Simultaneous Determination and Pharmacokinetic Study of Six Components in Rat Plasma by HPLC-MS/MS after Oral Administration of Acanthopanax sessiliflorus Fruit Extract
Abstract
:1. Introduction
2. Results and Discussion
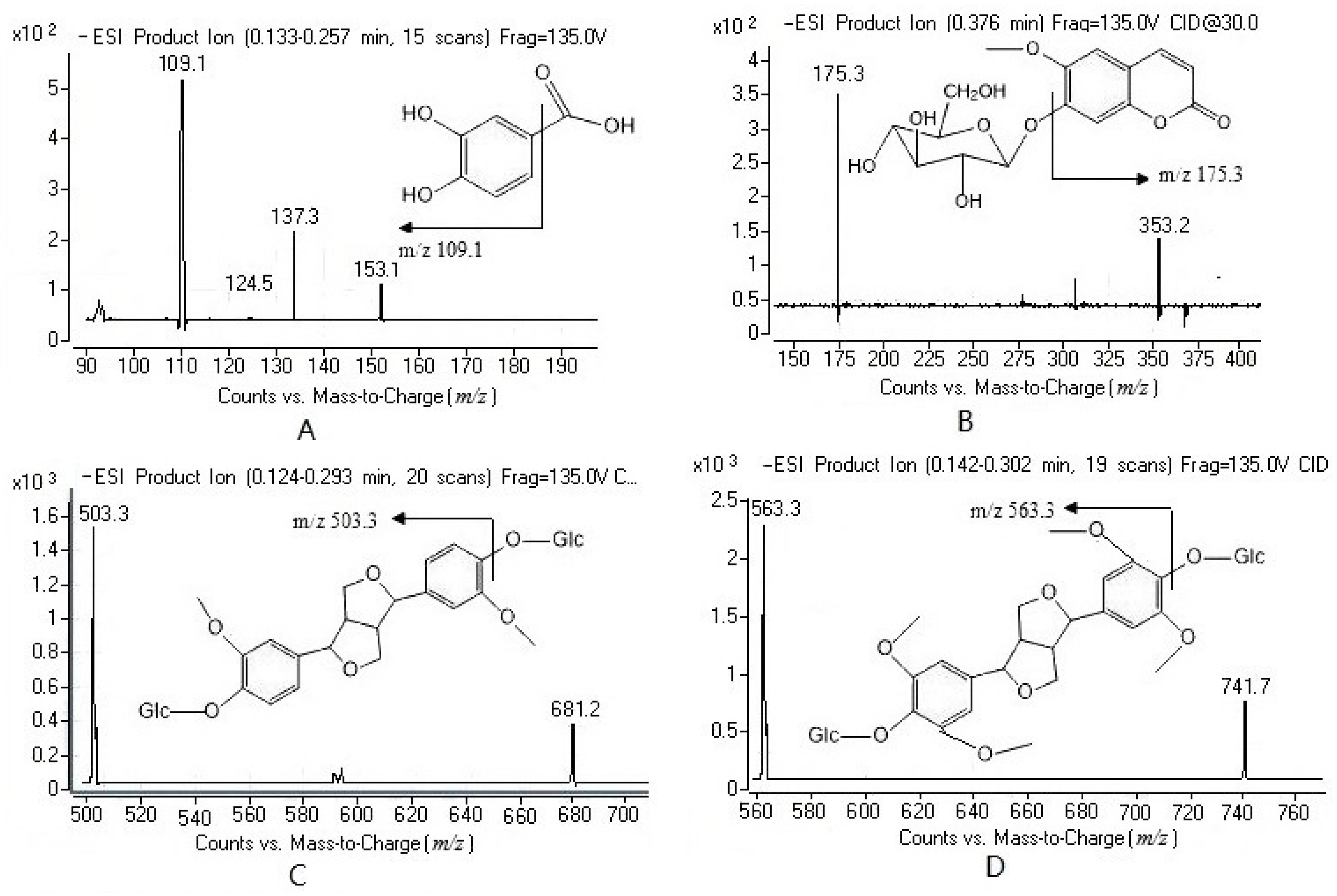
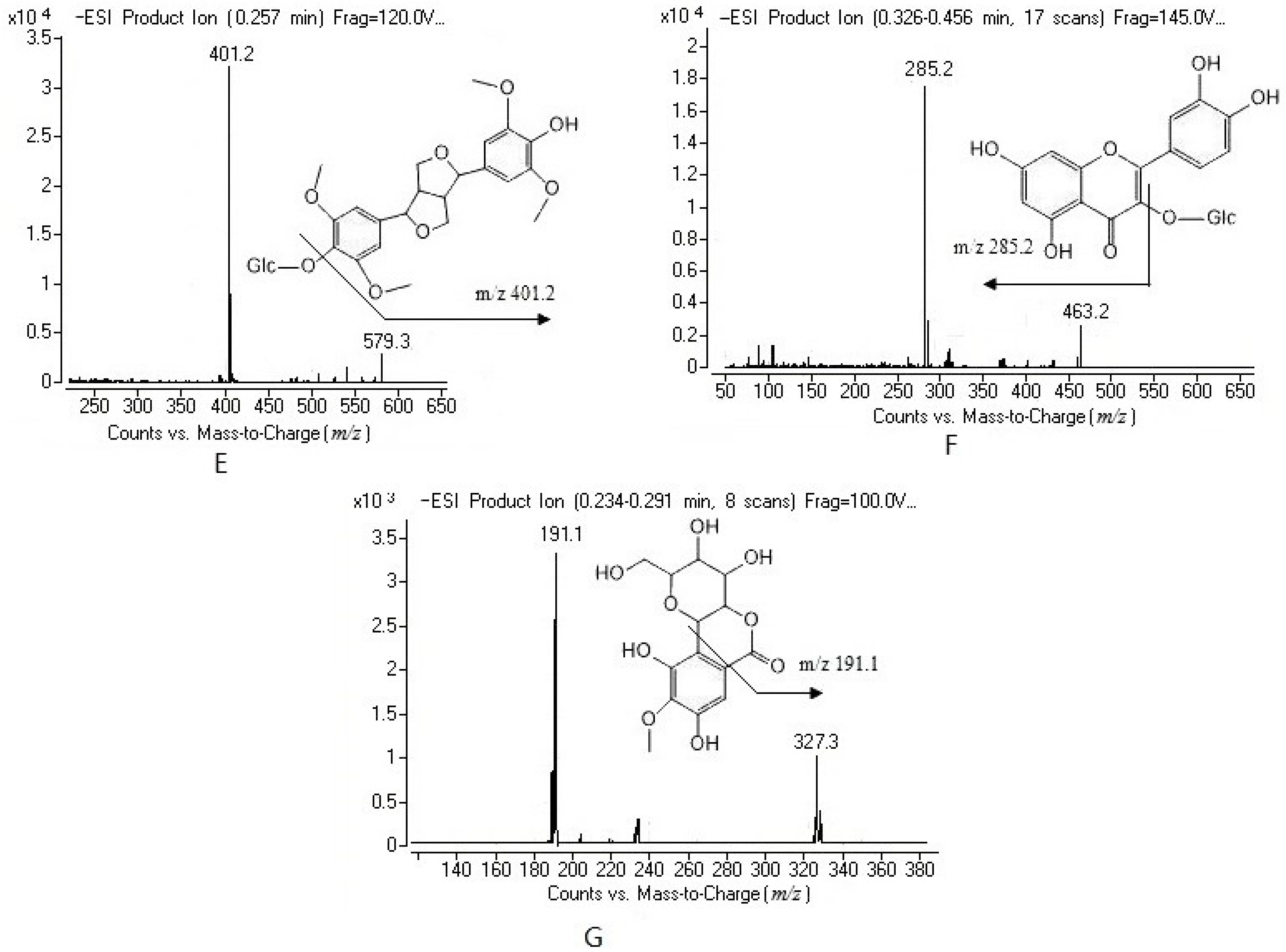
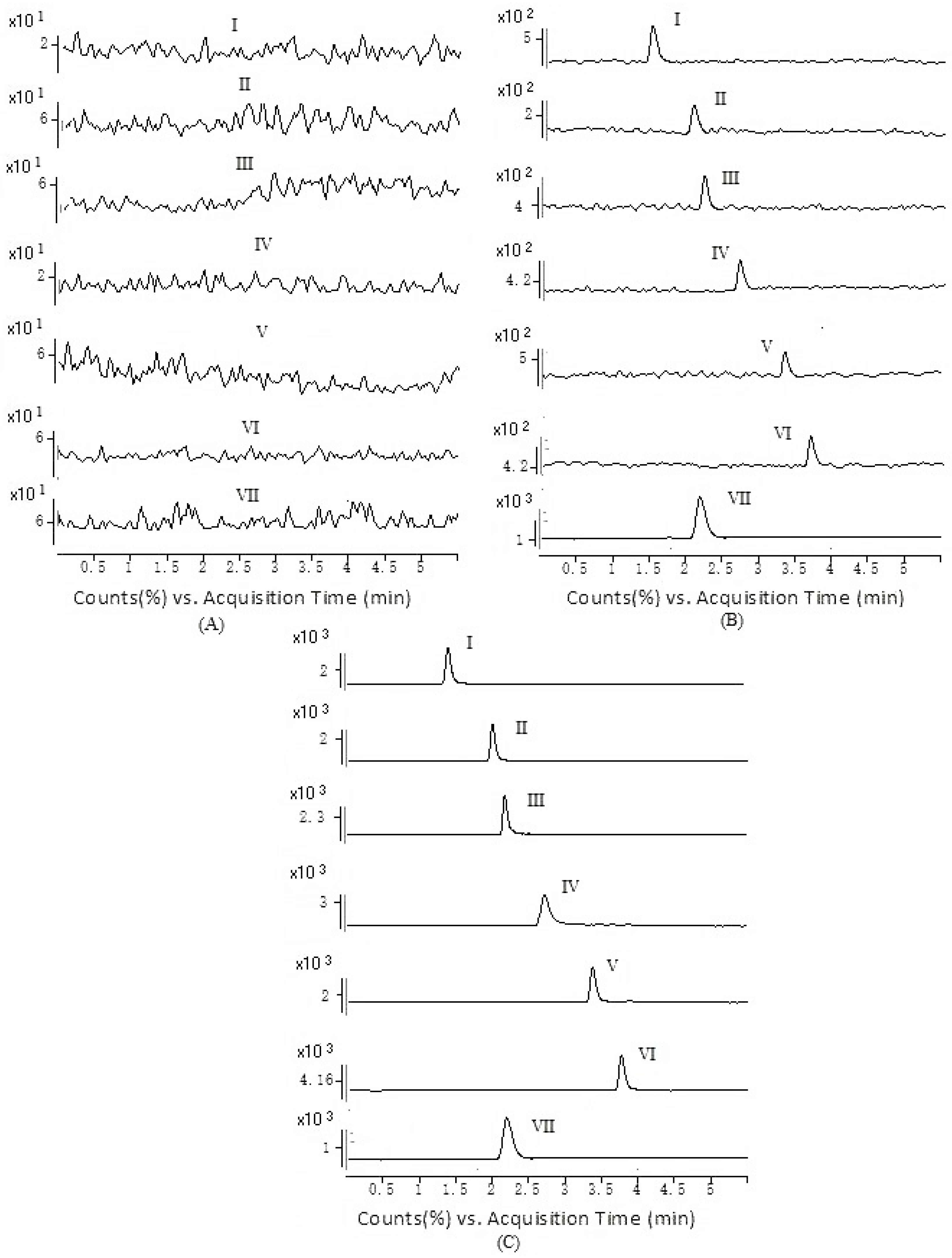
2.1. Method Development
2.2. Method Validation
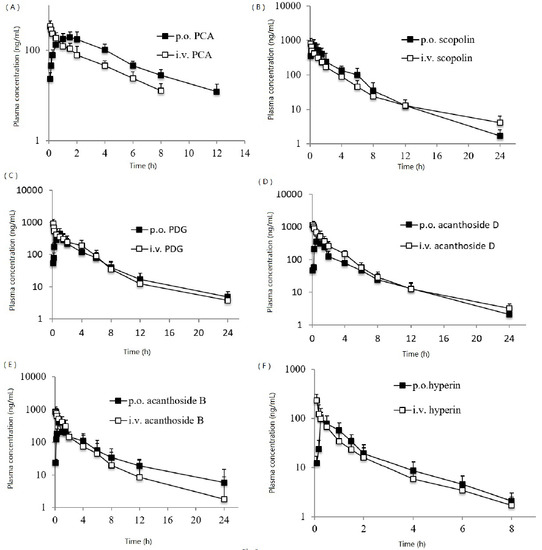
2.3. Application to the PK Study in the Sprague–Dawley Rats
3. Experimental
3.1. Chemicals and Reagents
3.2. Instrumentation
3.3. HPLC/MS/MS Conditions
3.4. Preparation of Standard and Quality Control Samples
3.5. Sample Preparation
3.6. Method Validation
3.7. Pharmacokinetic Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Billingham, M.E.; Davies, G.E. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1979; pp. 108–144. [Google Scholar]
- Lee, S.H.; Lee, Y.S.; Jung, S.H.; Ji, J.; Shin, K.H.; Kin, B.K.; Kang, S.S. Antitumor and Immunostimulating Activities of Acanthopanax sessiliflorus Fruits. Nat. Prod. Sci. 2003, 99, 112–116. [Google Scholar]
- An, Q.; Yang, C.J.; Song, Y.; Yu, K.; Xiong, Z.L.; Li, F.M. Studies on the chemical constituents of the fruit of Acanthopanax sessiliflorus (Rupr. Et Maxim.) Seem. Nat. Prod. Res. Dev. 2008, 20, 765–769. [Google Scholar]
- Yang, C.J.; An, Q.; Song, Y.; Xiong, Z.L.; Li, F.M. Isolation and identification of chemical constituents of fruits of Acanthopanax sessiliflorus. China J. Chin. Mater. Med. 2009, 34, 29–31. [Google Scholar]
- Yang, C.J.; An, Q.; Xiong, Z.L.; Song, Y.; Yu, K.; Li, F.M. Triterpenes from Acanthopanax sessiliflorus fruits and their antiplatelet aggregation activities. Planta Med. 2009, 75, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.W.; Gong, X.J.; Zhou, X.; Zhao, Y.; Li, M.L. An UHPLC–MS/MS method for simultaneous quantification of gallic acid and protocatechuic acid in rat plasma after oral administration of Polygonum capitatum extract and its application to pharmacokinetics. J. Enthopharmacol. 2005, 162, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Gong, Z.P.; Cao, X.; Wang, Y.L.; Wang, A.M.; Zheng, L.; Huang, Y.; Lan, Y.Y. A UPLC-MS method for simultaneous determination of geniposidic acid, two lignans and phenolics in rat plasma and its application to pharmacokinetics studies of Eucommia ulmoides extracts in rats. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Li, W.; Ma, X.H.; Chu, Y.; Li, S.M.; Guo, J.H.; Jia, Y.M.; Zhou, S.P.; Zhu, Y.H.; Liu, C.X. Simultaneous determination of caffeic acid and its major pharmacologically active metabolites in rat plasma by LC-MS/MS and its application in pharmacokinetic study. Biomed. Chromatogr. 2015, 29, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.F.; Dai, Y.; Wang, Q.; Cai, F. A high-performance liquid chromatographic method for determination of scopolin in rat plasma: Application to pharmacokinetic studies. Biomed. Chromatogr. 2008, 22, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Yuan, Z.Y.; Dai, Y.S.; Wang, C.; Yang, J.; Peng, L.M.; Xu, G.W. Identification and determination of glycosides in tobacco leaves by liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry. J. Sep. Sci. 2007, 30, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, F.; Zhang, Y.; Sun, L.; Yu, L.; Zhang, Z.; Peng, S.; Guo, J. Simultaneous quantification of hyperin, reynoutrin and guaijaverin in mice plasma by LC-MS/MS: Application to a pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, F.; Yu, X.L.; Lu, X.; Zheng, G.F. Pharmacokinetics of eupalinolide A, eupalinolide B and hyperoside from Eupatorium lindleyanum in rats by LC/MS/MS. J. Chromatogr. B. 2015, 995, 1–7. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: Bioanalytical Method Validation, Center for Drug Evaluation and Research; US Department of Health and Human Services: Rockville, MD, USA, 2001.

| Concentration (ng/mL) | RSD (%) | RE (%) | ||
|---|---|---|---|---|
| Added | Found (Mean) | Intra-Day | Inter-Day | |
| PCA | ||||
| 2.40 | 2.61 | 8.7 | 9.3 | 8.8 |
| 600.0 | 643.6 | 6.5 | 7.4 | 7.3 |
| 960.0 | 998.5 | 6.1 | 5.2 | 4.0 |
| Scopolin | ||||
| 1.92 | 2.02 | 7.5 | 6.3 | 5.2 |
| 480.0 | 510.2 | 5.1 | 8.2 | 6.3 |
| 768.0 | 805.1 | 3.7 | 5.5 | 4.8 |
| PDG | ||||
| 2.24 | 2.41 | 10.2 | 9.9 | 7.6 |
| 560.0 | 583.8 | 6.8 | 8.3 | 4.2 |
| 896.0 | 928.5 | 4.7 | 4.1 | 3.6 |
| Acanthoside D | ||||
| 2.64 | 2.87 | 10.1 | 11.4 | 8.7 |
| 660.0 | 634.5 | 5.3 | 6.4 | −3.9 |
| 1056.0 | 1123.6 | 4.6 | 5.9 | 6.4 |
| Acanthoside B | ||||
| 1.98 | 2.12 | 7.7 | 9.1 | 7.1 |
| 495.0 | 523.4 | 8.1 | 6.4 | 5.7 |
| 960.0 | 1003.2 | 3.9 | 4.7 | 4.5 |
| Hyperin | ||||
| 2.02 | 2.24 | 9.3 | 7.4 | 10.9 |
| 510.0 | 543.7 | 8.1 | 8.5 | 6.6 |
| 808.0 | 845.9 | 5.9 | 5.2 | 4.7 |
| Conditions | Concentration (ng/mL) | RSD (%) | RE (%) | |
|---|---|---|---|---|
| Added | Found (Mean) | |||
| Bench-Top (23 °C for 2 h) | ||||
| PCA | 2.40 | 2.54 | 9.4 | 5.8 |
| 960.0 | 993.2 | 6.7 | 3.5 | |
| Scopolin | 1.92 | 2.04 | 10.3 | 6.3 |
| 768.0 | 798.2 | 5.3 | 3.9 | |
| PDG | 2.24 | 2.45 | 8.5 | 9.4 |
| 896.0 | 934.7 | 4.9 | 4.3 | |
| Acanthoside D | 2.64 | 2.45 | 8.2 | −7.2 |
| 1056.0 | 1103.2 | 3.7 | 4.5 | |
| Acanthoside B | 1.98 | 2.12 | 9.3 | 7.1 |
| 960.0 | 989.5 | 6.4 | 3.1 | |
| Hyperin | 2.02 | 2.19 | 11.3 | 8.4 |
| 808.0 | 843.8 | 6.2 | 4.4 | |
| Three freeze/thaw cycles(−40 to 23 °C) | ||||
| PCA | 2.40 | 2.56 | 8.8 | 6.7 |
| 960.0 | 988.4 | 6.1 | 3.0 | |
| Scopolin | 1.92 | 2.08 | 9.5 | 8.3 |
| 768.0 | 803.2 | 5.7 | 4.6 | |
| PDG | 2.24 | 2.41 | 10.3 | 7.6 |
| 896.0 | 932.1 | 4.9 | 4.0 | |
| Acanthoside D | 2.64 | 2.87 | 7.9 | 8.7 |
| 1056.0 | 1098.5 | 7.1 | 4.0 | |
| Acanthoside B | 1.98 | 1.78 | 11.5 | −10.1 |
| 960.0 | 904.6 | 6.2 | −5.8 | |
| Hyperin | 2.02 | 2.14 | 7.6 | 5.9 |
| 808.0 | 832.6 | 5.2 | 3.0 | |
| Autosampler rack at 4 °C for 6 h | ||||
| PCA | 2.40 | 2.19 | 8.3 | −8.8 |
| 960.0 | 998.4 | 4.8 | 4.0 | |
| Scopolin | 1.92 | 1.78 | 8.1 | −7.3 |
| 768.0 | 813.5 | 6.2 | 5.9 | |
| PDG | 2.24 | 2.41 | 10.7 | 7.6 |
| 896.0 | 934.2 | 4.9 | 4.3 | |
| Acanthoside D | 2.64 | 2.87 | 9.8 | 8.7 |
| 1056.0 | 1098.5 | 5.4 | 4.0 | |
| Acanthoside B | 1.98 | 2.18 | 9.7 | 10.1 |
| 960.0 | 1004.2 | 9.4 | 4.6 | |
| Hyperin | 2.02 | 2.14 | 10.3 | 5.9 |
| 808.0 | 845.6 | 6.9 | 4.7 | |
| Freezing at −40 °C for 30 days | ||||
| PCA | 2.40 | 2.59 | 7.3 | 7.9 |
| 960.0 | 993.6 | 8.1 | 3.5 | |
| Scopolin | 1.92 | 2.04 | 8.5 | 6.3 |
| 768.0 | 804.3 | 6.2 | 4.7 | |
| PDG | 2.24 | 2.37 | 9.4 | 5.8 |
| 896.0 | 923.7 | 7.5 | 3.1 | |
| Acanthoside D | 2.64 | 2.88 | 11.3 | 9.1 |
| 1056.0 | 1089.4 | 9.2 | 3.2 | |
| Acanthoside B | 1.98 | 2.15 | 9.6 | 8.6 |
| 960.0 | 1003.8 | 4.7 | 4.6 | |
| Hyperin | 2.02 | 2.19 | 8.2 | 8.4 |
| 808.0 | 843.8 | 6.1 | 4.4 | |
| Compound | Cmax (ng/mL) | tmax (h) | t1/2 (h) | AUC0–∞ (ng·h/mL) | F (%) |
|---|---|---|---|---|---|
| PCA | 198.1 ± 60.3 | 1.5 ± 0.5 | 1.0 ± 0.2 | 901.3 ± 132.1 | 11.9 |
| Scopolin | 734.1 ± 203.4 | 0.5 ± 0.3 | 1.4 ± 0.5 | 1876.9 ± 398.4 | 25.4 |
| PDG | 447.1 ± 104.5 | 1.0 ± 0.4 | 2.3 ± 0.8 | 1470.9 ± 350.5 | 7.7 |
| Acanthoside D | 356.7 ± 145.2 | 0.5 ± 0.3 | 2.0 ± 0.6 | 1042.0 ± 285.1 | 6.0 |
| Acanthoside B | 409.5 ± 133.2 | 0.5 ± 0.2 | 1.2 ± 1.0 | 1216.9 ± 315.0 | 6.2 |
| Hyperin | 103.7 ± 46.3 | 0.25 ± 0.3 | 0.9 ± 0.5 | 151.0 ± 26.1 | 5.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, P.; Lei, M.; Liu, Y.; Yang, S. Simultaneous Determination and Pharmacokinetic Study of Six Components in Rat Plasma by HPLC-MS/MS after Oral Administration of Acanthopanax sessiliflorus Fruit Extract. Int. J. Mol. Sci. 2017, 18, 45. https://doi.org/10.3390/ijms18010045
Du P, Lei M, Liu Y, Yang S. Simultaneous Determination and Pharmacokinetic Study of Six Components in Rat Plasma by HPLC-MS/MS after Oral Administration of Acanthopanax sessiliflorus Fruit Extract. International Journal of Molecular Sciences. 2017; 18(1):45. https://doi.org/10.3390/ijms18010045
Chicago/Turabian StyleDu, Peng, Mingdao Lei, Yu Liu, and Shilin Yang. 2017. "Simultaneous Determination and Pharmacokinetic Study of Six Components in Rat Plasma by HPLC-MS/MS after Oral Administration of Acanthopanax sessiliflorus Fruit Extract" International Journal of Molecular Sciences 18, no. 1: 45. https://doi.org/10.3390/ijms18010045