Glucocorticoids Protect Neonatal Rat Brain in Model of Hypoxic-Ischemic Encephalopathy (HIE)
Abstract
:1. Introduction
2. Results
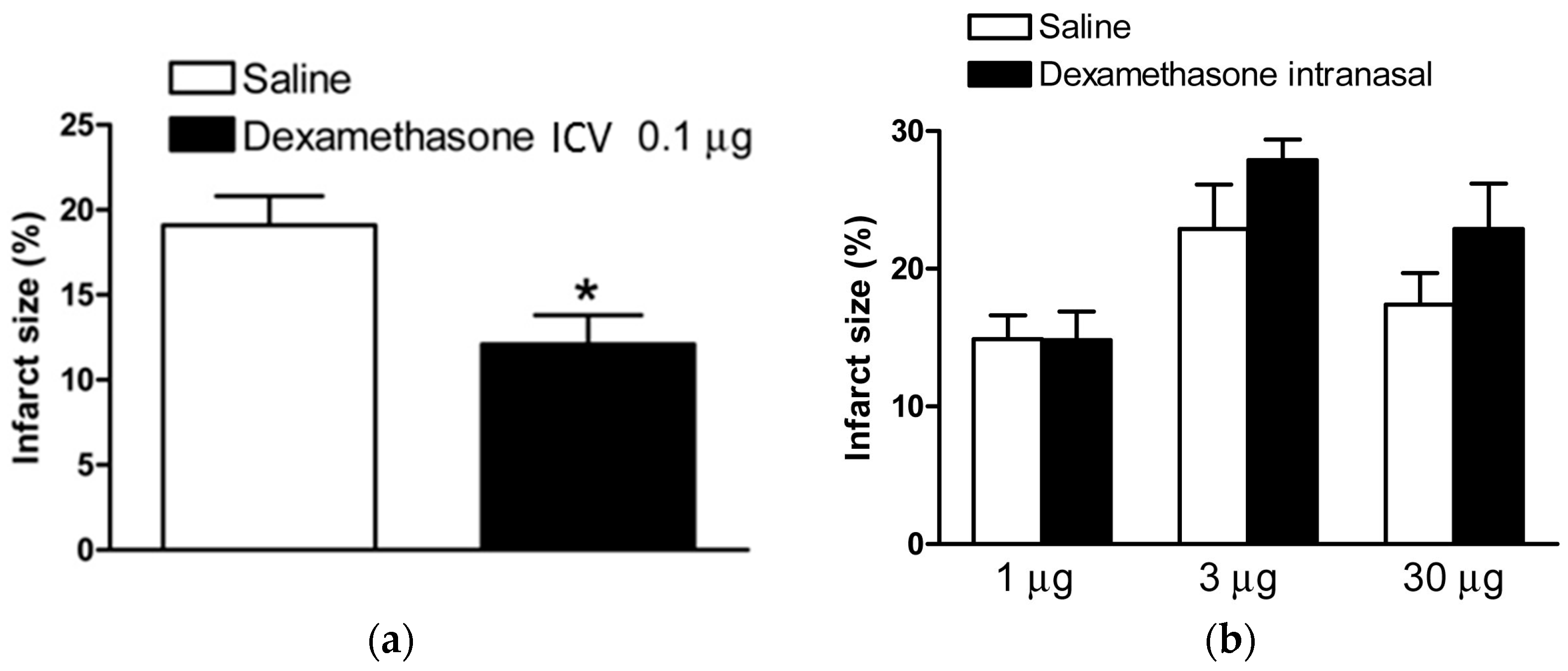
2.1. Dexamethasone after HI Injury via ICV Injection and Intranasal Administration
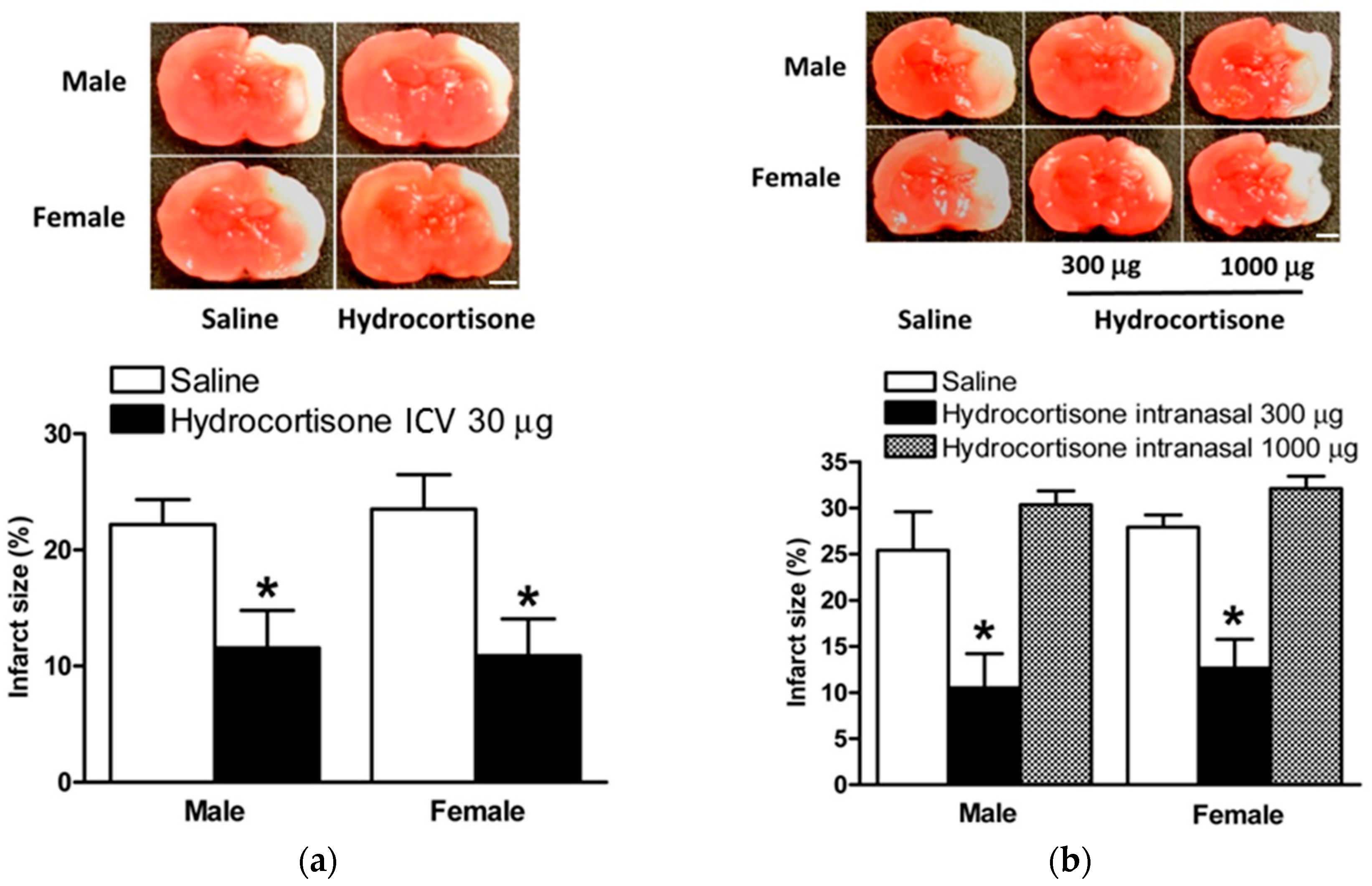
2.2. Hydrocortisone after HI Injury via ICV Injection and Intranasal Administration
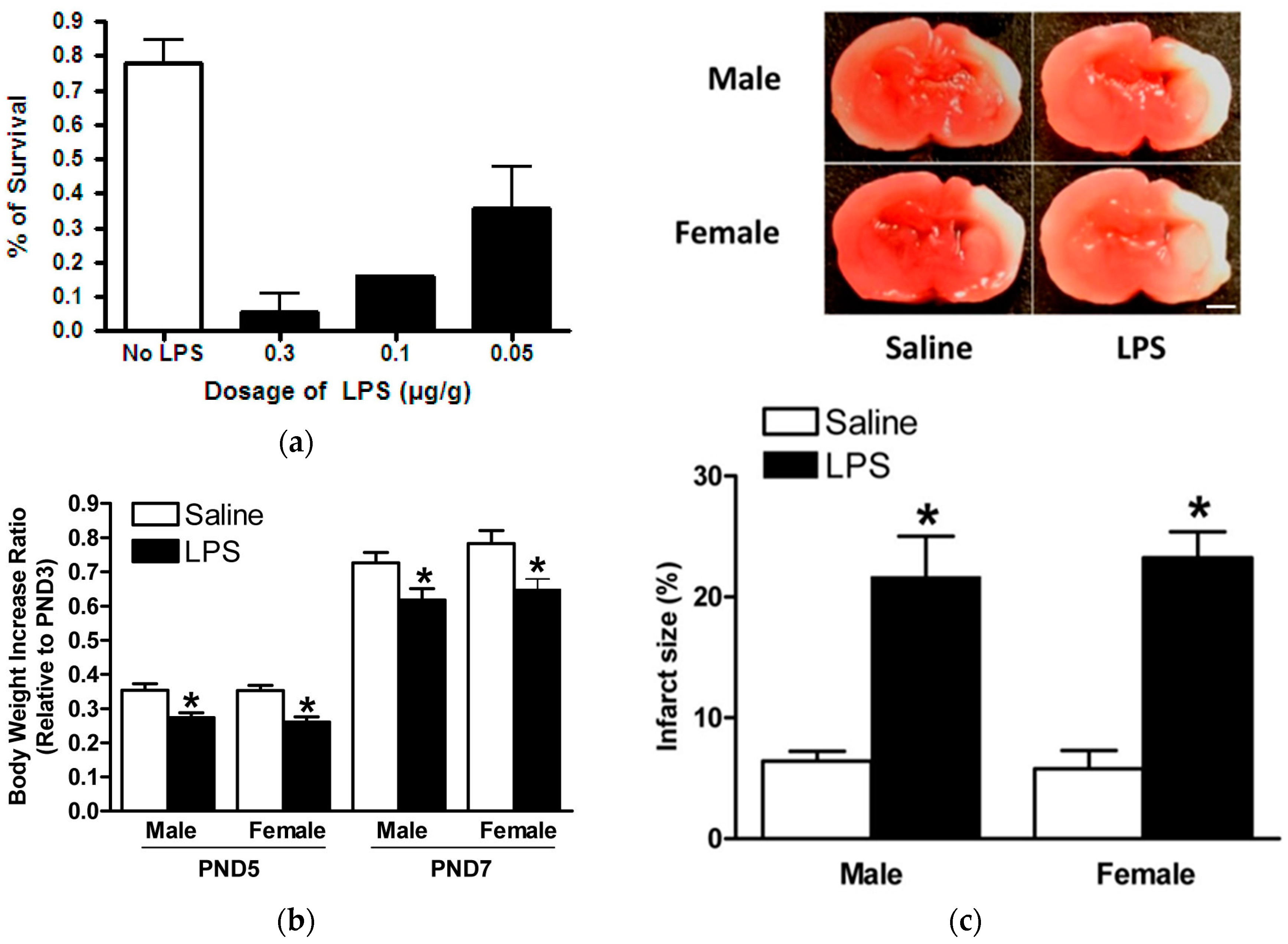
2.3. Model of LPS Sensitization in Neonatal Rat Brains with HIE
2.4. Hydrocortisone via ICV Injection and Intranasal Administration in Model of HIE with LPS Sensitization
3. Discussion
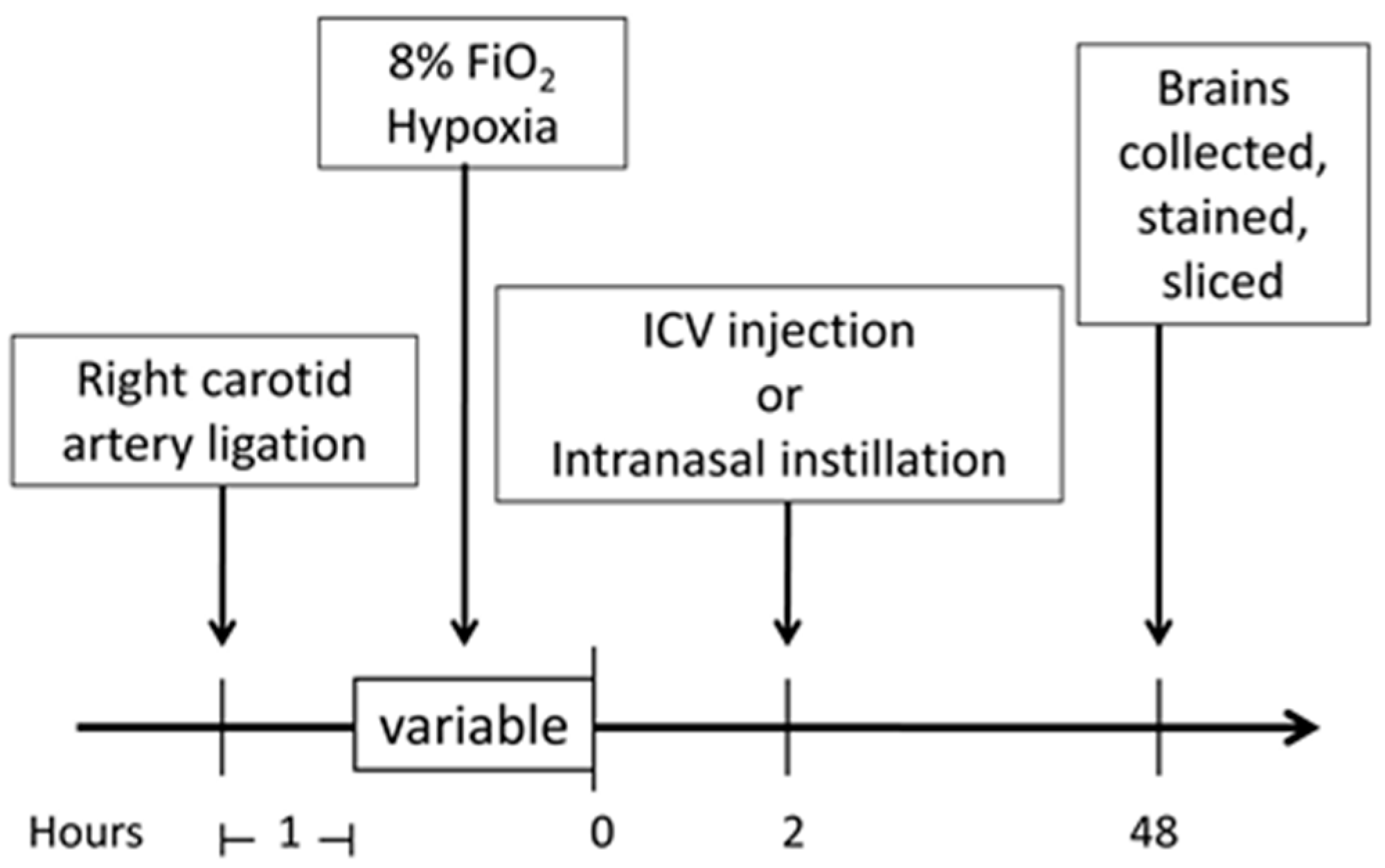
4. Materials and Methods
4.1. Experimental Animals
4.2. Neonatal Hypoxic–Ischemic Encephalopathy (HIE) Rat Model
4.3. Lipopolysaccharides (LPS) Sensitization of Neonatal HIE Rat Model
4.4. Intracerebroventricular (ICV) Injection
4.5. Intranasal Treatment
4.6. Measurement of Infarction Size
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2014, 385, 430–440. [Google Scholar] [CrossRef]
- Vannucci, R.C. Current and potentially new management strategies for perinatal hypoxic-ischemic encephalopathy. Pediatrics 1990, 85, 961–968. [Google Scholar] [PubMed]
- Shalak, L.F.; Laptook, A.R.; Jafri, H.S.; Ramilo, O.; Perlman, J.M. Clinical chorioamnionitis, elevated cytokines, and brain injury in term infants. Pediatrics 2002, 110, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Morley, C.J.; Inder, T.E.; Stewart, M.J.; Smith, K.R.; McNamara, P.J.; Wright, I.M.; Kirpalani, H.M.; Darlow, B.A.; Doyle, L.W.; et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: A randomized controlled trial. Arch. Pediatr. Adolesc. Med. 2011, 165, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C.; Perlman, J.M. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics 1997, 100, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Lear, C.A.; Koome, M.E.; Davidson, J.O.; Drury, P.P.; Quaedackers, J.S.; Galinsky, R.; Gunn, A.J.; Bennet, L. The effects of dexamethasone on post-asphyxial cerebral oxygenation in the preterm fetal sheep. J. Physiol. 2014, 592, 5493–5505. [Google Scholar] [CrossRef] [PubMed]
- Abraham, I.M.; Harkany, T.; Horvath, K.M.; Luiten, P.G. Action of glucocorticoids on survival of nerve cells: Promoting neurodegeneration or neuroprotection? J. Neuroendocrinol. 2001, 13, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Rhodes, P.G.; Bhatt, A.J. Dexamethasone pre-treatment protects brain against hypoxic-ischemic injury partially through up-regulation of vascular endothelial growth factor a in neonatal rats. Neuroscience 2011, 179, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodriguez, P.J.; Li, Y.; Martinez, F.; Zhang, L.B. Dexamethasone protects neonatal hypoxic-ischemic brain injury via l-PGDS-dependent PGD2-DP1-pERK signaling pathway. PLoS ONE 2014, 9, e114470. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Dasgupta, C.; Li, Y.; Bajwa, N.M.; Xiong, F.; Harding, B.; Hartman, R.; Zhang, L. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol. Dis. 2016, 89, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.E., 3rd; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Karemaker, R.; Heijnen, C.J.; Veen, S.; Baerts, W.; Samsom, J.; Visser, G.H.; Kavelaars, A.; van Doornen, L.J.; van Bel, F. Differences in behavioral outcome and motor development at school age after neonatal treatment for chronic lung disease with dexamethasone versus hydrocortisone. Pediatr. Res. 2006, 60, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Laptook, A.R. Birth asphyxia and hypoxic-ischemic brain injury in the preterm infant. Clin. Perinatol. 2016, 43, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H. What is early brain injury? Transl. Stroke Res. 2015, 6, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ranchhod, S.M.; Gunn, K.C.; Fowke, T.M.; Davidson, J.O.; Lear, C.A.; Bai, J.; Bennet, L.; Mallard, C.; Gunn, A.J.; Dean, J.M. Potential neuroprotective strategies for perinatal infection and inflammation. Int. J. Dev. Neurosci. 2015, 45, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C.; Vannucci, S.J. A model of perinatal hypoxic-ischemic brain damage. Ann. N. Y. Acad. Sci. 1997, 835, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, C.E.; Boylan, G.B.; Murray, D.M. Short and long term prognosis in perinatal asphyxia: An update. World J. Clin. Pediatr. 2016, 5, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Flagel, S.B.; Vazquez, D.M.; Watson, S.J., Jr.; Neal, C.R., Jr. Effects of tapering neonatal dexamethasone on rat growth, neurodevelopment, and stress response. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, 55–63. [Google Scholar]
- Neal, C.R., Jr.; Weidemann, G.; Kabbaj, M.; Vazquez, D.M. Effect of neonatal dexamethasone exposure on growth and neurological development in the adult rat. American journal of physiology. Regul. Integr. Comp. Physiol. 2004, 287, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Navarro, H.A.; Lachowicz, J.; Bartolome, J.; Whitmore, W.L.; Slotkin, T.A. Effects of prenatal dexamethasone on development of ornithine decarboxylase activity in brain and peripheral tissues of rats. Pediatr. Res. 1988, 24, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.A.; Huttenlocher, P.R. The effect of corticosteroids on dendritic development in the rat brain. Yale J. Biol. Med. 1974, 47, 155–165. [Google Scholar] [PubMed]
- Whitelaw, A.; Thoresen, M. Antenatal steroids and the developing brain. Arch. Dis. Child Fetal Neonatal Ed. 2000, 83, 154–157. [Google Scholar] [CrossRef]
- Feng, Y.; Kumar, P.; Wang, J.; Bhatt, A.J. Dexamethasone but not the equivalent doses of hydrocortisone induces neurotoxicity in neonatal rat brain. Pediatr. Res. 2015, 77, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Z.; Wen, X.H.; Liu, H. Sex differences in brain mri abnormalities and neurodevelopmental outcomes in a rat model of neonatal hypoxia-ischemia. Int. J. Neurosci. 2016, 126, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Ferreira, E.; Phillips, E.; Francesch-Domenech, E.; Thei, L.; Peebles, D.M.; Raivich, G.; Hristova, M. The role of different strain backgrounds in bacterial endotoxin-mediated sensitization to neonatal hypoxic-ischemic brain damage. Neuroscience 2015, 311, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.R.; Denson, J.L.; Joste, N.E.; Robinson, S.; Jantzie, L.L. Combined in utero hypoxia-ischemia and lipopolysaccharide administration in rats induces chorioamnionitis and a fetal inflammatory response syndrome. Placenta 2015, 36, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Jantzie, L.L.; Corbett, C.J.; Berglass, J.; Firl, D.J.; Flores, J.; Mannix, R.; Robinson, S. Complex pattern of interaction between in utero hypoxia-ischemia and intra-amniotic inflammation disrupts brain development and motor function. J. Neuroinflamm. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Kim, K.D.; Yang, X.; Auh, S.; Fu, Y.X.; Tang, H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc. Natl. Acad. Sci. USA 2008, 105, 7528–7533. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Chang, Y.C.; Lin, C.Y.; Hong, J.S.; Huang, C.C. Low-dose lipopolysaccharide selectively sensitizes hypoxic ischemia-induced white matter injury in the immature brain. Pediatr. Res. 2010, 68, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Stigger, F.; Lovatel, G.; Marques, M.; Bertoldi, K.; Moyses, F.; Elsner, V.; Siqueira, I.R.; Achaval, M.; Marcuzzo, S. Inflammatory response and oxidative stress in developing rat brain and its consequences on motor behavior following maternal administration of LPS and perinatal anoxia. Int. J. Dev. Neurosci. 2013, 31, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, D.; Thoresen, M.; Maes, E.; Flatebo, T.; Elstad, M.; Sabir, H. Hypothermia is not neuroprotective after infection-sensitized neonatal hypoxic-ischemic brain injury. Resuscitation 2014, 85, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Roohey, T.; Raju, T.N.; Moustogiannis, A.N. Animal models for the study of perinatal hypoxic-ischemic encephalopathy: A critical analysis. Early Hum. Dev. 1997, 47, 115–146. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, D.; Dasgupta, C.; Xiong, F.; Tong, W.; Yang, S.; Zhang, L. Perinatal nicotine exposure increases vulnerability of hypoxic-ischemic brain injury in neonatal rats: Role of angiotensin ii receptors. Stroke 2012, 43, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Bederson, J.B.; Pitts, L.H.; Germano, S.M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H.M. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 1986, 17, 1304–1308. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harding, B.; Conception, K.; Li, Y.; Zhang, L. Glucocorticoids Protect Neonatal Rat Brain in Model of Hypoxic-Ischemic Encephalopathy (HIE). Int. J. Mol. Sci. 2017, 18, 17. https://doi.org/10.3390/ijms18010017
Harding B, Conception K, Li Y, Zhang L. Glucocorticoids Protect Neonatal Rat Brain in Model of Hypoxic-Ischemic Encephalopathy (HIE). International Journal of Molecular Sciences. 2017; 18(1):17. https://doi.org/10.3390/ijms18010017
Chicago/Turabian StyleHarding, Benjamin, Katherine Conception, Yong Li, and Lubo Zhang. 2017. "Glucocorticoids Protect Neonatal Rat Brain in Model of Hypoxic-Ischemic Encephalopathy (HIE)" International Journal of Molecular Sciences 18, no. 1: 17. https://doi.org/10.3390/ijms18010017




