Neurotrophin Promotes Neurite Outgrowth by Inhibiting Rif GTPase Activation Downstream of MAPKs and PI3K Signaling
Abstract
:1. Introduction
2. Results
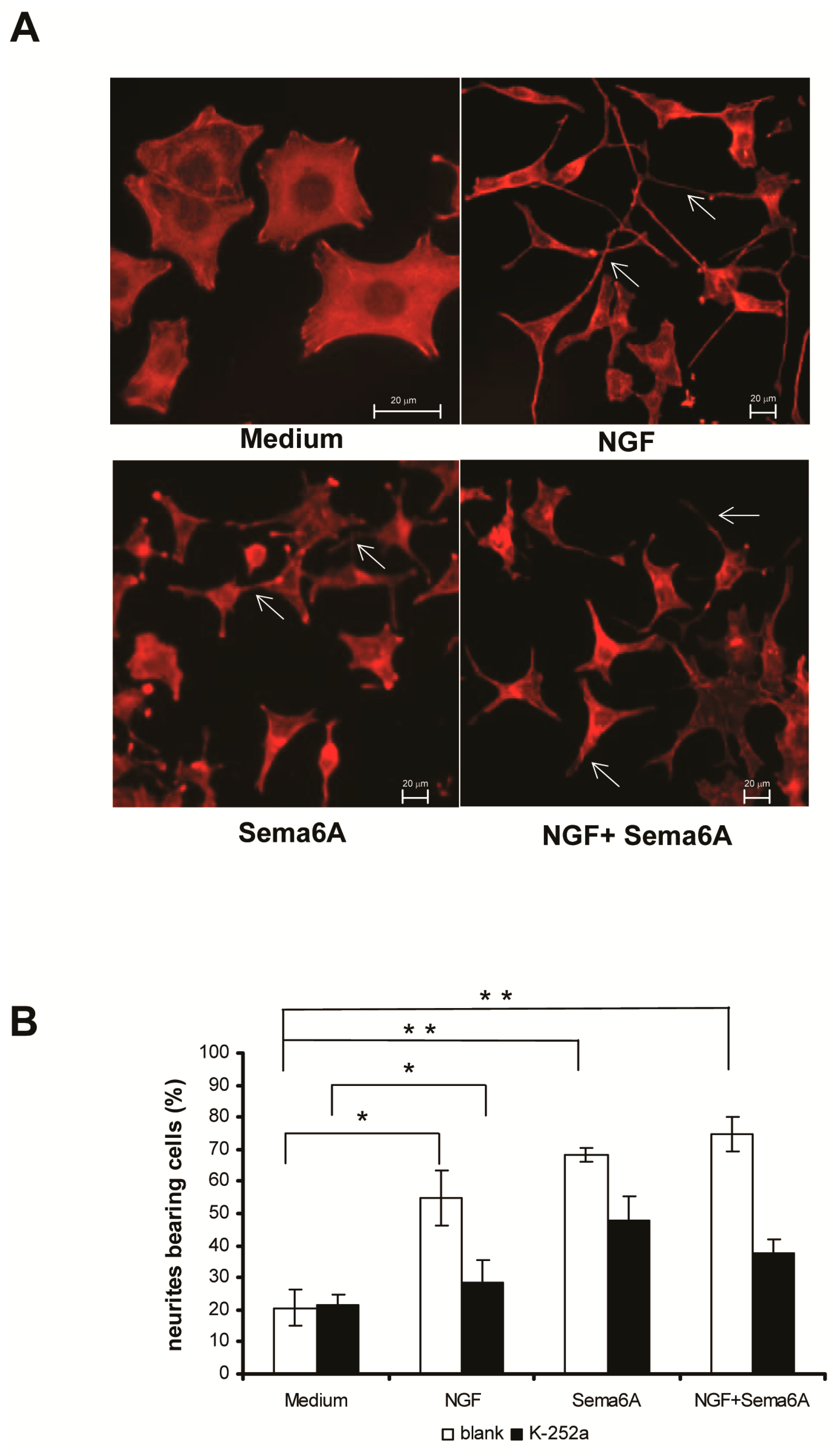
2.1. Semaphorin 6A (Sema6A) Was as Effectively Neurotrophic as Nerve Growth Factor (NGF) in PC12 Cells
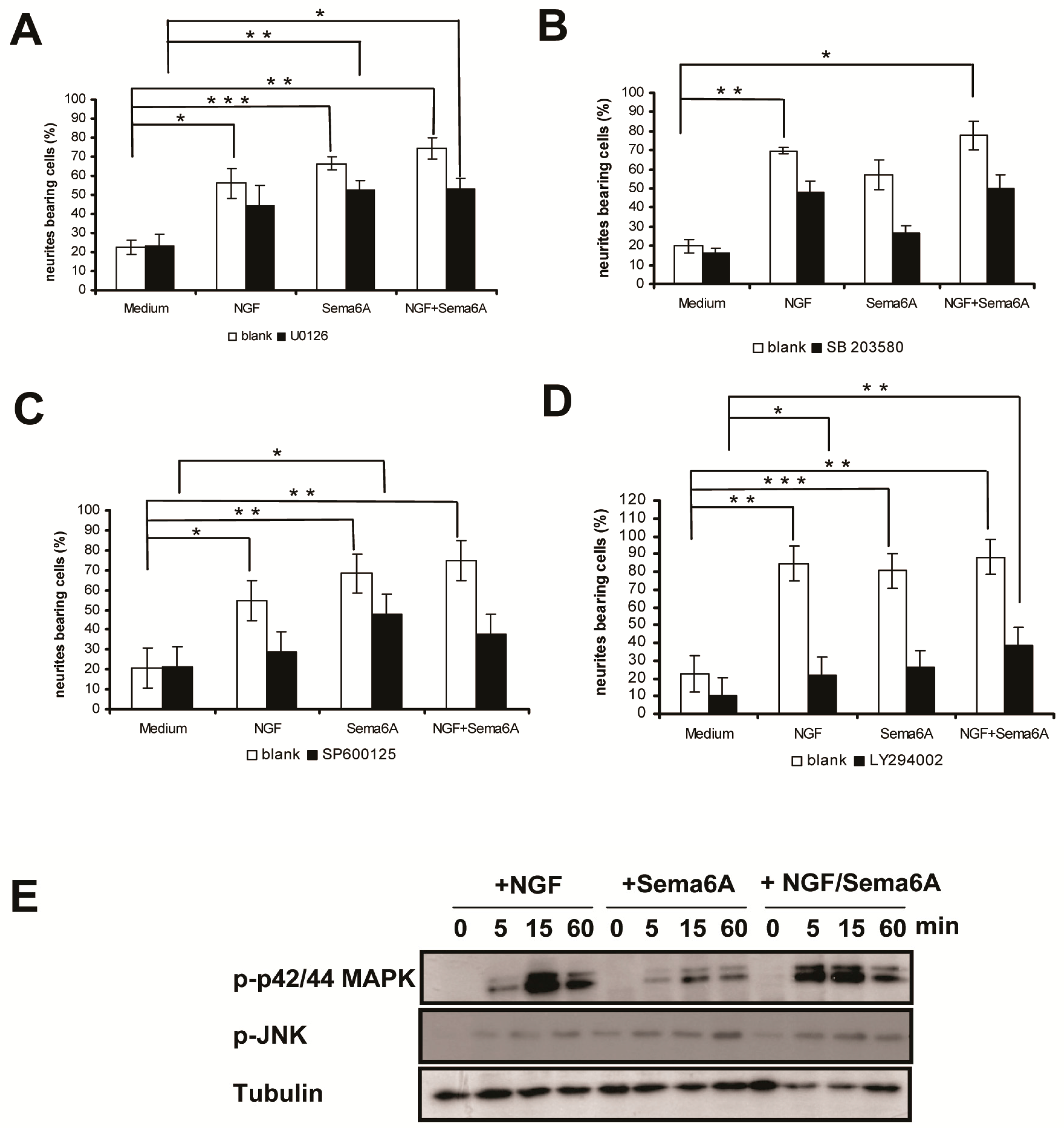
2.2. Mitogen-Activated Protein Kinase and Phosphoinositide 3-Kinase Signaling Were Necessary for Sema6A-Stimulated NGF-Induced Neurite Outgrowth in PC12 Cells
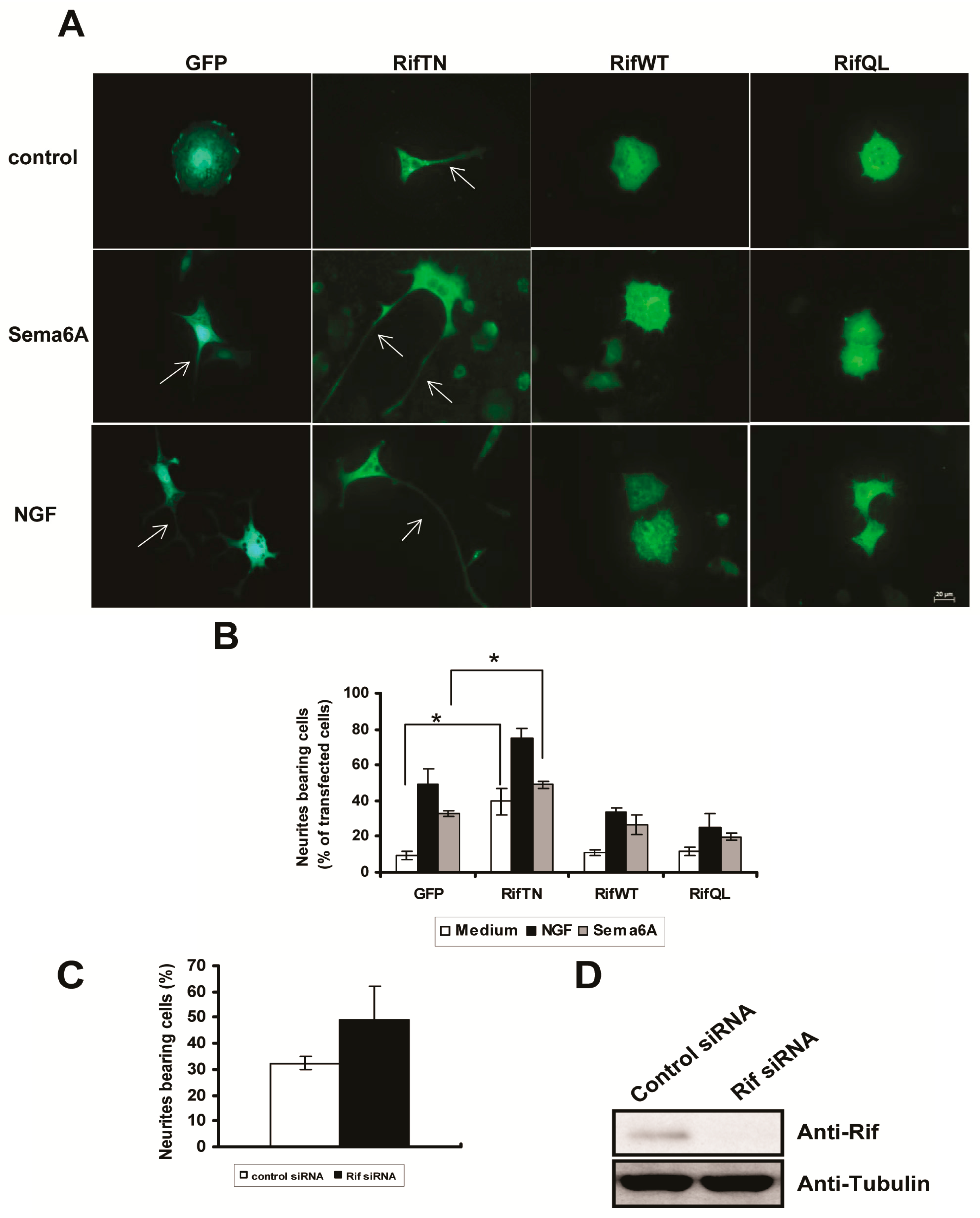
2.3. Rif Expression Antagonized Neurotrophin-Induced Neurite Outgrowth in PC12 Cells
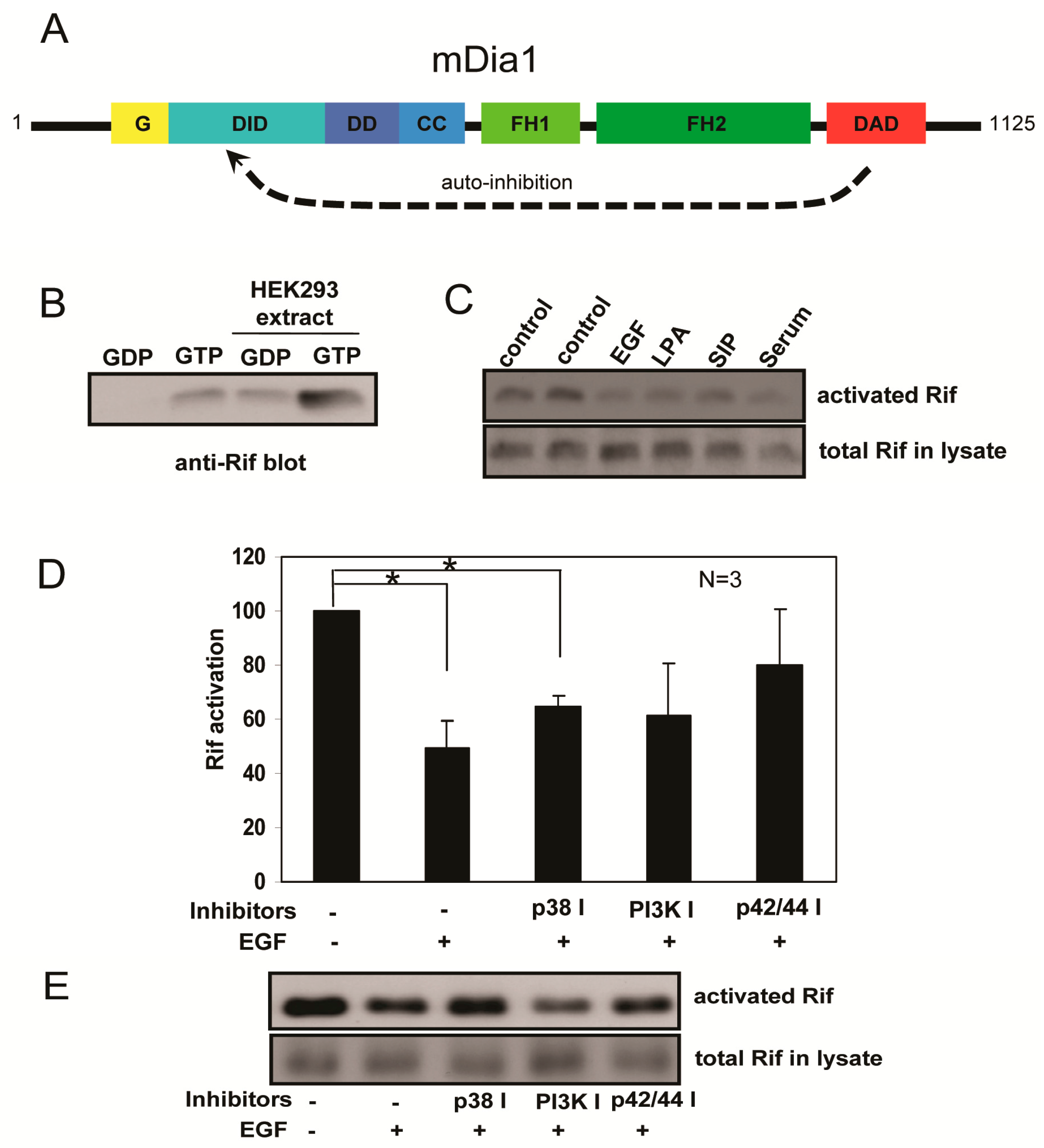
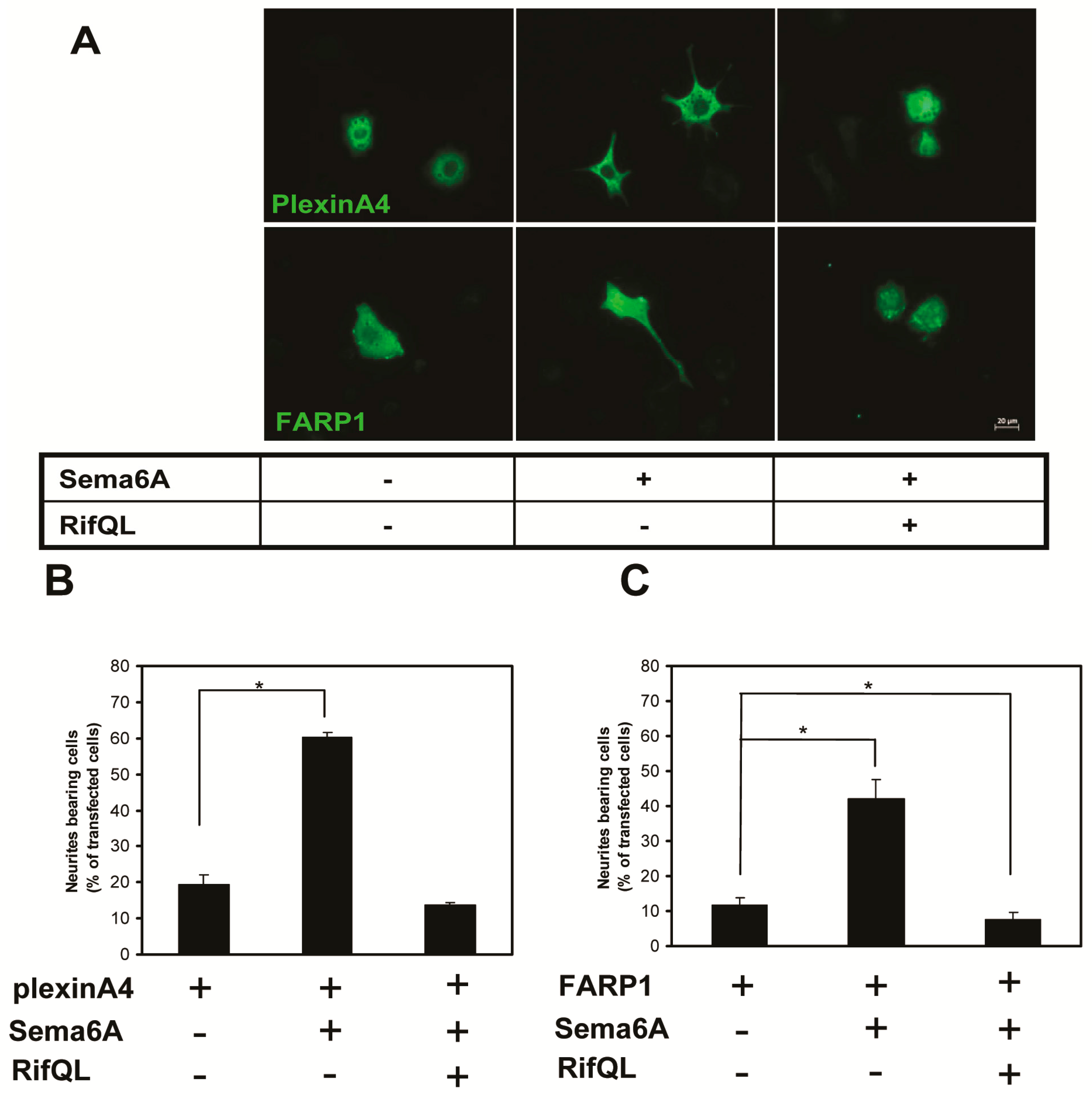
2.4. Rif Activity Inhibited the Mitogenic Stimulation Mediated by Mitogen-Activated Protein Kinases (MAPKs) and Phosphatidylinositol-3-Kinase (PI3K) Activation
2.5. The Roles of Rif in PC12 Cell Neurite Formation
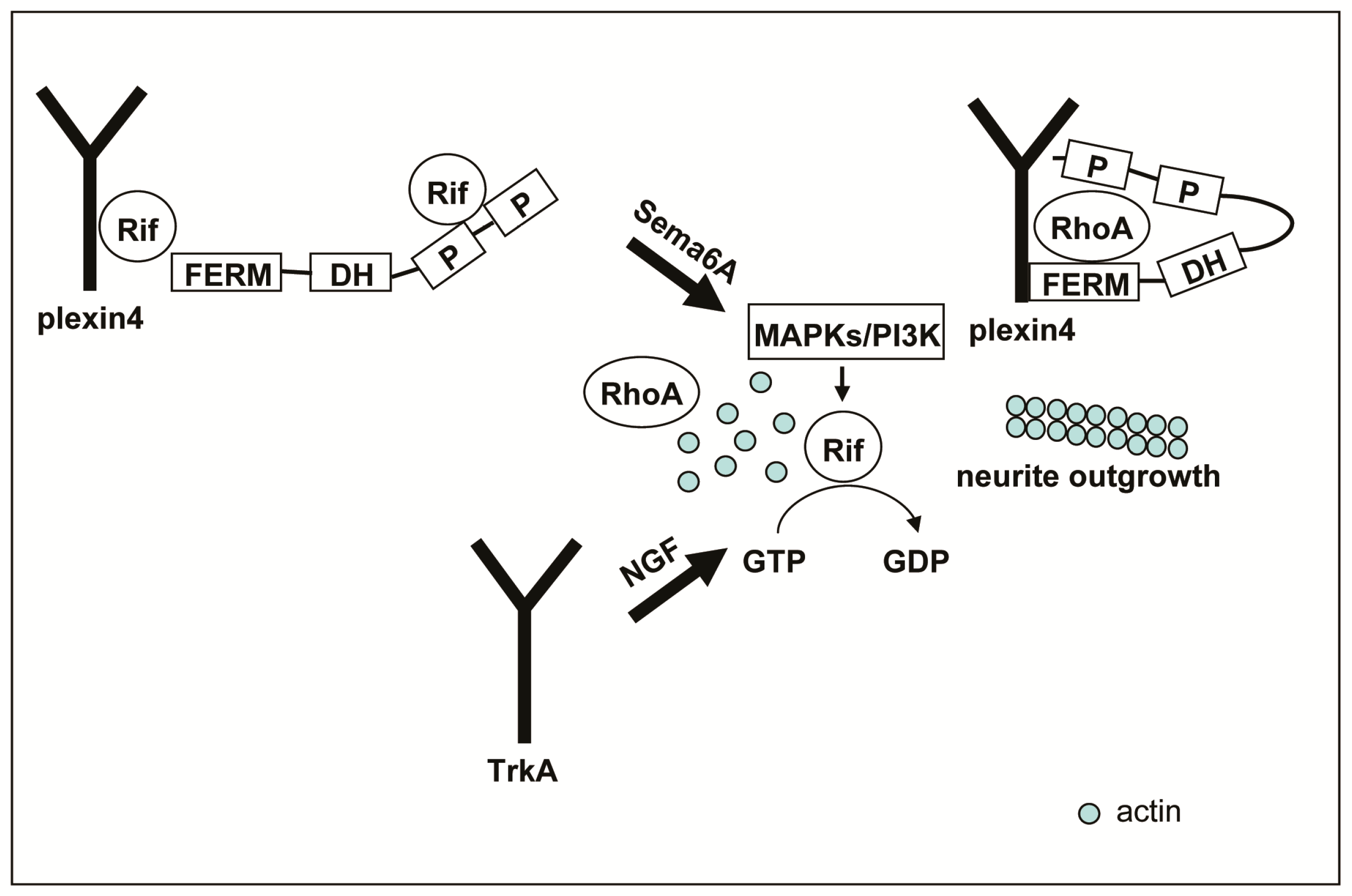
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Determination of Neurite Outgrowth
4.4. siRNA Transfection
4.5. Preparation of Recombinant Protein and Rif Activation Assay
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, Y.; Raible, D.; Raper, J.A. Collapsin: A protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 1993, 75, 217–227. [Google Scholar] [CrossRef]
- Tran, T.S.; Kolodkin, A.L.; Bharadwaj, R. Semaphorin regulation of cellular morphology. Annu. Rev. Cell Dev. Biol. 2007, 23, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Fisher, D.A.; Zhou, L.; White, F.A.; Ng, S.; Snider, W.D.; Luo, Y. The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J. Neurosci. 2000, 20, 2638–2648. [Google Scholar] [PubMed]
- Zhuang, B.; Su, Y.S.; Sockanathan, S. FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane semaphorin 6A and PlexinA4 signaling. Neuron 2009, 61, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Gross, I.; Bassit, B.; Benezra, M.; Licht, J.D. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J. Biol. Chem. 2001, 276, 46460–46468. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, V.; Grasso, S.; Tibullo, D.; Giallongo, C.; Raciti, G.; Viola, M.; Avola, R. Modulation of extracellular signal-related kinase, cyclin D1, glial fibrillary acidic protein, and vimentin expression in estradiol-pretreated astrocyte cultures treated with competence and progression growth factors. J. Neurosci. Res. 2015, 93, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, V.; Grasso, S.; Tibullo, D.; Giallongo, C.; Pappa, R.; Brundo, M.V.; Tomassoni, D.; Viola, M.; Amenta, F.; Avola, R. Neuroactive molecules and growth factors modulate cytoskeletal protein expression during astroglial cell proliferation and differentiation in culture. J. Neurosci. Res. 2016, 94, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, V.; Grasso, S.; Tomassoni, D.; Traini, E.; Raciti, G.; Viola, M.; Li Volti, G.; Campisi, A.; Amenta, F.; Avola, R. Effect of growth factors and steroid hormones on heme oxygenase and cyclin D1 expression in primary astroglial cell cultures. J. Neurosci. Res. 2015, 93, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, V.; Bronzi, D.; Raciti, G.; Avitabile, M.; Avola, R. Neurosteroid-growth factor cross-talk induces up and down regulation of GFAP and vimentin expression in serum free astrocyte cultures. Ital. J. Biochem. 2007, 56, 302–306. [Google Scholar] [PubMed]
- Schwamborn, J.C.; Fiore, R.; Bagnard, D.; Kappler, J.; Kaltschmidt, C.; Puschel, A.W. Semaphorin 3A stimulates neurite extension and regulates gene expression in PC12 cells. J. Biol. Chem. 2004, 279, 30923–30926. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Masuda, K.; Toguchi, M.; Ohoka, Y.; Sakai, T.; Furuyama, T.; Inagaki, S. Neurotrophic effect of semaphorin 4D in PC12 cells. Biochem. Biophys. Res. Commun. 2003, 301, 304–310. [Google Scholar] [CrossRef]
- Chen, X.Q.; Tan, I.; Leung, T.; Lim, L. The myotonic dystrophy kinase-related Cdc42-binding kinase is involved in the regulation of neurite outgrowth in PC12 cells. J. Biol. Chem. 1999, 274, 19901–19905. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Katoh, H.; Negishi, M. Direct interaction of Rnd1 with FRS2 β regulates Rnd1-induced down-regulation of RhoA activity and is involved in fibroblast growth factor-induced neurite outgrowth in PC12 cells. J. Biol. Chem. 2005, 280, 18418–18424. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Katoh, H.; Mori, K.; Negishi, M. Rnd1, a novel rho family GTPase, induces the formation of neuritic processes in PC12 cells. Biochem. Biophys. Res. Commun. 2000, 278, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Katoh, H.; Negishi, M. Pragmin, a novel effector of Rnd2 GTPase, stimulates RhoA activity. J. Biol. Chem. 2006, 281, 10355–10364. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.E.; Moura Costa, M.D.; Bilek, E.S.; Lopes, M.H.; Martins, V.R.; Puschel, A.W.; Mercadante, A.F.; Nakao, L.S.; Zanata, S.M. STI1 antagonizes cytoskeleton collapse mediated by small GTPase Rnd1 and regulates neurite growth. Exp. Cell Res. 2014, 324, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Talens-Visconti, R.; Peris, B.; Guerri, C.; Guasch, R.M. RhoE stimulates neurite-like outgrowth in PC12 cells through inhibition of the RhoA/ROCK-I signalling. J. Neurochem. 2010, 112, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Yasui, H.; Yamaguchi, Y.; Aoki, J.; Fujita, H.; Mori, K.; Negishi, M. Small GTPase RhoG is a key regulator for neurite outgrowth in PC12 cells. Mol. Cell. Biol. 2000, 20, 7378–7387. [Google Scholar] [CrossRef] [PubMed]
- Estrach, S.; Schmidt, S.; Diriong, S.; Penna, A.; Blangy, A.; Fort, P.; Debant, A. The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth. Curr. Biol. 2002, 12, 307–312. [Google Scholar] [CrossRef]
- Katoh, H.; Negishi, M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature 2003, 424, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Pellegrin, S.; Scott, A.; Mellor, H. The small GTPase Rif is an alternative trigger for the formation of actin stress fibers in epithelial cells. J. Cell Sci. 2010, 123 Pt 8, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Mellor, H. The novel Rho-family GTPase rif regulates coordinated actin-based membrane rearrangements. Curr. Biol. 2000, 10, 1387–1390. [Google Scholar] [CrossRef]
- Hotulainen, P.; Llano, O.; Smirnov, S.; Tanhuanpaa, K.; Faix, J.; Rivera, C.; Lappalainen, P. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J. Cell Biol. 2009, 185, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Gouw, L.G.; Reading, N.S.; Jenson, S.D.; Lim, M.S.; Elenitoba-Johnson, K.S. Expression of the Rho-family GTPase gene RHOF in lymphocyte subsets and malignant lymphomas. Br. J. Haematol. 2005, 129, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Bernardini, L.; Valenzano, F.; Bottillo, I.; de Simone, C.; Capizzi, R.; Capalbo, A.; Romano, F.; Novelli, A.; Dallapiccola, B.; et al. Array-based comparative genomic hybridization in early-stage mycosis fungoides: Recurrent deletion of tumor suppressor genes BCL7A, SMAC/DIABLO, and RHOF. Genes Chromosomes Cancer 2008, 47, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yan, H.; Pellegrin, S.; Morigen; Mellor, H. The Rif GTPase regulates cytoskeletal signaling from plexinA4 to promote neurite retraction. Neurosci. Lett. 2015, 590, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.Y.; Moon, M.Y.; Kim, J.H.; Kim, H.J.; Kim, J.G.; Li, Y.; Jin, J.K.; Kim, P.H.; Kim, H.C.; Meier, K.E.; et al. Control of neurite outgrowth by RhoA inactivation. J. Neurochem. 2012, 120, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Gotoh, Y.; Tachibana, T.; Dell, K.; Hattori, S.; Yoneda, Y.; Nishida, E. Induction of neurite outgrowth by MAP kinase in PC12 cells. Oncogene 1995, 11, 239–244. [Google Scholar] [PubMed]
- Vaudry, D.; Stork, P.J.; Lazarovici, P.; Eiden, L.E. Signaling pathways for PC12 cell differentiation: Making the right connections. Science 2002, 296, 1648–1649. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Furuyama, T.; Ohoka, Y.; Miyazaki, N.; Fujioka, S.; Sugimoto, H.; Amasaki, M.; Hattori, S.; Matsuya, T.; Inagaki, S. Mouse semaphorin H induces PC12 cell neurite outgrowth activating Ras-mitogen-activated protein kinase signaling pathway via Ca2+ influx. J. Biol. Chem. 1999, 274, 29666–29671. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, T.; Yoshida, J.; Sugimoto, T.; Zhang, H.; Kumanogoh, A.; Hori, M.; Kikutani, H. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat. Neurosci. 2005, 8, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Vikis, H.G.; Li, W.; Guan, K.L. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev. 2002, 16, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Nagata, S.; Kita, Y.; Nakatsu, N.; Ihara, S.; Kaibuchi, K.; Kuroda, S.; Ui, M.; Iba, H.; Konishi, H.; et al. Expression of a constitutively active phosphatidylinositol 3-kinase induces process formation in rat PC12 cells. Use of Cre/loxP recombination system. J. Biol. Chem. 1997, 272, 16089–16092. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Kimura, K.D.; Kobayashi, M.; Ihara, S.; Kaibuchi, K.; Kuroda, S.; Ui, M.; Iba, H.; Konishi, H.; Kikkawa, U.; et al. Microinjection of activated phosphatidylinositol-3 kinase induces process outgrowth in rat PC12 cells through the Rac-JNK signal transduction pathway. J. Cell Sci. 1998, 111 Pt 7, 907–915. [Google Scholar] [PubMed]
- Jalink, K.; van Corven, E.J.; Hengeveld, T.; Morii, N.; Narumiya, S.; Moolenaar, W.H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 1994, 126, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Otomo, T.; Otomo, C.; Tomchick, D.R.; Machius, M.; Rosen, M.K. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol. Cell 2005, 18, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, S.; Mellor, H. Rho GTPase activation assays. In Current Protocols in Cell Biology/Editorial Board; Bonifacino, J.S., Harford, J.B., Schwartz, J.L., Yamada, K.M., Eds.; Chapter 14, Unit 14.8; John Wiley and Sons, Inc.: New York, NY, USA, 2008. [Google Scholar]
- Jennings, R.T.; Knaus, U.G. Rho family and Rap GTPase activation assays. Methods Mol. Biol. 2014, 1124, 79–88. [Google Scholar] [PubMed]
- Knaus, U.G.; Bamberg, A.; Bokoch, G.M. Rac and Rap GTPase activation assays. Methods Mol. Biol. 2007, 412, 59–67. [Google Scholar] [PubMed]
- Oinuma, I.; Katoh, H.; Negishi, M. Molecular dissection of the semaphorin 4D receptor plexin-B1-stimulated R-Ras GTPase-activating protein activity and neurite remodeling in hippocampal neurons. J. Neurosci. 2004, 24, 11473–11480. [Google Scholar] [CrossRef] [PubMed]
- Zanata, S.M.; Hovatta, I.; Rohm, B.; Puschel, A.W. Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in semaphorin 3A-induced cytoskeletal collapse. J. Neurosci. 2002, 22, 471–477. [Google Scholar] [PubMed]
- Fiore, R.; Puschel, A.W. The function of semaphorins during nervous system development. Front. Biosci. 2003, 8, s484–s499. [Google Scholar] [PubMed]
- Luo, L. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 2000, 1, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Cherfils, J.; Chardin, P. GEFs: Structural basis for their activation of small GTP-binding proteins. Trends Biochem. Sci. 1999, 24, 306–311. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dvorsky, R.; Ahmadian, M.R. Deciphering the molecular and functional basis of Dbl family proteins: A novel systematic approach toward classification of selective activation of the Rho family proteins. J. Biol. Chem. 2013, 288, 4486–4500. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Q.; Heron, P.; Mashburn, C.; Smith, G.M. Targeting sensory axon regeneration in adult spinal cord. J. Neurosci. 2007, 27, 6068–6078. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Hunter, D.; Mackinnon, S.E.; Sakiyama-Elbert, S.E. Heparin-binding-affinity-based delivery systems releasing nerve growth factor enhance sciatic nerve regeneration. J. Biomater. Sci. Polym. Ed. 2010, 21, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Ben-Gigi, L.; Yagil, Z.; Lerman, O.; Behar, O. Semaphorin 3A regulates axon growth independently of growth cone repulsion via modulation of TrkA signaling. Cell Signal. 2008, 20, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Dontchev, V.D.; Letourneau, P.C. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J. Neurosci. 2002, 22, 6659–6669. [Google Scholar] [PubMed]
- Pellegrin, S.; Mellor, H. The Rho family GTPase Rif induces filopodia through mDia2. Curr. Biol. 2005, 15, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.C.; Zheng, J.Y.; Tang, L.J.; Huang, B.S.; Li, K.; Tao, Y.; Yu, W.; Zhu, R.L.; Li, S.; Li, L.X. miR-133b Promotes neurite outgrowth by targeting RhoA expression. Cell. Physiol. Biochem. 2015, 35, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Kato, T.; Fujita, A.; Ishizaki, T.; Narumiya, S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1999, 1, 136–143. [Google Scholar] [PubMed]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Yan, H.; Li, J.; Wu, S.; Wang, J.; Fan, L. Neurotrophin Promotes Neurite Outgrowth by Inhibiting Rif GTPase Activation Downstream of MAPKs and PI3K Signaling. Int. J. Mol. Sci. 2017, 18, 148. https://doi.org/10.3390/ijms18010148
Tian X, Yan H, Li J, Wu S, Wang J, Fan L. Neurotrophin Promotes Neurite Outgrowth by Inhibiting Rif GTPase Activation Downstream of MAPKs and PI3K Signaling. International Journal of Molecular Sciences. 2017; 18(1):148. https://doi.org/10.3390/ijms18010148
Chicago/Turabian StyleTian, Xiaoxia, Huijuan Yan, Jiayi Li, Shuang Wu, Junyu Wang, and Lifei Fan. 2017. "Neurotrophin Promotes Neurite Outgrowth by Inhibiting Rif GTPase Activation Downstream of MAPKs and PI3K Signaling" International Journal of Molecular Sciences 18, no. 1: 148. https://doi.org/10.3390/ijms18010148




