1. Introduction
Multiple sclerosis (MS) is a demyelination disease affecting the central nervous system (CNS), typically characterized by motor and sensory dysfunction, and optic neuritis. Previous studies reported that 40%–70% of MS patients also exhibit cognitive impairments (CI), primarily in learning, memory, information processing speed, and executive functions [
1].
CI can occur in the early stages of the disease course, even among newly diagnosed patients, and typically has severe detrimental impacts on patients’ lives [
2,
3]. Previous studies hypothesized that CI is associated with the extent of white matter (WM) lesion load in MS patients [
4], based on the volume of T2 (transverse relaxation time) lesions visible on conventional MR imaging. However, several studies reported a weak relationship between WM damage and cognitive changes [
5,
6,
7]. Later, several studies using diffusion tensor imaging (DTI) reported that widespread subtle pathological changes in WM microstructures (normal appearing WM; NAWM) exhibited a significant correlation with CI, whereas T2-hyperintense lesions of MS did not [
8]. In addition, researchers proposed that fractional anisotropy (FA) in the uncinate fasciculus was associated with processing speed and spatial memory [
9], and Hulst et al. found that WM integrity was related to cognition in CI–MS patients [
10].
Extensive gray matter (GM) loss has been widely reported in patients with MS, including cortical and deep GM. Asymmetric left-sided loss of GM in the left fronto-temporal cortex and precuneus, as well as bilateral anterior cingulate gyrus and caudate nuclei atrophy, were reported in relapsing-remitting MS (RRMS) [
11]. Moreover, an association between decreased GM volume and cognitive impairment was also reported. Several studies of MS patients have revealed a relationship between CI and deep GM atrophy in a range of areas, including the thalamus, hippocampus, caudate nucleus, globus pallidus, and basal ganglia, based on voxel-based morphometric (VBM) analysis, which allows quantification of GM volumes in vivo [
12,
13,
14]. However, Hulst et al. reported that only WM integrity was significantly different between CI and cognitive preservation (CP) patients, while no difference in regional GM volume was found [
10]. However, both RRMS and secondary progressive MS (SPMS) patients were enrolled in Hulst’s study [
10]. Previous research indicated that cognition in SPMS patients was more frequently and severely impaired than among RRMS patients [
15]. Thus, the inclusion of different disease subtypes of MS may have affected the results.
The neural mechanisms underlying CI in MS remain controversial. The discrepancy between previous results may be related to the different types of MS included in study samples. Thus, the current study aimed to further explore the neural basis of cognitive impairment by distinguishing CI and CP groups of RRMS patients according to cognitive ability, and examining them with a combination of DTI and VBM techniques.
3. Discussion
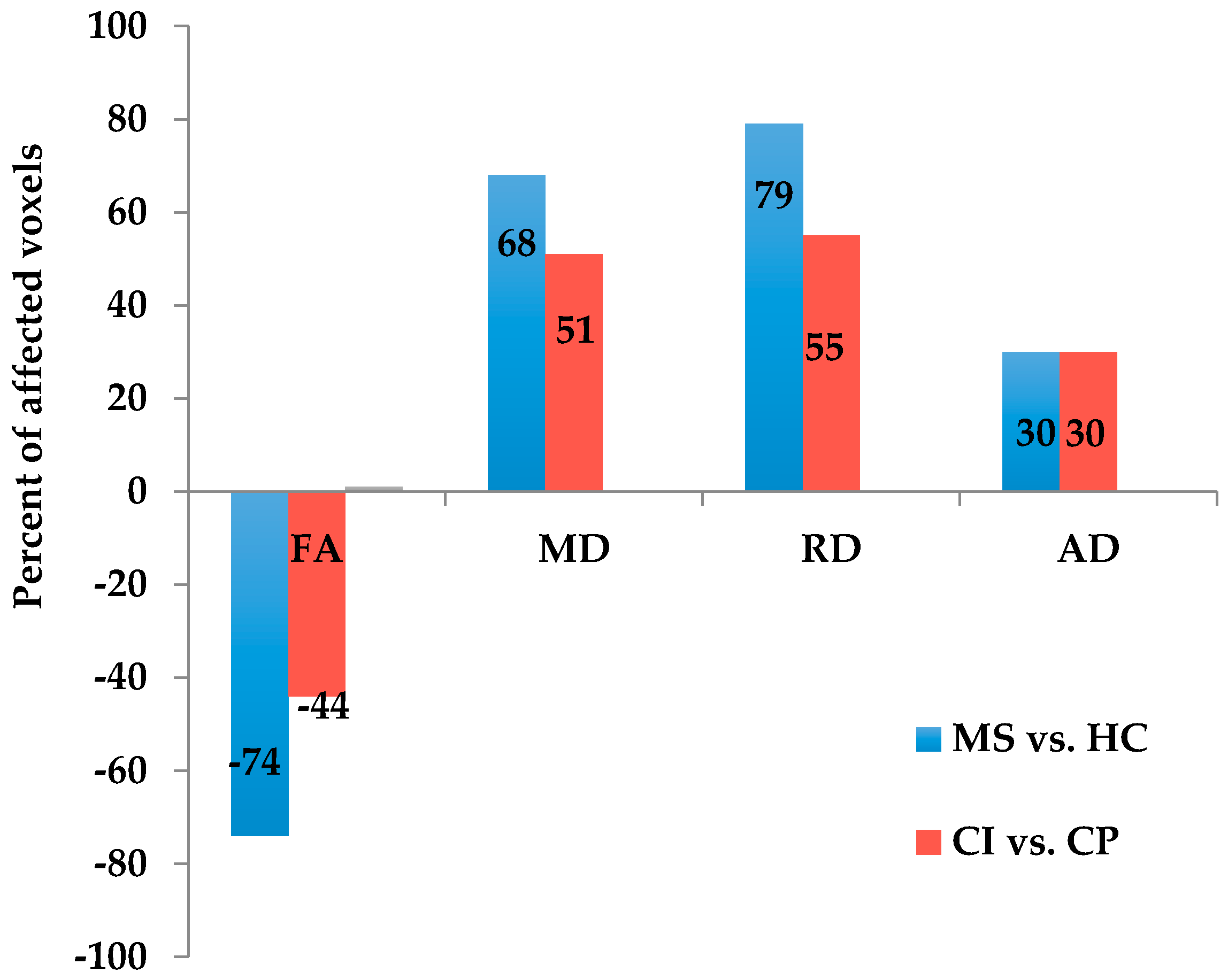
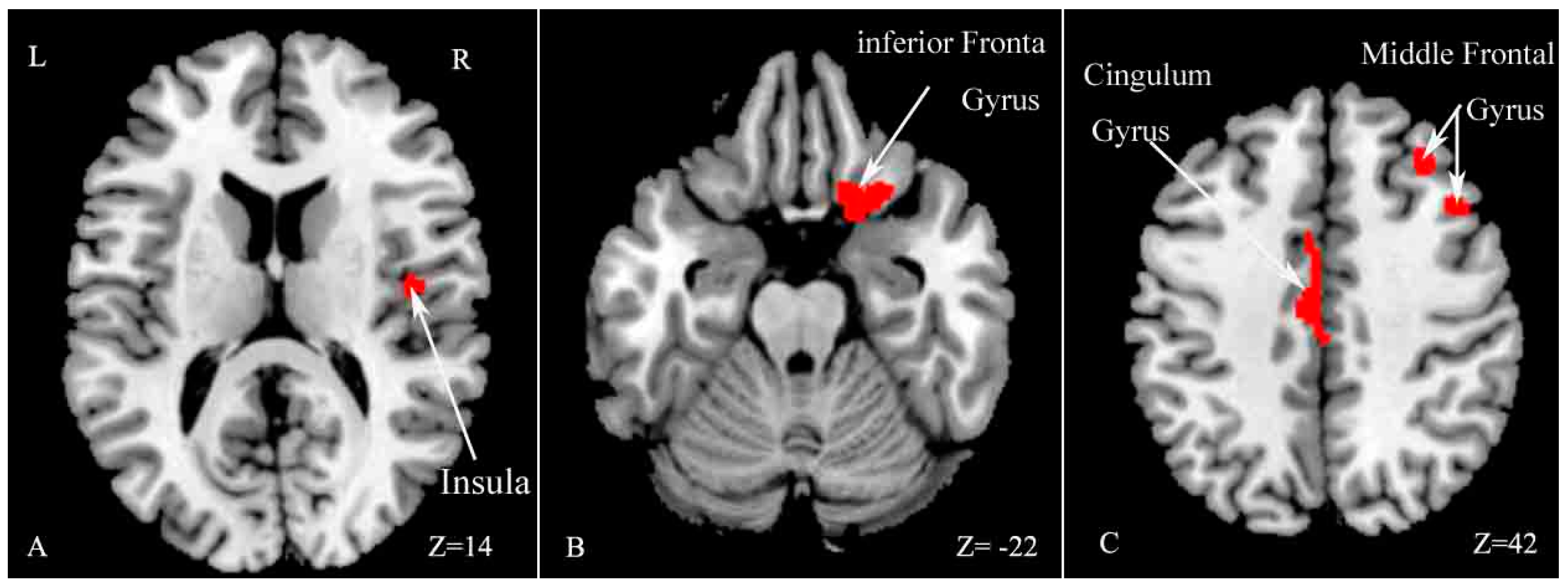
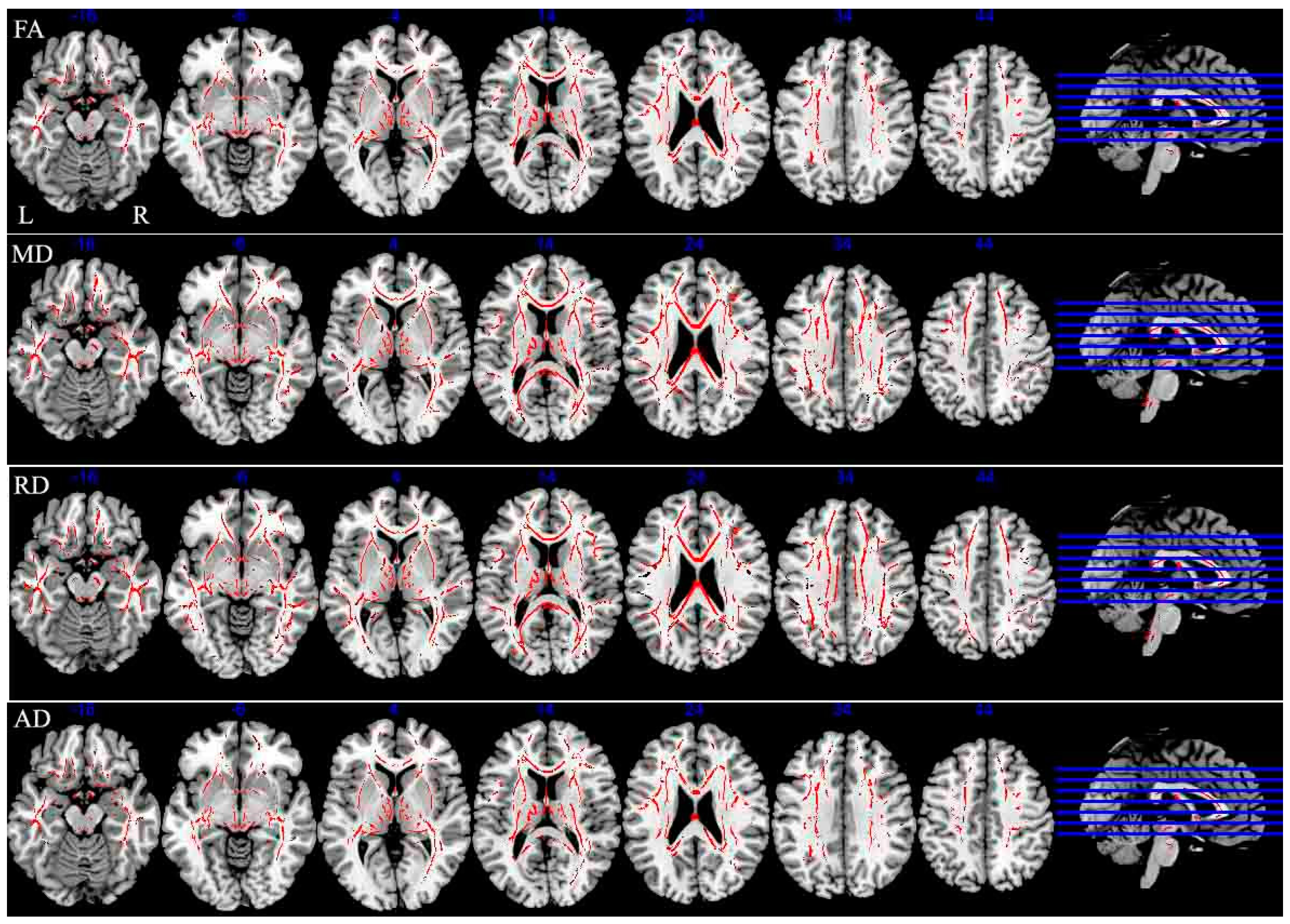
The current study revealed several main findings. First, MS patients exhibited worse performance on the SDMT and PASAT-3″ compared with HCs. We found extensive WM damage, including cortical and subcortical GM atrophy in the right insula, middle frontal gyrus, inferior frontal gyrus and left cingulate gyrus in MS patients compared with HCs. In addition, CI–MS patients exhibited worse performance on the Auditory Verbal Learning Test (AVLT), Verbal Fluency Test (VFT), PASAT-3″, Brief Visuospatial Memory Test (BVMT), and Trail making test (TMT) compared with CP–MS patients. CI–MS patients showed a widespread reduction in FA values in many WM tracts throughout the skeletons, compared with CP–MS patients. The results also revealed regional GM atrophy in several regions, including the right temporal lobe, left insula and bilateral caudate.
The current results revealed a significant difference in SDMT and PASAT-3″ performance between MS and HC, which primarily reflects information processing speed and working memory performance. This finding is consistent with previous reports [
1,
16]. Moreover, we found widespread WM impairment, as well as cortical and subcortical GM loss in a number of areas, including the insula, frontal gyrus and cingulate gyrus, in accord with a number of previous studies [
10,
11,
12,
17,
18,
19,
20]. Both WM and GM abnormities are known to be involved in MS.
Further analyses revealed significant differences in verbal fluency, speed of information processing, executive function, and spatial memory between CI–MS and CP–MS patients, in accord with previous studies [
10,
21]. In addition, the CI group exhibited widespread WM microstructure dysfunction compared with the CP group in a range of brain regions, including the corpus callosum, cerebellar peduncle, corona radiata, optic radiation, superior longitudinal fasciculus, the anterior limb of the internal capsule, and the cingulate. The corpus callosum has previously been implicated in the speed of information processing, executive function, and verbal fluency [
22]. In addition, damage to the optic radiation and superior longitudinal fasciculus have been associated with spatial memory deficits [
23,
24]. Hulst et al. reported a 76% decrease in FA in the WM of CI patients, and a 49% FA decrease in the WM of CP patients. On the basis of these results, the authors speculated that WM damage was related to CI–MS [
10]. The current results further support the notion that WM damage is associated with cognitive dysfunction in MS.
However, no significant difference in GM volume was found between CI and CP patients in Hulst’s study, and the authors proposed that GM was not related to cognitive deficits in MS [
10]. The current results revealed significant decreased GM volume, including in the right temporal lobe, left insula and bilateral caudate nucleus in CI patients compared with CP patients. The caudate nucleus has been found to be related to verbal fluency, language learning and memory [
25], and the temporal lobe is implicated in memory function and information processing speed [
12,
24,
26]. The current results indicate that atrophy of the right temporal pole, left insula and bilateral caudate nucleus may be related to CI in RRMS. The discrepancy may be related to the different disease subtypes of MS patients between the two studies.
In addition, the results revealed that CI–MS patients had a lower educational level, as well as more severe clinical disability and mental dysfunction, including anxiety and depression, compared with CP–MS patients. These findings are consistent with several previous reports suggesting that lower educational level, higher Expanded Disability Status Scale (EDSS)score and more severe mental dysfunction are related to CI [
27,
28,
29]. A 5-year neuropsychological longitudinal study of patients with MS reported no significant change in the speed of information processing in a group of patients with more than 14 years of education, whereas patients with less than 14 years of education exhibited significant declines in information processing speed [
30]. Similarly, a recent 4.5-year follow-up study indicated that increased intellectual enrichment contributed to resilience against disease-related cognitive decline [
31]. In addition, compared with CP–MS patients, CI–MS patients exhibited more severe physical disabilities and abnormal moods including anxiety and depression. Previous studies reported that fatigue and abnormal mood, particularly depression, can affect patients’ cognitive function, quality of life and medical compliance [
27,
32]. Taken together, these findings highlight the importance of treatment for abnormal mood, particularly depression, among MS patients.
The present study involved several limitations. First, the CI patient sample size was relatively small; Second, the study did not implement a uniform set of criteria for distinguishing CI–MS patients. Most previous studies used >1.5 or 2 standard deviations (SDs) below normal data in at least two cognitive domains for identifying CI. In the current study, we used 1.5 SDs as the threshold, to enable the detection of mild CI; Third, this was a single center study. Since all patients were enrolled in the study via tertiary referrals, selection bias may have caused the sample to be non-representative of the wider population. It would be useful for future studies involving multiple centers and a larger study population to validate the current results. In addition, future studies should use more strict criteria for identifying CI (e.g., >2 SDs, or impairment in at least three cognitive domains).
It should be noted that cortical lesions were not measured in our studies and lesion-filling was not implemented either. Previous investigations have shown that lesion-filling could improve the accuracy of WM and GM volume measurements [
33]. However, there is controversy about the lesion filling approach in MS. Some researchers have argued that hypointensity WM lesions in MS could be misclassified as GM in tissue segmentation that increase the measured GM volume [
34,
35]. In contrast, some others believe that all GM volumes except for hippocampus had smaller volumes in the original image compared to the lesion-filled image in MS patients [
36]. Moreover, lesion volumes were usually identified manually, and this process might increase the subjective errors.