Changes of Photosynthetic Behaviors and Photoprotection during Cell Transformation and Astaxanthin Accumulation in Haematococcus pluvialis Grown Outdoors in Tubular Photobioreactors
Abstract
:1. Introduction
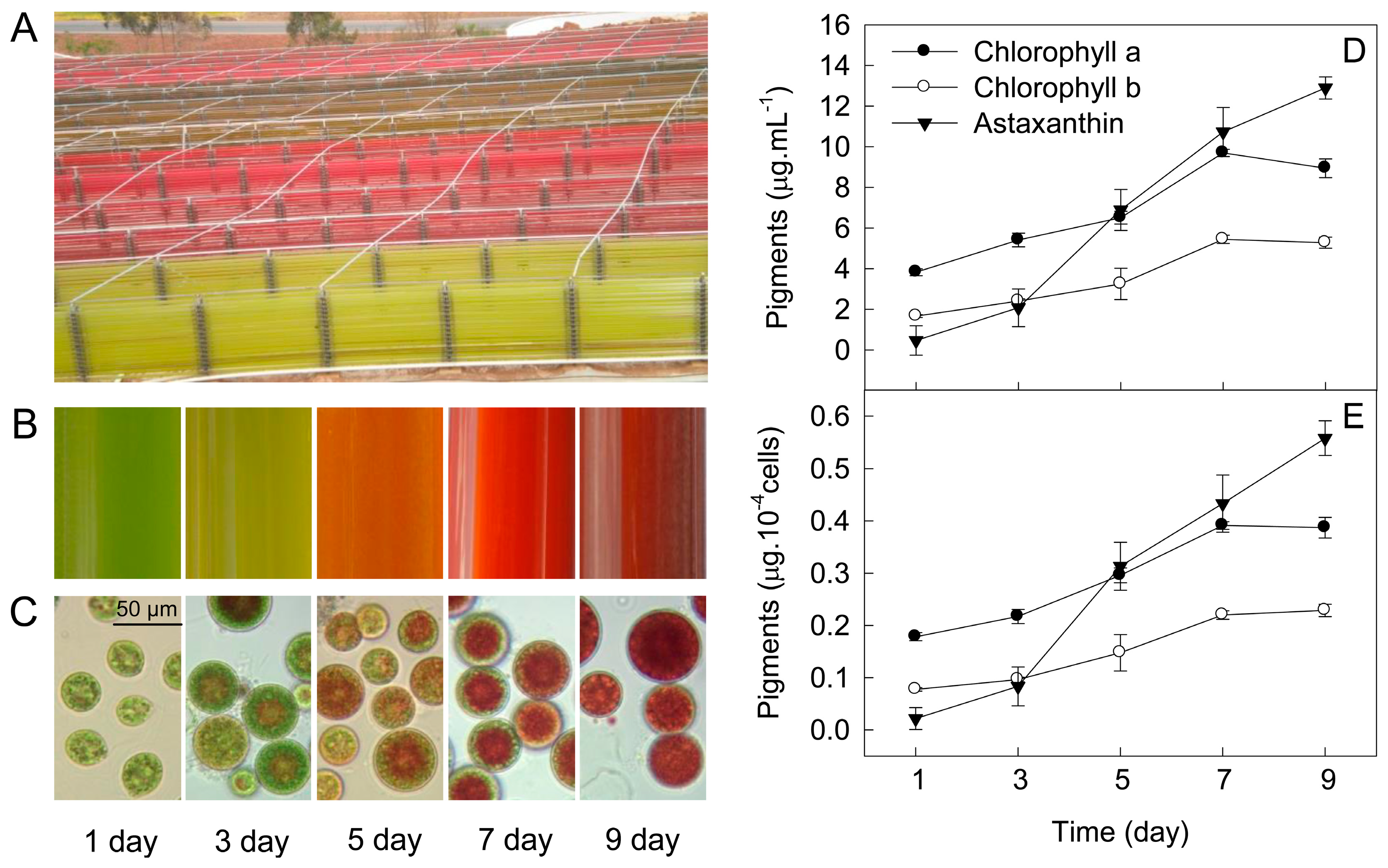
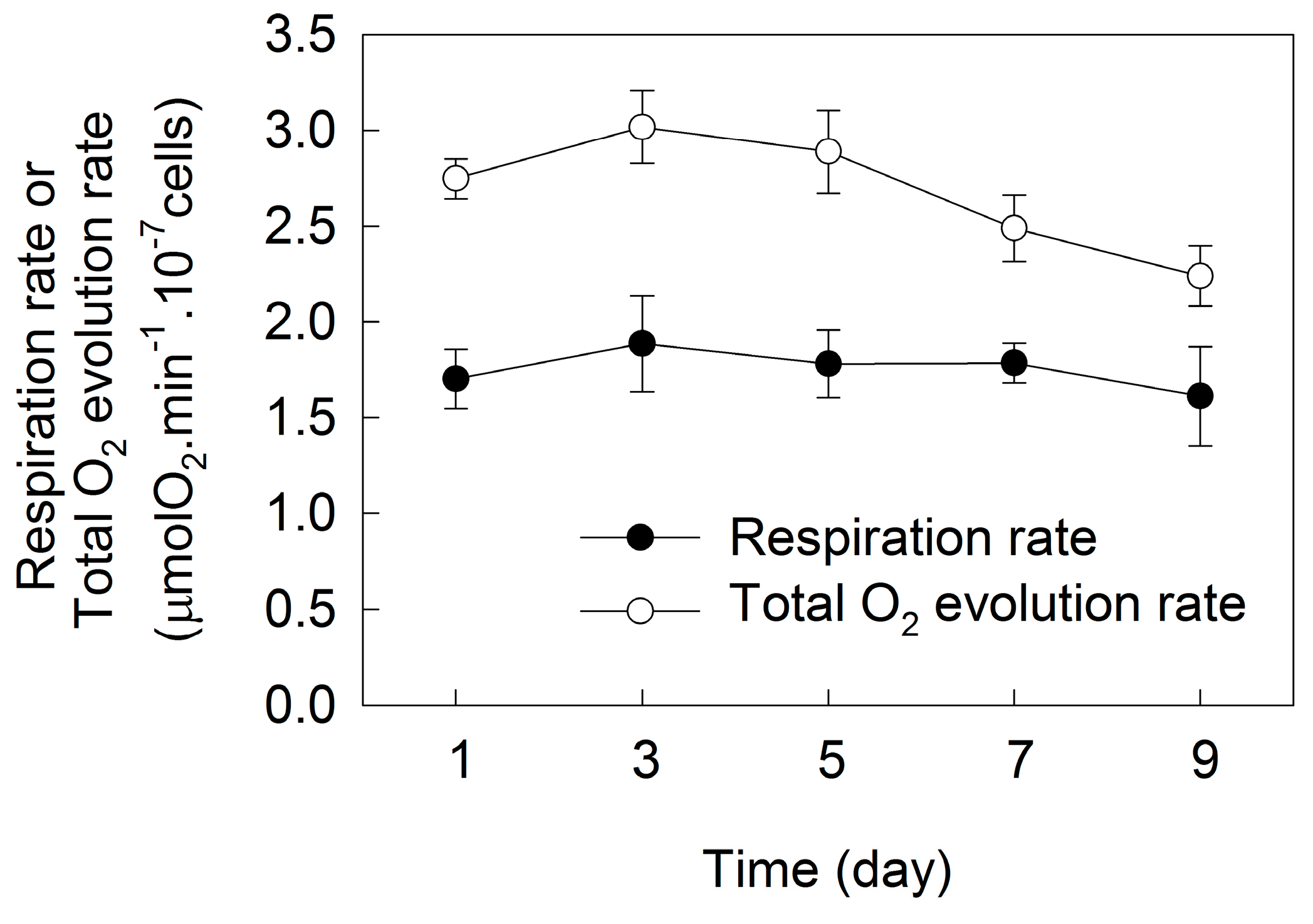
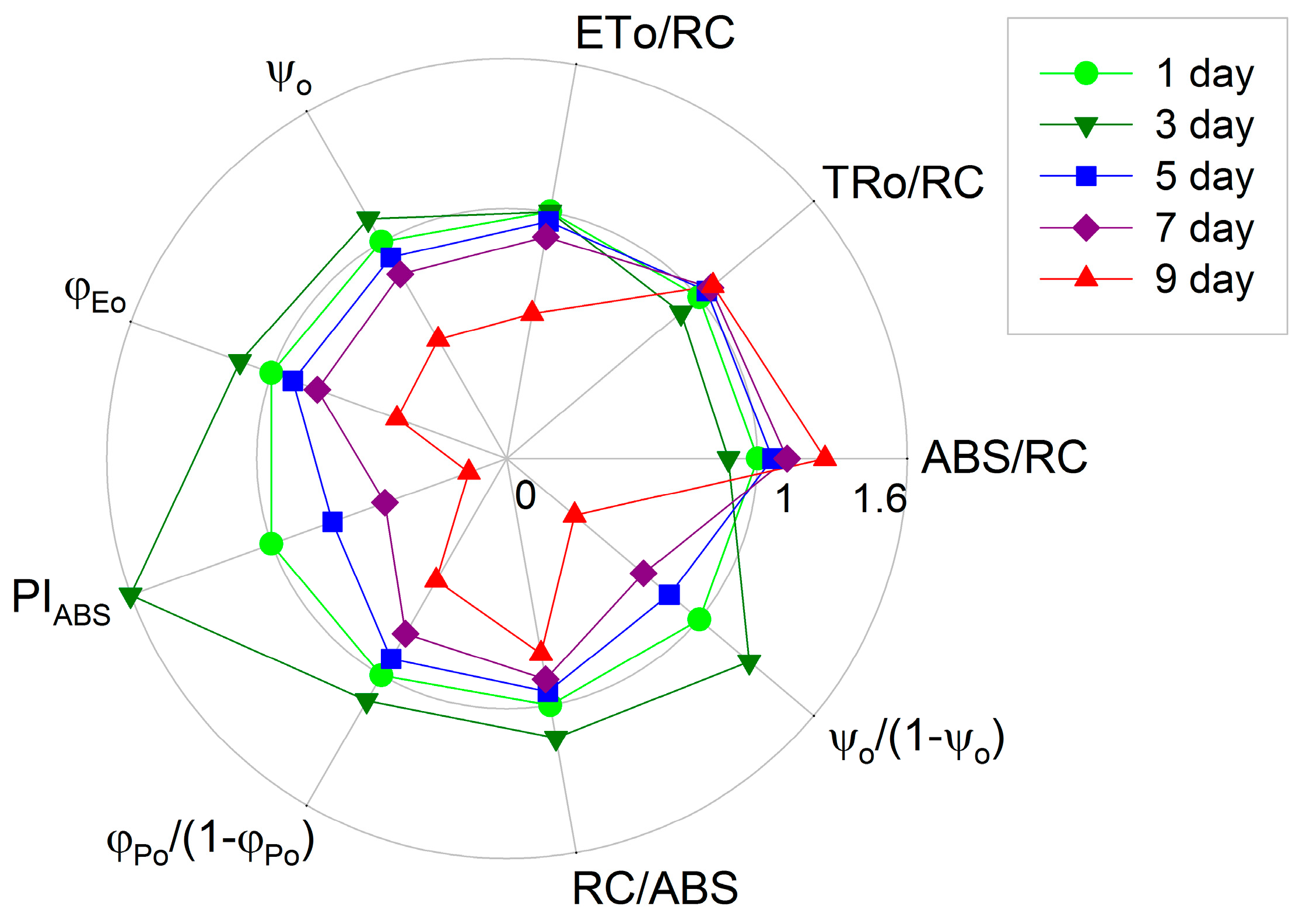
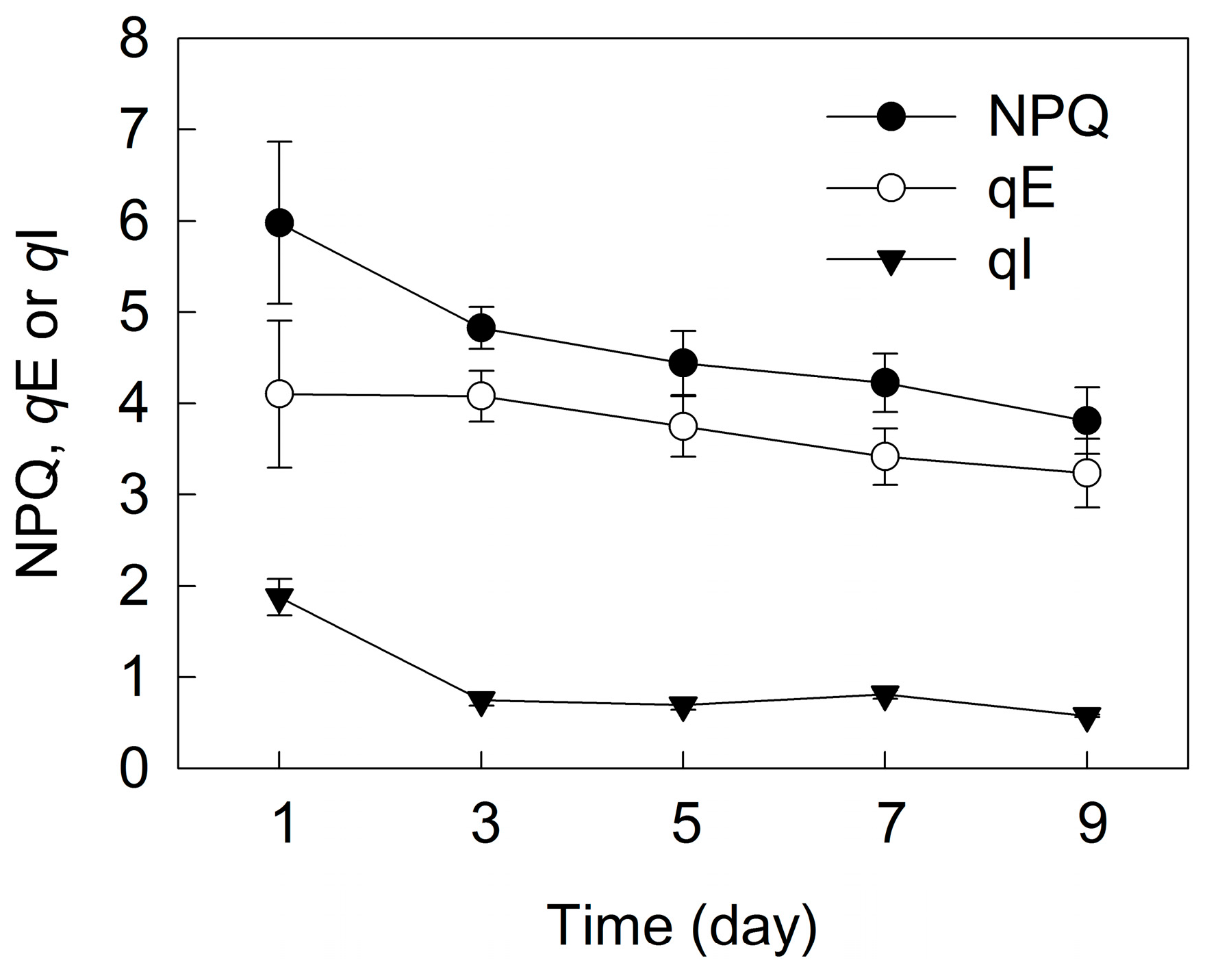
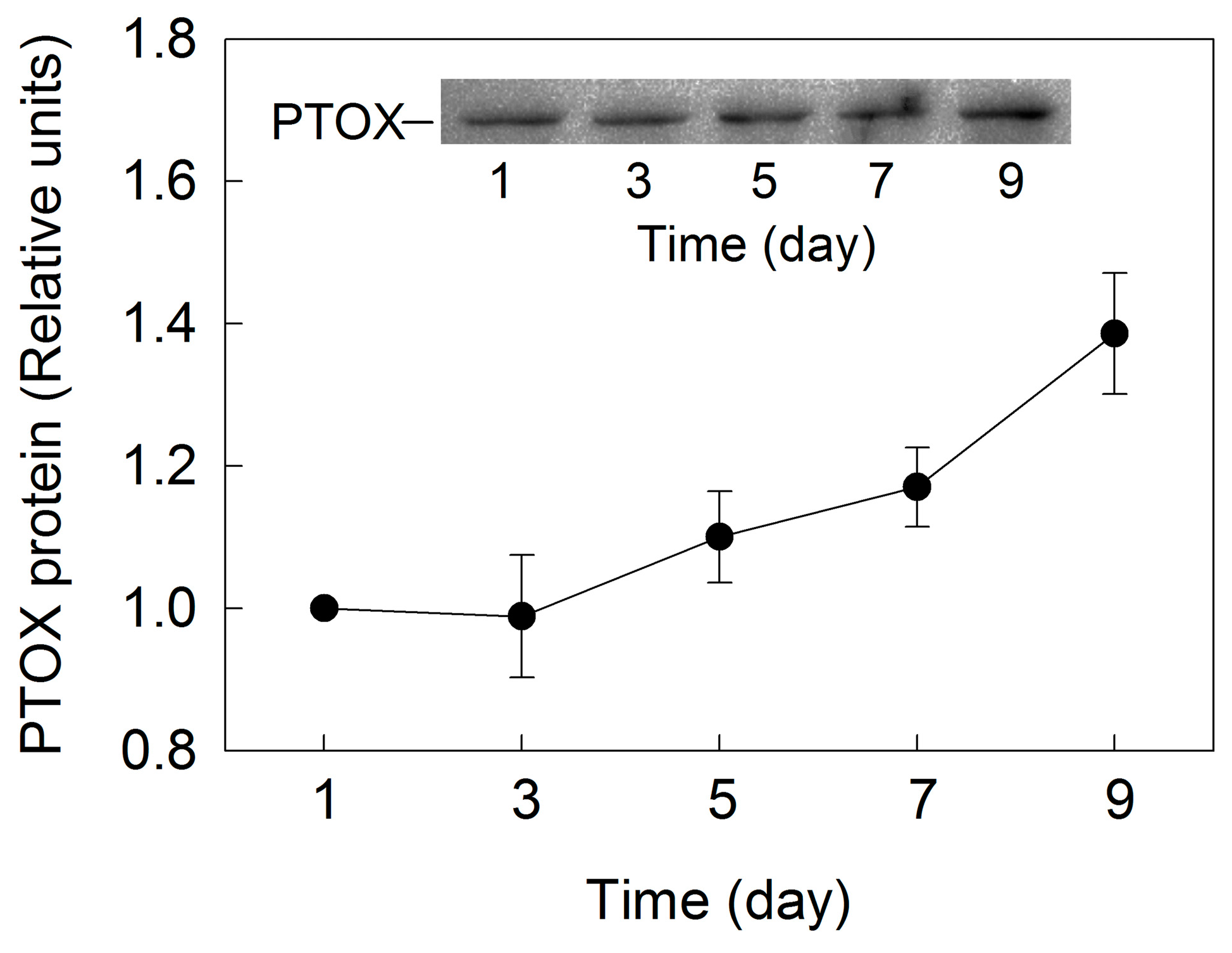
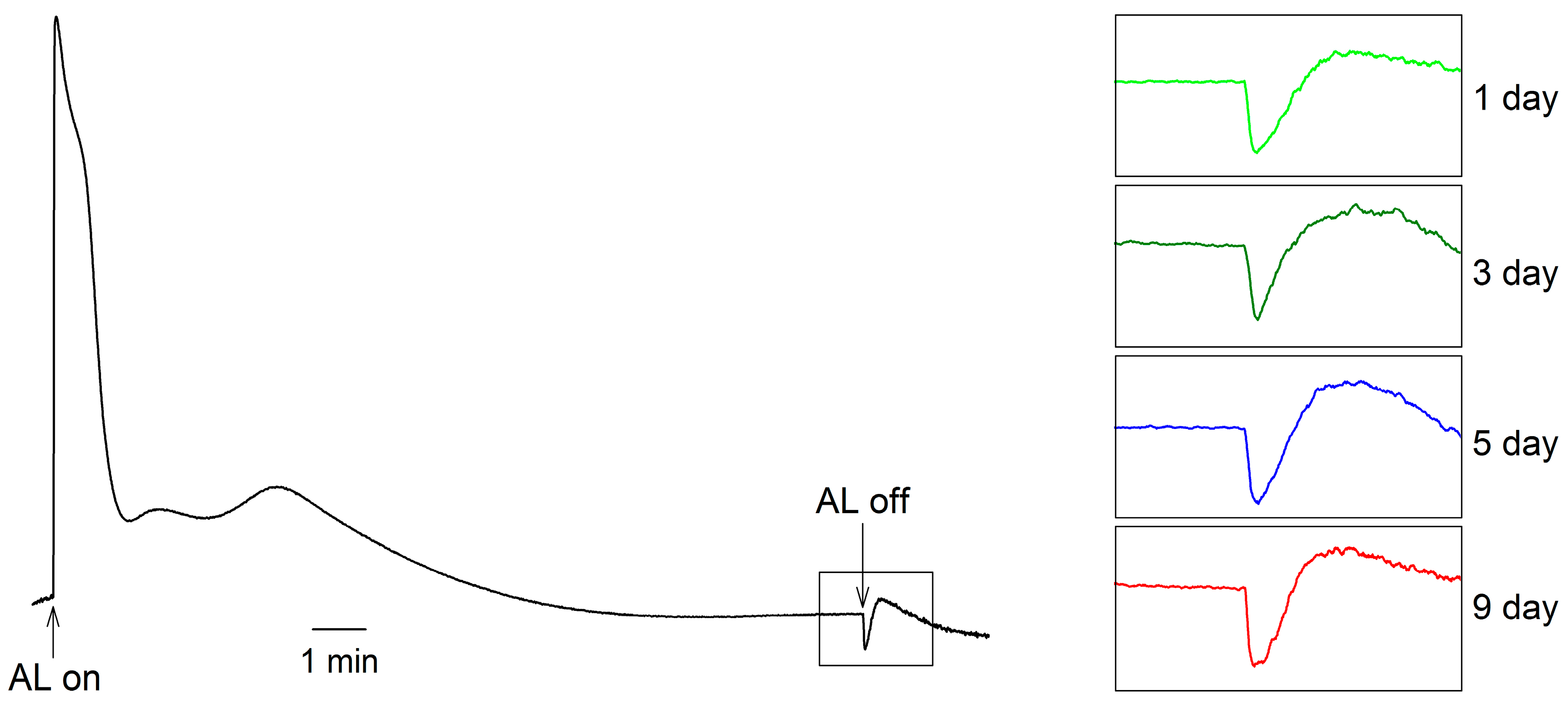
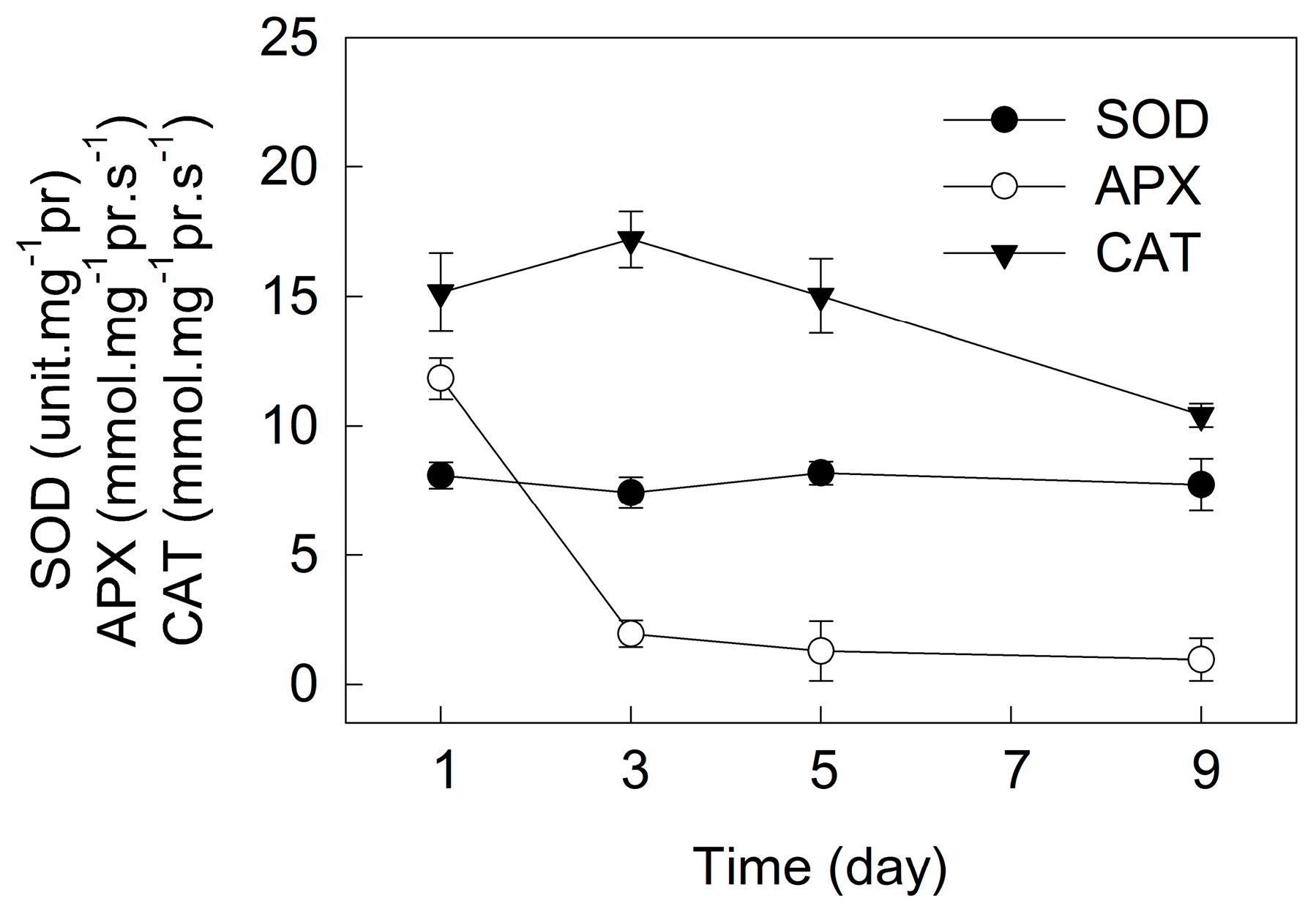
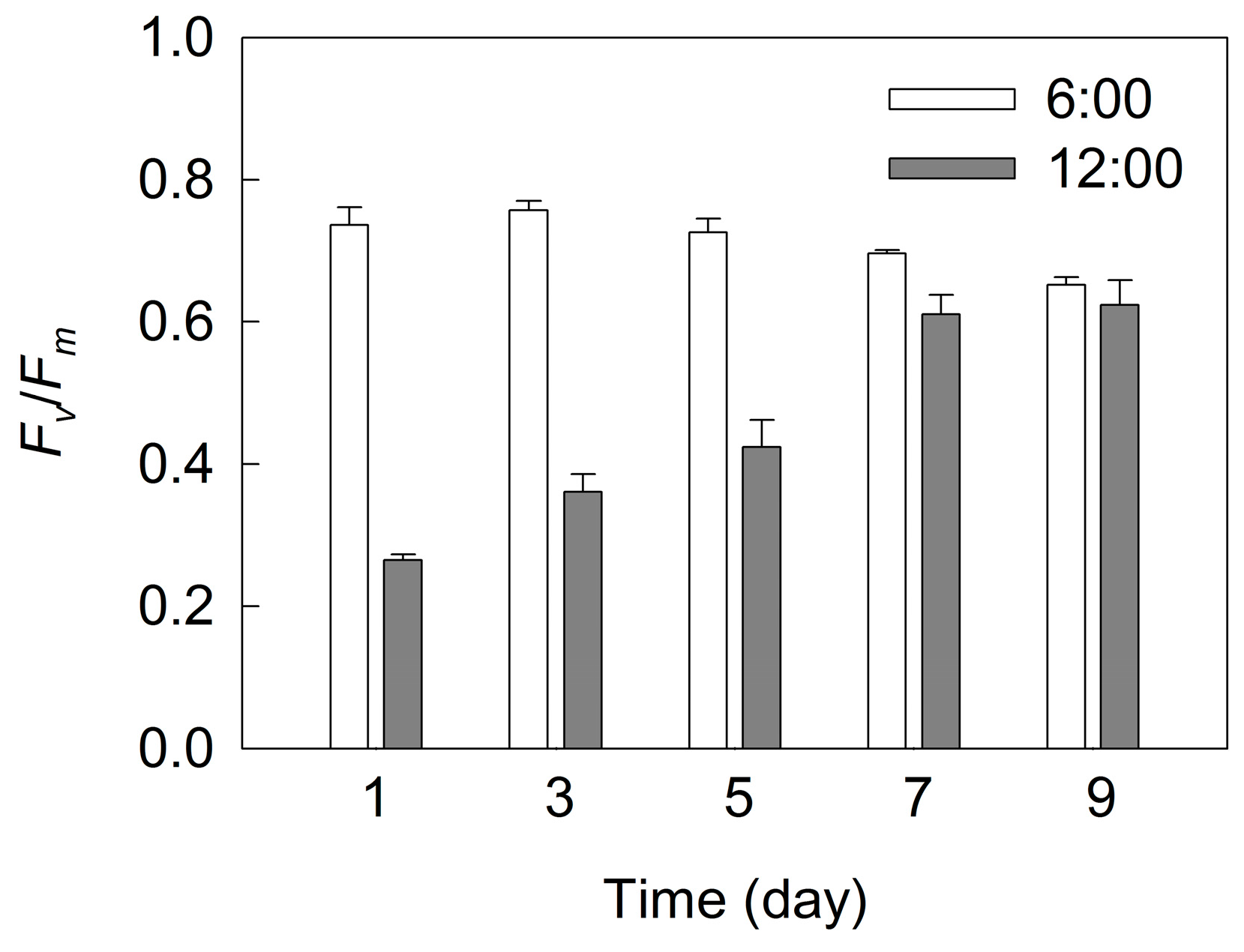
2. Results
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Analytical Procedures
- (1)
- The specific energy fluxes, i.e. the light absorption per active reaction center (ABS/RC, the average antenna size), the trapping of excitation energy per active reaction center (TRo/RC), the electron transport per active reaction center (ETo/RC).
- (2)
- The flux ratios or yields, i.e., the efficiency of electron transport (ψo), and the quantum yield of electron transport (φEo).
- (3)
- The photosynthetic performance index PIABS as well as its individual partial components, the efficiency of light absorption (RC/ABS), the performance due to the quantum efficiency of primary photochemistry (φPo/(1 − φPo)) and the performance due to the quantum efficiency of the conversion of excitation energy to electron transport (ψo/(1 − ψo)), were also calculated according to the JIP-test equations.
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, G.Q.; Wang, B.B.; Han, D.X.; Sommerfeld, M.; Lu, Y.H.; Chen, F.; Hu, Q. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant J. 2015, 81, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, L.; Yamada, H.; Katsuda, T.; Yamaji, H. Effects of temperature on the astaxanthin productivity and light harvesting characteristics of the green alga Haematococcus pluvialis. J. Biosci. Bioeng. 2015, 119, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Han, D.X.; Sommerfeld, M.R.; Lu, C.M.; Hu, Q. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J. Appl. Phycol. 2013, 25, 253–260. [Google Scholar] [CrossRef]
- Benemann, J.R. Microalgae aquaculture feeds. J. Appl. Phycol. 1992, 4, 233–245. [Google Scholar] [CrossRef]
- Sommer, T.R.; Potts, W.T.; Morrissy, N.M. Utilization of microalgal astaxanthin by rainbow trout (Oncorhyncus mykiss). Aquaculture 1991, 94, 79–88. [Google Scholar] [CrossRef]
- Goswami, G.; Chaudhuri, S.; Dutta, D. The present perspective of astaxanthin with reference to biosynthesis and pharmacological importance. World J. Microbiol. Biotechnol. 2010, 26, 1925–1939. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Liu, J.G.; van der Meer, J.P.; Zhang, L.T.; Zhang, Y. Cultivation of Haematococcus pluvialis for astaxanthin production. In Micro-Algal Production for Biomass and High-Value Products; Slocombe, S.P., Benemann, J.R., Eds.; Taylor & Francis: New York, NY, USA, 2016; pp. 267–293. [Google Scholar]
- Kobayashi, M.; Kakizono, T.; Nishio, N.; Nagai, S. Effects of light intensity, light quality, and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. J. Ferment. Bioeng. 1992, 74, 61–63. [Google Scholar] [CrossRef]
- Steinbrenner, J.; Linden, H. Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin biosynthesis in the green alga Haematococcus pluvialis. Plant Physiol. 2000, 125, 810–817. [Google Scholar] [CrossRef]
- Steinbrenner, J.; Linden, H. Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: Regulation by photosynthetic redox control. Plant Mol. Biol. 2003, 52, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Scibilia, L.; Girolomoni, L.; Berteotti, S.; Alboresi, A.; Ballottari, M. Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res. 2015, 12, 170–181. [Google Scholar] [CrossRef]
- Chekanov, K.; Lukyanov, A.; Boussiba, S.; Aflalo, C.; Solovchenko, A. Modulation of photosynthetic activity and photoprotection in Haematococcus pluvialis cells during their conversion into haematocysts and back. Photosynth. Res. 2016, 128, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Strasser, R.J.; Qiang, S. In vivo assessment of effect of phytotoxin tenuazonic acid on PSII reaction centers. Plant Physiol. Biochem. 2014, 84, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Sarin, N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.T.; Liu, J.G. Effects of heat stress on photosynthetic electron transport in a marine cyanobacterium Arthrospira sp. J. Appl. Phycol. 2016, 28, 757–763. [Google Scholar] [CrossRef]
- Strasser, B.J.; Strasser, R.J. Measuring fast fluorescence transients to address environmental questions: The JIP-test. In Photosynthesis: From Light to Biosphere; Mathis, P., Ed.; Kluwer Academic Publishers Press: Dordrecht, The Netherlands, 1995; pp. 977–980. [Google Scholar]
- Strasser, R.J.; Srivatava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Ed.; Kluwer Academic Publishers Press: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Zhang, L.T.; Zhang, Z.S.; Gao, H.Y.; Xue, Z.C.; Yang, C.; Meng, X.L.; Meng, Q.W. Mitochondrial alternative oxdiase pathway protects plants against photoinhibition by alleviating inhibition of the repair of photodamaged PSII through preventing formation of reactive oxygen species in Rumex K-1 leaves. Physiol. Plant. 2011, 143, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, R.; Okuda, K.; Kobayashi, Y.; Shikanai, T. A eukaryotic factor required for accumulation of the chloroplast NAD(P)H dehydrogenase complex in arabidopsis. Plant Physiol. 2006, 142, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Duan, W.; Takabayashi, A.; Endo, T.; Shikanai, T.; Ye, J.Y.; Mi, H.L. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol. 2006, 141, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Milward, S.E.; Fan, D.Y.; Chow, W.S.; Badger, M.R. How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol. 2009, 149, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.T.; He, M.L.; Liu, J.G.; Li, L. Role of the mitochondrial alternative oxidase pathway in hydrogen photoproduction in Chlorella protothecoides. Planta 2015, 241, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Kalachanis, D.; Manetas, Y. Analysis of fast chlorophyll fluorescence rise (O-K-J-I-P) curves in green fruits indicates electron flow limitations at the donor side of PSII and the acceptor sides of both photosystems. Physiol. Plant. 2010, 139, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Vass, I. Role of charge recombination processes in photodamage and photoprotection of the photosystem II complex. Physiol. Plant. 2011, 142, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Ruban, A.V.; Walters, R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Mol. Biol. 1996, 47, 655–684. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef]
- Rumeau, D.; Peltier, G.; Cournac, L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ. 2007, 30, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M. In vivo antioxidant role of astaxanthin under oxidative stress in the green alga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2000, 54, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Okada, T. Protective role of astaxanthin against u.v.-B irradiation in the green alga Haematococcus pluvialis. Biotechnol. Lett. 2000, 22, 177–181. [Google Scholar] [CrossRef]
- Liu, J.G.; Zhang, X.L.; Sun, Y.H.; Lin, W. Antioxidative capacity and enzyme activity in Haematococcus pluvialis cells exposed to superoxide free radicals. Chin. J. Oceanol. Limnol. 2010, 28, 1–9. [Google Scholar] [CrossRef]
- Zhang, C.H.; Zhang, L.T.; Liu, J.G. The role of photorespiration during astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae). Plant Physiol. Biochem. 2016, 107, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, F.; Liu, X.; Li, X. Carotenoid composition in the green microalga Chlorococcum. Food Chem. 2002, 76, 319–325. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.G.; Qiu, N.W.; Lu, Q.T.; Lu, C.M. Enhanced thermotolerance of photosystem II in salt-adapted plants of the halophyte Artemisia anethifolia. Planta 2005, 220, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.D.; Gao, H.Y.; Zou, Q.; Jiang, G.M.; Li, L.H. Leaf orientation, photorespiration and xanthophyll cycle protect young soybean leaves against high irradiance in field. Environ. Exp. Bot. 2004, 55, 87–96. [Google Scholar] [CrossRef]
- Johnson, G.N.; Young, A.J.; Scholes, J.D.; Horton, P. The dissipation of excess excitation energy in British plant species. Plant Cell Environ. 1993, 16, 673–679. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Z.S.; Gao, H.Y.; Yang, C.; Liu, M.J.; Li, Y.T.; Li, P.M. Photoinhibition-like damage to the photosynthetic apparatus in plant leaves induced by submergence treatment in the dark. PLoS ONE 2014, 9, e89067. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.J.; Cheng, D.D.; Wang, W.B.; Gao, H.Y.; Liu, A.X.; Li, X.M.; Meng, Q.W. Different enhancement of senescence induced by metabolic products of Alternaria alternata in tobacco leaves of different ages. Physiol. Plant. 2010, 138, 164–175. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Su, F.; Zhang, C.; Gong, F.; Liu, J. Changes of Photosynthetic Behaviors and Photoprotection during Cell Transformation and Astaxanthin Accumulation in Haematococcus pluvialis Grown Outdoors in Tubular Photobioreactors. Int. J. Mol. Sci. 2017, 18, 33. https://doi.org/10.3390/ijms18010033
Zhang L, Su F, Zhang C, Gong F, Liu J. Changes of Photosynthetic Behaviors and Photoprotection during Cell Transformation and Astaxanthin Accumulation in Haematococcus pluvialis Grown Outdoors in Tubular Photobioreactors. International Journal of Molecular Sciences. 2017; 18(1):33. https://doi.org/10.3390/ijms18010033
Chicago/Turabian StyleZhang, Litao, Fang Su, Chunhui Zhang, Fengying Gong, and Jianguo Liu. 2017. "Changes of Photosynthetic Behaviors and Photoprotection during Cell Transformation and Astaxanthin Accumulation in Haematococcus pluvialis Grown Outdoors in Tubular Photobioreactors" International Journal of Molecular Sciences 18, no. 1: 33. https://doi.org/10.3390/ijms18010033