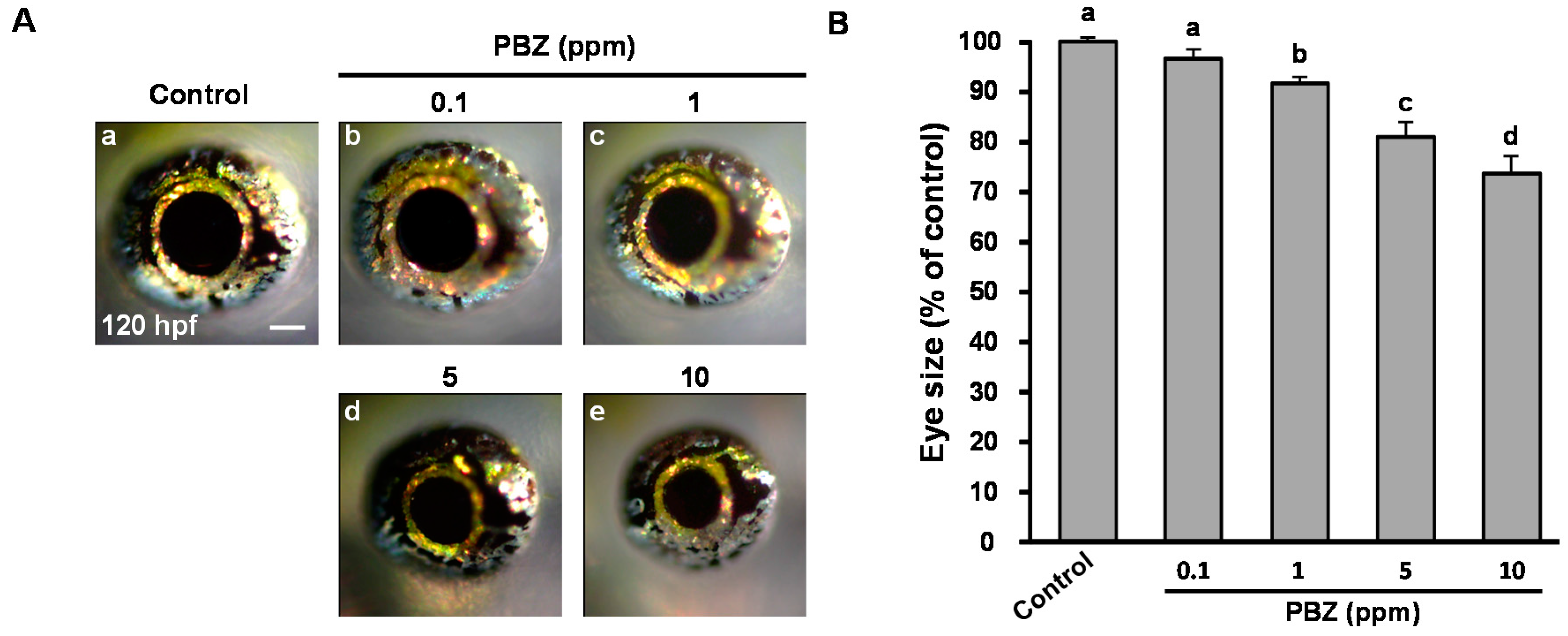
2.1. Paclobutrazol Exposure Dose-Dependently Reduces Eye Size in Zebrafish Embryos
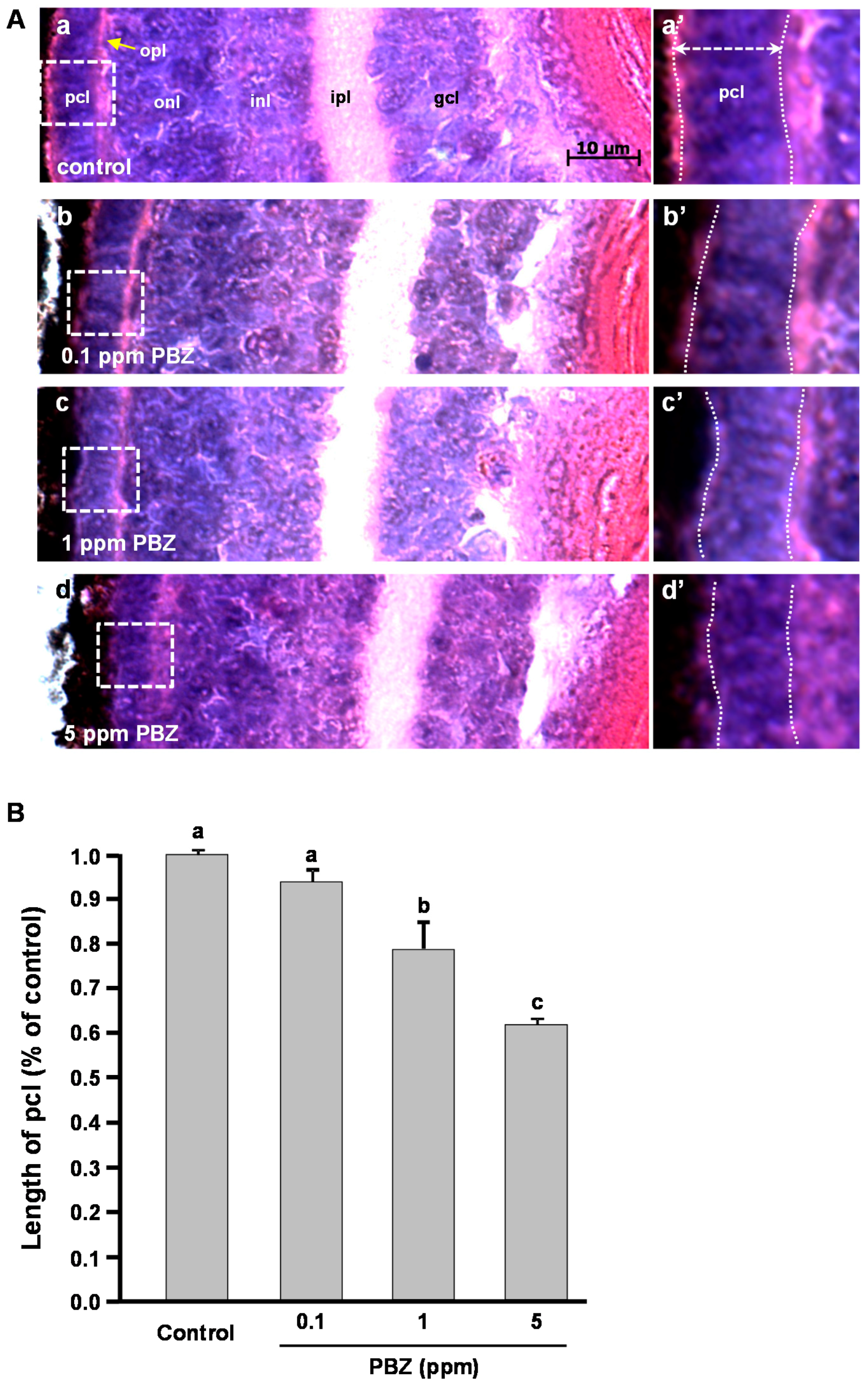
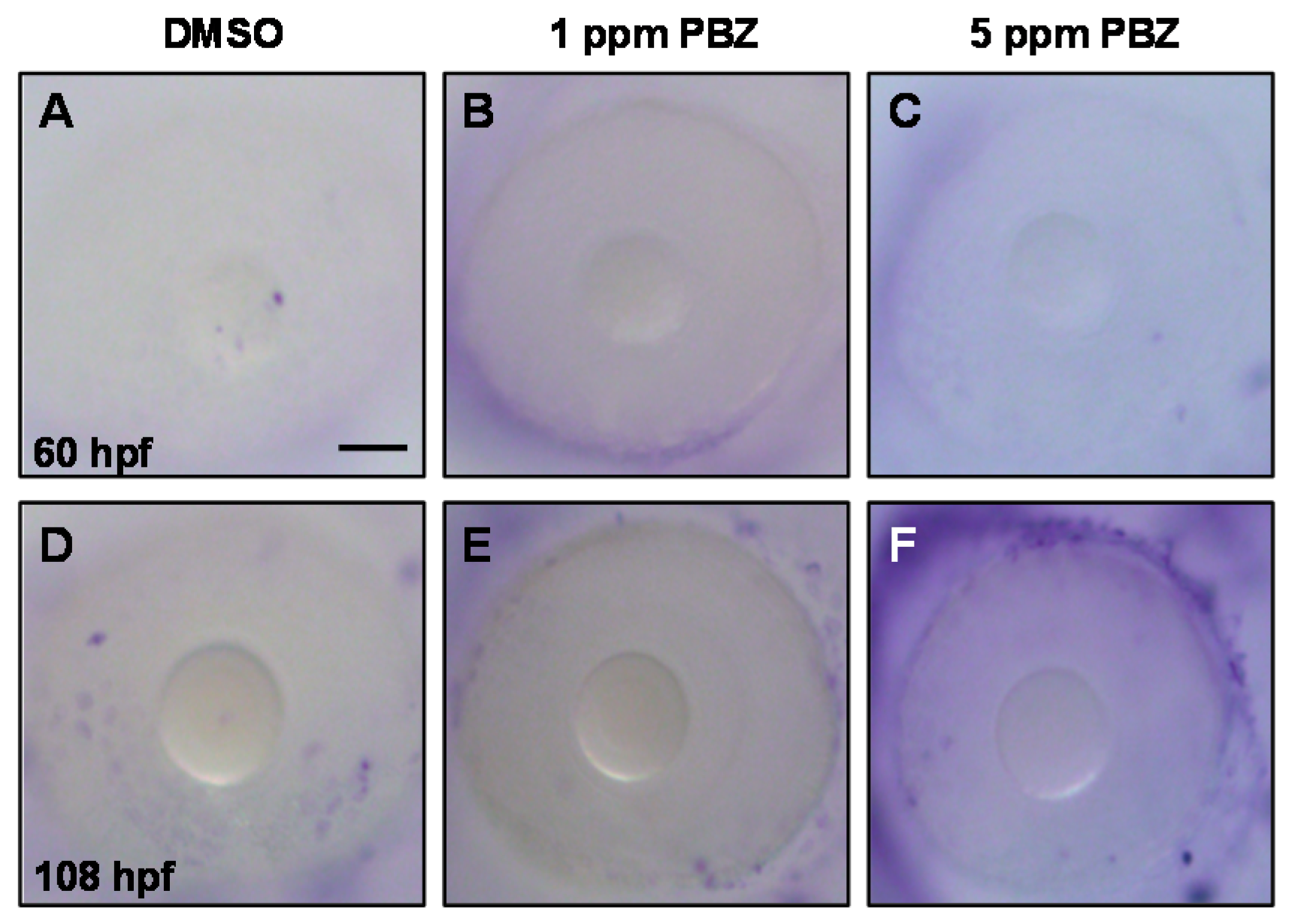
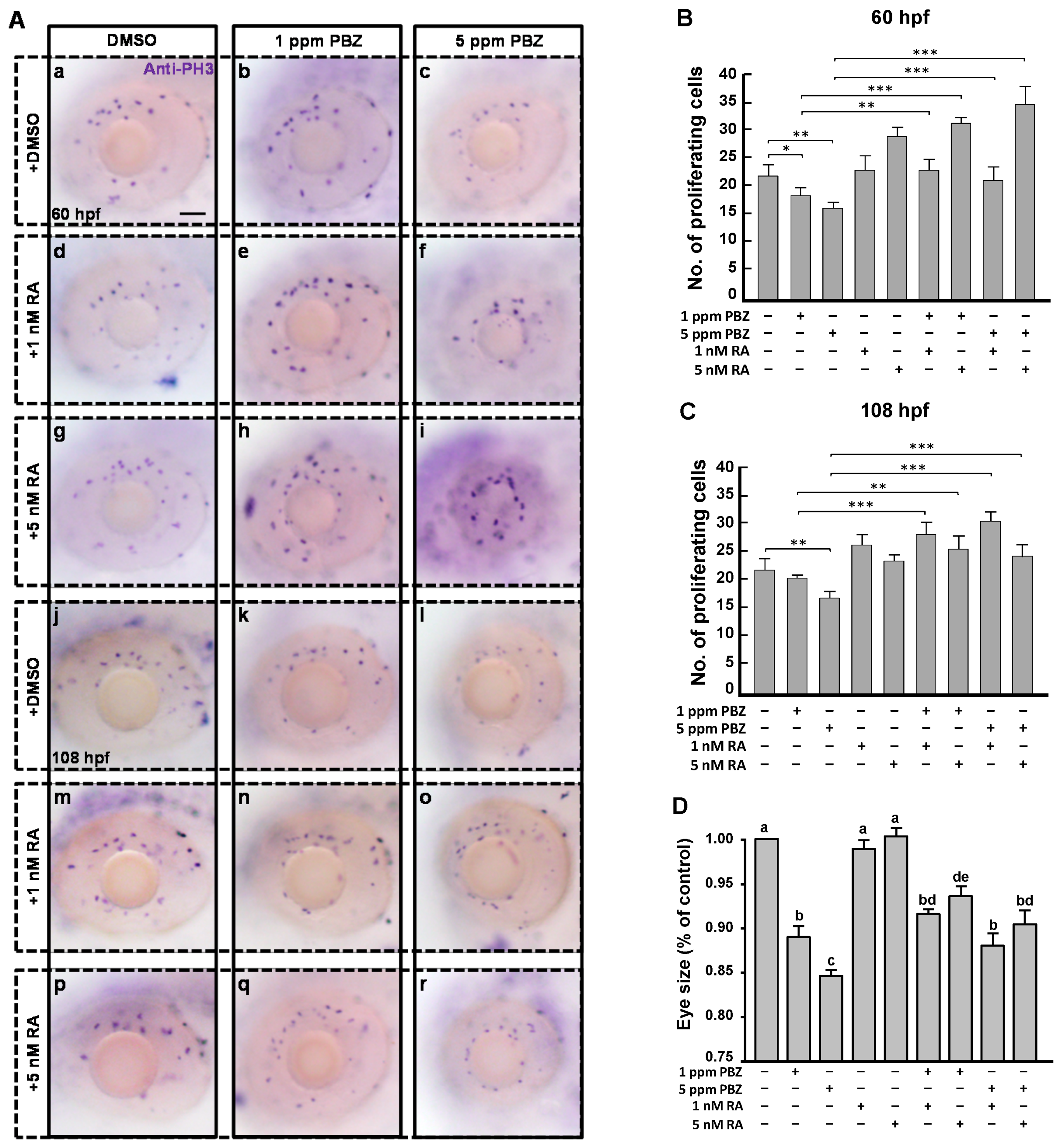
2.2. Toxic Effects of PBZ on the Development of Retinal Photoreceptor Cells
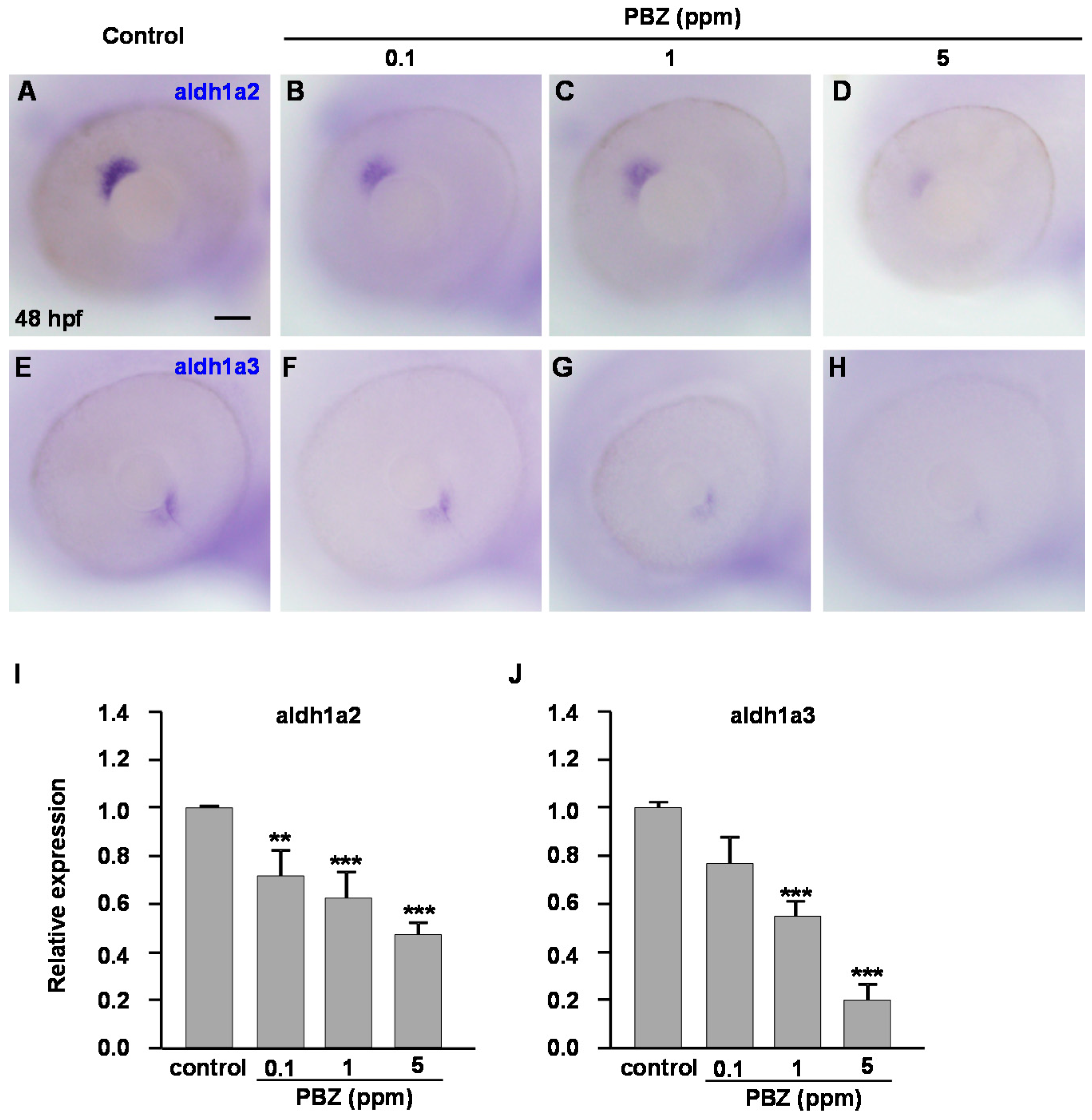
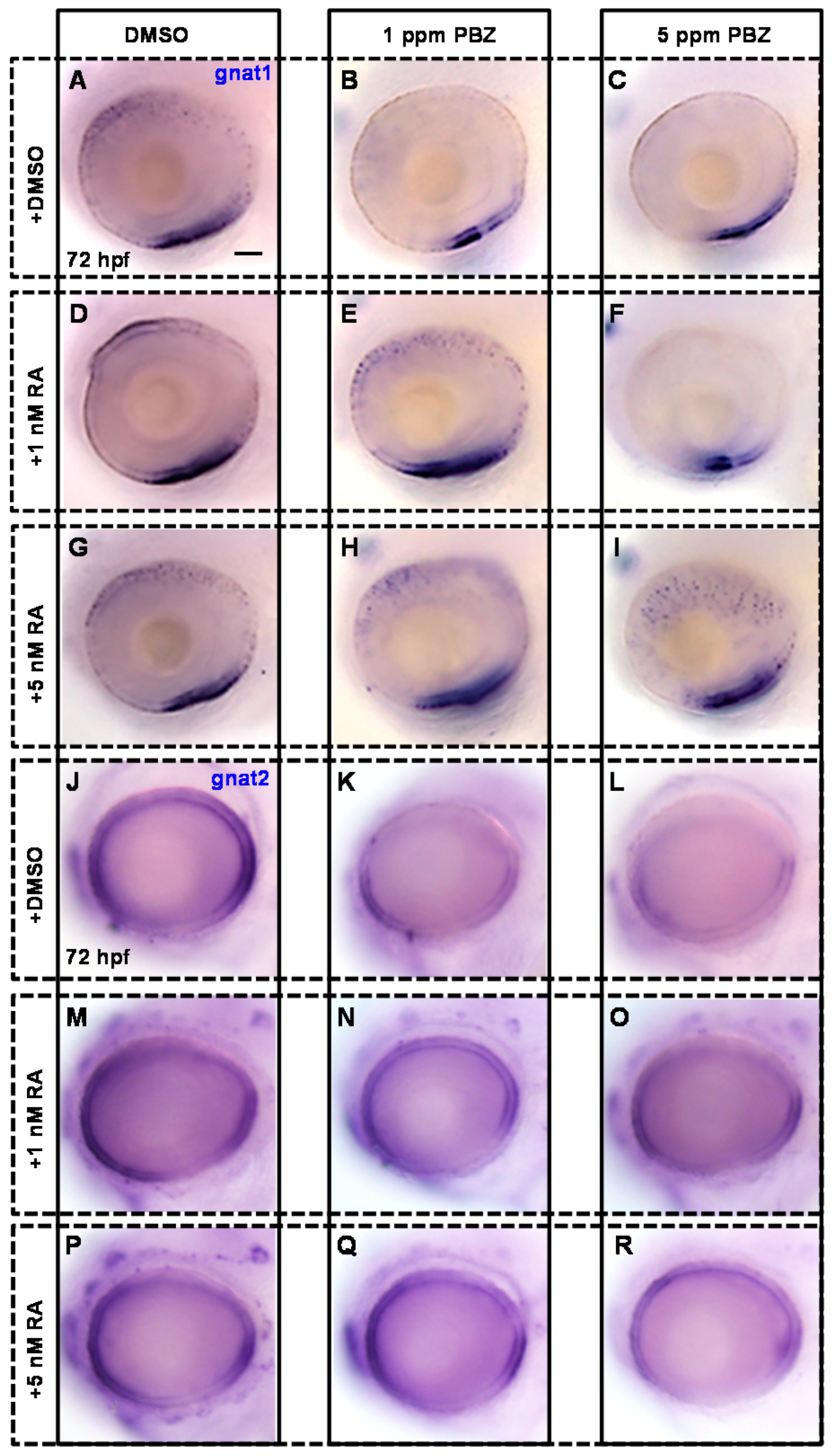
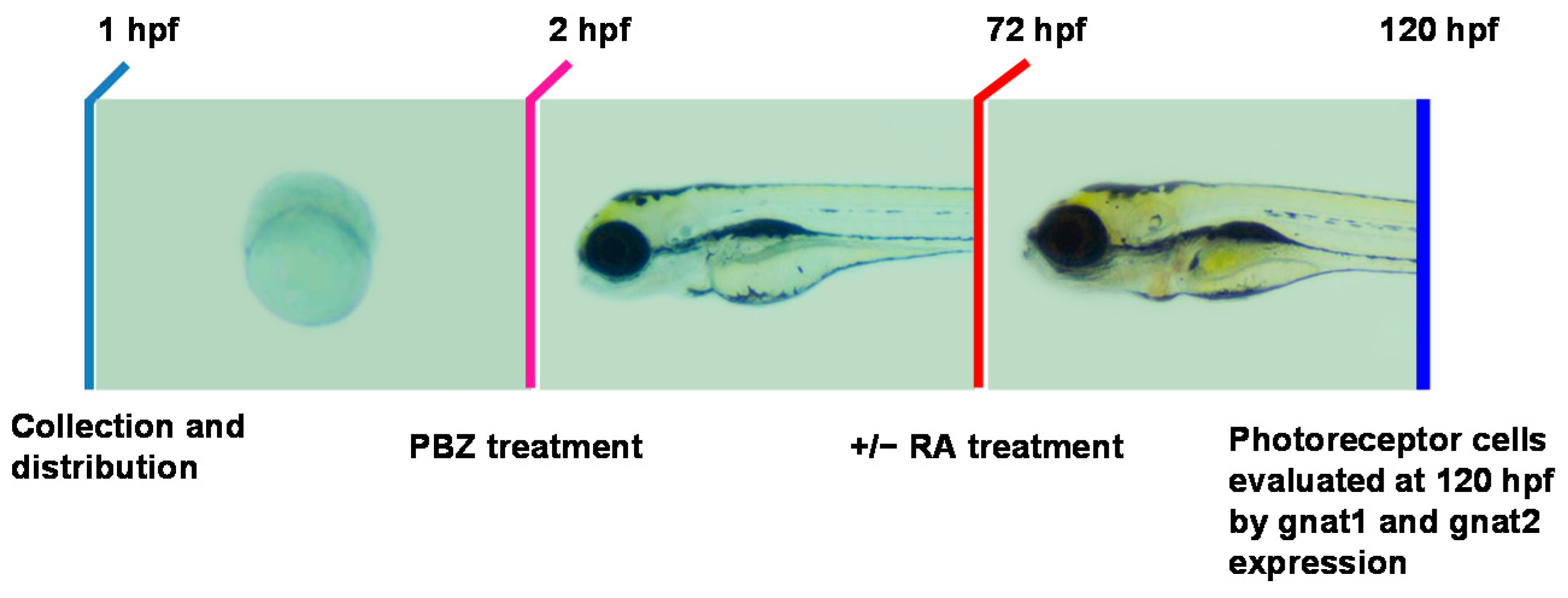
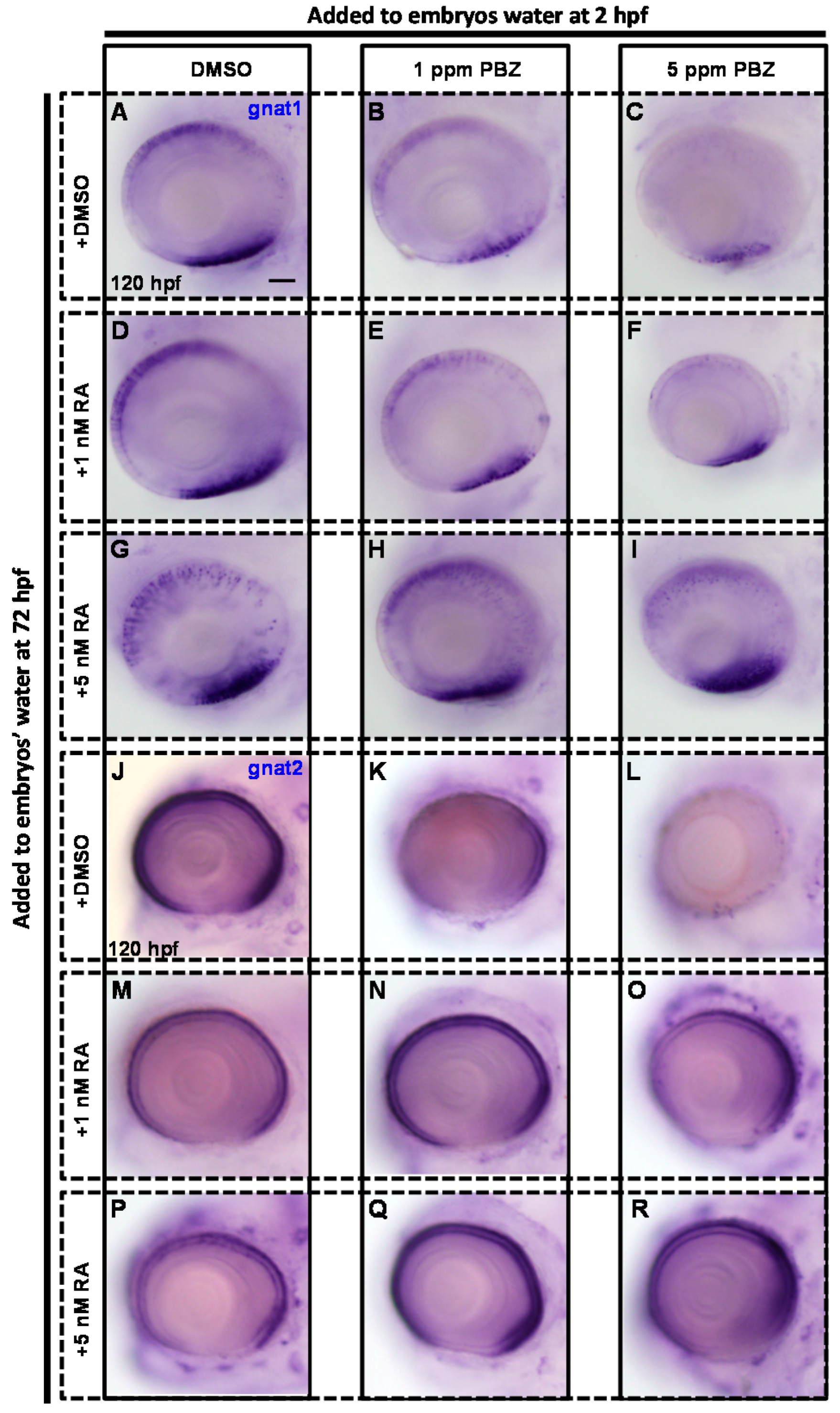
2.3. Reduction of Retinoic Acid Synthesis Is Involved in the Defect in Photoreceptor Cells of Paclobutrazol-Treated Embryos
3. Discussion
3.1. Paclobutrazol Has Wild Toxic Effects on Fish Embryo Development
3.2. Paclobutrazol Affects Retinoic Acid Synthesis
4. Materials and Methods
4.1. Ethics Statement
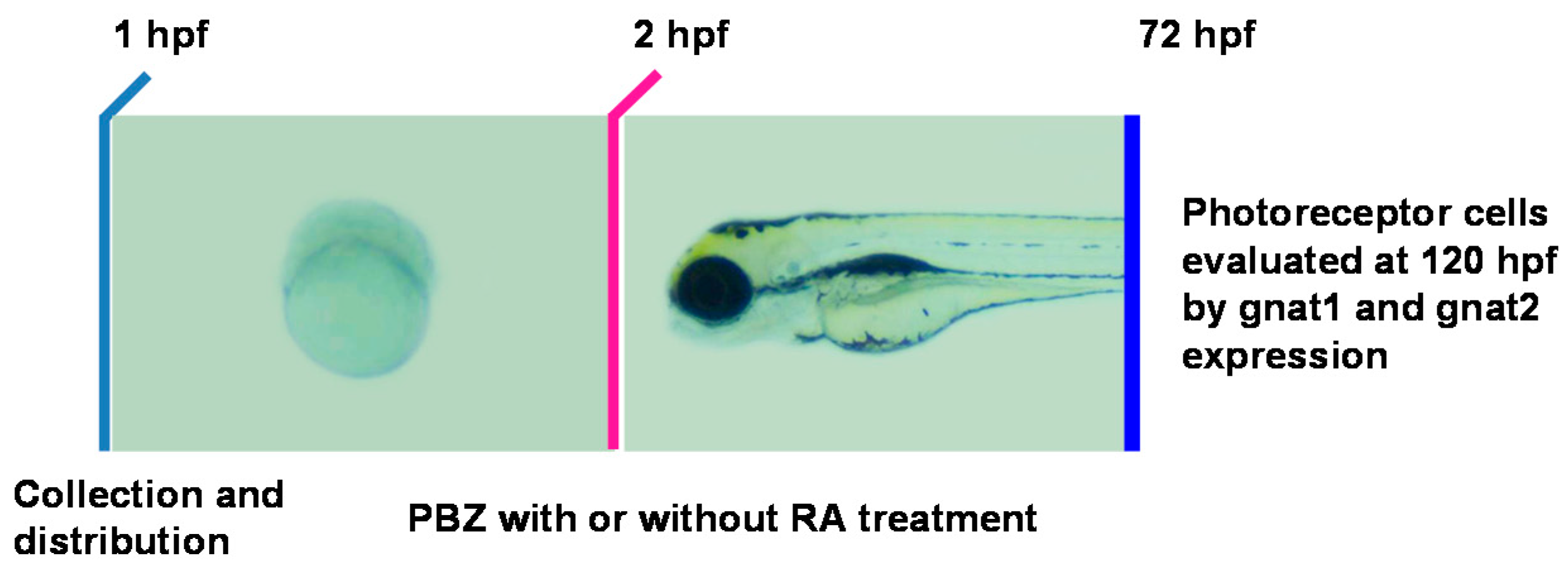
4.2. Fish Maintenance, Embryo Collection, and Treatments
4.3. Chemicals
4.4. Whole-Mount In Situ Hybridization, Immunochemistry, and Cell Death Assay
4.5. Histological Study
4.6. Quantitative Reverse Transcription Polymerase Chain Reaction Analysis
4.7. Image and Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Konwick, B.J.; Garrison, A.W.; Avants, J.K.; Fisk, A.T. Bioaccumulation and biotransformation of chiral triazole fungicides in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2006, 80, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, Q.; Wu, C.; Wang, Z. Application of dispersion-solidification liquid-liquid microextraction for the determination of triazole fungicides in environmental water samples by high-performance liquid chromatography. J. Hazard. Mater. 2011, 185, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Zlabek, V.; Grabic, R.; Li, P.; Randak, T. Modulation of glutathione-related antioxidant defense system of fish chronically treated by the fungicide propiconazole. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Norman, S.M.; Bennett, R.D.; Poling, S.M.; Maier, V.P.; Nelson, M.D. Paclobutrazol inhibits abscisic acid biosynthesis in cercospora rosicola. Plant Physiol. 1986, 80, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, L.; Zuo, Z.; Chen, M.; Wang, C. Effects of paclobutrazol exposure on antioxidant defense system in Sebastiscus marmoratus. Bull. Environ. Contam. Toxicol. 2012, 89, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Song, W.H.; Guo, J.; Gao, M.L.; Hu, W.X. Oxidative stress and structure-activity relationship in the zebrafish (Danio rerio) under exposure to paclobutrazol. J. Environ. Sci. Health B 2009, 44, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, L.; Zuo, Z.; Chen, M.; Geng, H.; Wang, C. Exposure to paclobutrazol disrupts spermatogenesis in male Sebastiscus marmoratus. Aquat. Toxicol. 2012, 122–123, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Zuo, Z.; Chen, M.; Wang, C. Chronic exposure to paclobutrazol causes hepatic steatosis in male rockfish Sebastiscus marmoratus and the mechanism involved. Aquat. Toxicol. 2013, 126, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Yekti, A.P.; Hsu, H.J.; Wang, W.D. The effect of paclobutrazol on the development of zebrafish (Danio rerio) embryos. Zebrafish 2014, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Chen, G.T.; Hsu, H.J.; Wu, C.Y. Aryl hydrocarbon receptor 2 mediates the toxicity of paclobutrazol on the digestive system of zebrafish embryos. Aquat. Toxicol. 2015, 159, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Lamb, T.D. Evolution of phototransduction, vertebrate photoreceptors and retina. Prog. Retin. Eye Res. 2013, 36, 52–119. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y. Phototransduction in rods and cones. In Webvision: The organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 2010; pp. 1–47. [Google Scholar]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Loosli, F.; Winkler, S.; Wittbrodt, J. Six3 overexpression initiates the formation of ectopic retina. Genes Dev. 1999, 13, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Loosli, F.; Staub, W.; Finger-Baier, K.C.; Ober, E.A.; Verkade, H.; Wittbrodt, J.; Baier, H. Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 2003, 4, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Nornes, S.; Clarkson, M.; Mikkola, I.; Pedersen, M.; Bardsley, A.; Martinez, J.P.; Krauss, S.; Johansen, T. Zebrafish contains two Pax6 genes involved in eye development. Mech. Dev. 1998, 77, 185–196. [Google Scholar] [CrossRef]
- Seo, H.C.; Drivenes, O.; Ellingsen, S.; Fjose, A. Transient expression of a novel Six3-related zebrafish gene during gastrulation and eye formation. Gene 1998, 216, 39–46. [Google Scholar] [CrossRef]
- Wargelius, A.; Seo, H.C.; Austbo, L.; Fjose, A. Retinal expression of zebrafish six3.1 and its regulation by Pax6. Biochem. Biophys. Res. Commun. 2003, 309, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.A.; Dowling, J.E. Early eye morphogenesis in the zebrafish, Brachydanio rerio. J. Comp. Neurol. 1994, 344, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.A.; Dowling, J.E. Early retinal development in the zebrafish, Danio rerio: Light and electron microscopic analyses. J. Comp. Neurol. 1999, 404, 515–536. [Google Scholar] [CrossRef]
- Lister, J.A.; Close, J.; Raible, D.W. Duplicate mitf genes in zebrafish: Complementary expression and conservation of melanogenic potential. Dev. Biol. 2001, 237, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Branchek, T.; Bremiller, R. The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. J. Comp. Neurol. 1984, 224, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Raymond, P.A.; Barthel, L.K.; Curran, G.A. Developmental patterning of rod and cone photoreceptors in embryonic zebrafish. J. Comp. Neurol. 1995, 359, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Dolle, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [PubMed]
- Cvekl, A.; Wang, W.L. Retinoic acid signaling in mammalian eye development. Exp. Eye Res. 2009, 89, 280–291. [Google Scholar] [CrossRef]
- Duester, G. Keeping an eye on retinoic acid signaling during eye development. Chem. Biol. Interact. 2009, 178, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, G.A.; Dowling, J.E. Retinoic acid. A key molecule for eye and photoreceptor development. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1471–1475. [Google Scholar]
- Hyatt, G.A.; Schmitt, E.A.; Marsh-Armstrong, N.R.; Dowling, J.E. Retinoic acid-induced duplication of the zebrafish retina. Proc. Natl. Acad. Sci. USA 1992, 89, 8293–8297. [Google Scholar] [CrossRef] [PubMed]
- Prabhudesai, S.N.; Cameron, D.A.; Stenkamp, D.L. Targeted effects of retinoic acid signaling upon photoreceptor development in zebrafish. Dev. Biol. 2005, 287, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, G.A.; Schmitt, E.A.; Fadool, J.M.; Dowling, J.E. Retinoic acid alters photoreceptor development in vivo. Proc. Natl. Acad. Sci. USA 1996, 93, 13298–13303. [Google Scholar] [CrossRef] [PubMed]
- Kam, R.K.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci. 2012, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Marsh-Armstrong, N.; McCaffery, P.; Gilbert, W.; Dowling, J.E.; Drager, U.C. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc. Natl. Acad. Sci. USA 1994, 91, 7286–7290. [Google Scholar] [CrossRef] [PubMed]
- Drager, U.C.; Wagner, E.; McCaffery, P. Aldehyde dehydrogenases in the generation of retinoic acid in the developing vertebrate: A central role of the eye. J. Nutr. 1998, 128, 463S–466S. [Google Scholar] [PubMed]
- Carter-Dawson, L.; Kuwabara, T.; O’Brien, P.J.; Bieri, J.G. Structural and biochemical changes in vitamin A–deficient rat retinas. Investig. Ophthalmol. Vis. Sci. 1979, 18, 437–446. [Google Scholar]
- Carter-Dawson, L.; Kuwabara, T.; Bieri, J.G. Intrinsic, light-independent, regional differences in photoreceptor cell degeneration in vitamin A-deficient rat retinas. Investig. Ophthalmol. Vis. Sci. 1982, 22, 249–252. [Google Scholar]
- Kolb, H. Photoreceptors. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 2012; pp. 1–35. [Google Scholar]
- Cullen, M.C.; Connell, D.W. Pesticide bioaccumulation in cattle. Ecotoxicol. Environ. Saf. 1994, 28, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Jessiman, B.J.; Qadri, S.U. Bioaccumulation kinetics of the organochlorine pesticide mirex in amphipods. Ecotoxicol. Environ. Saf. 1983, 7, 295–305. [Google Scholar] [CrossRef]
- Palacio, J.A.; Henao, B.; Velez, J.H.; Gonzalez, J.; Parra, C.M. Acute toxicity and bioaccumulation of pesticide diazinon in red tilapia (Oreochromis niloticus x Mossambicus albina). Environ. Toxicol. 2002, 17, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Pucher, J.; Gut, T.; Mayrhofer, R.; El-Matbouli, M.; Viet, P.H.; Ngoc, N.T.; Lamers, M.; Streck, T.; Focken, U. Pesticide-contaminated feeds in integrated grass carp aquaculture: Toxicology and bioaccumulation. Dis. Aquat. Organ. 2014, 108, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Day, K.E. Pesticide residues in freshwater and marine zooplankton: A review. Environ. Pollut. 1990, 67, 205–222. [Google Scholar] [CrossRef]
- Wolf, D.C.; Allen, J.W.; George, M.H.; Hester, S.D.; Sun, G.; Moore, T.; Thai, S.F.; Delker, D.; Winkfield, E.; Leavitt, S.; et al. Toxicity profiles in rats treated with tumorigenic and nontumorigenic triazole conazole fungicides: Propiconazole, triadimefon, and myclobutanil. Toxicol. Pathol. 2006, 34, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Itoh, K.; Mizuno, N.; Konishi, H.; Tanimura, T. Alterations in migrating cranial neural crest cells in embryos of mice fed retinoic acid. Anal. Cell. Pathol. 1989, 2, 23–40. [Google Scholar] [PubMed]
- Old, R.W.; Smith, D.P.; Mason, C.S.; Marklew, S.; Jones, E.A. Effects of retinoic acid on xenopus embryos. Biochem. Soc. Symp. 1996, 62, 157–174. [Google Scholar] [PubMed]
- Lyn, S.D. Whole-mount in situ hybridization of mouse embryos exposed to teratogenic levels of retinoic acid. Methods Mol. Biol. 1998, 89, 67–79. [Google Scholar] [PubMed]
- Morriss-Kay, G. Retinoic acid and development. Pathobiology 1992, 60, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; Van Veldhuizen, P.; et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014, 22, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Luo, T.; McCaffery, P.; Hamada, H.; Drager, U.C. CYP26A1 and CYP26C1 cooperate in degrading retinoic acid within the equatorial retina during later eye development. Dev. Biol. 2004, 276, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Weiler, R.; Pottek, M.; Schultz, K.; Janssen-Bienhold, U. Retinoic acid, a neuromodulator in the retina. Prog. Brain Res. 2001, 131, 309–318. [Google Scholar] [PubMed]
- Kelley, M.W.; Williams, R.C.; Turner, J.K.; Creech-Kraft, J.M.; Reh, T.A. Retinoic acid promotes rod photoreceptor differentiation in rat retina in vivo. Neuroreport 1999, 10, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Pares, X.; Farres, J.; Kedishvili, N.; Duester, G. Medium- and short-chain dehydrogenase/reductase gene and protein families : Medium-chain and short-chain dehydrogenases/reductases in retinoid metabolism. Cell. Mol. Life Sci. 2008, 65, 3936–3949. [Google Scholar] [CrossRef] [PubMed]
- Matt, N.; Dupe, V.; Garnier, J.M.; Dennefeld, C.; Chambon, P.; Mark, M.; Ghyselinck, N.B. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 2005, 132, 4789–4800. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The zebrafish book. In A Guide for the Laboratory Use of Zebrafish (Danio Rerio); University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M.; Park, L.; Stenkamp, D.L. Retinal homeobox 1 is required for retinal neurogenesis and photoreceptor differentiation in embryonic zebrafish. Dev. Biol. 2009, 328, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Wilm, T.P.; Solnica-Krezel, L. Essential roles of a zebrafish prdm1/blimp1 homolog in embryo patterning and organogenesis. Development 2005, 132, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Pittlik, S.; Domingues, S.; Meyer, A.; Begemann, G. Expression of zebrafish aldh1a3 (raldh3) and absence of aldh1a1 in teleosts. Gene Expr. Patterns 2008, 8, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-D.; Hsu, H.-J.; Li, Y.-F.; Wu, C.-Y. Retinoic Acid Protects and Rescues the Development of Zebrafish Embryonic Retinal Photoreceptor Cells from Exposure to Paclobutrazol. Int. J. Mol. Sci. 2017, 18, 130. https://doi.org/10.3390/ijms18010130
Wang W-D, Hsu H-J, Li Y-F, Wu C-Y. Retinoic Acid Protects and Rescues the Development of Zebrafish Embryonic Retinal Photoreceptor Cells from Exposure to Paclobutrazol. International Journal of Molecular Sciences. 2017; 18(1):130. https://doi.org/10.3390/ijms18010130
Chicago/Turabian StyleWang, Wen-Der, Hwei-Jan Hsu, Yi-Fang Li, and Chang-Yi Wu. 2017. "Retinoic Acid Protects and Rescues the Development of Zebrafish Embryonic Retinal Photoreceptor Cells from Exposure to Paclobutrazol" International Journal of Molecular Sciences 18, no. 1: 130. https://doi.org/10.3390/ijms18010130





