A Multiple Reaction Modelling Framework for Microbial Electrochemical Technologies
Abstract
:1. Introduction
- Predict kinetics in terms of current and voltage of the system as functions of operation conditions.
- Describe and study the mechanism of extracellular electron transport: conductive biofilms, nanowires, electric gradient, electron shuttling, etc.
- Investigate the ecology of the microbial communities that perform extracellular electron transport (EET).
2. Results and Discussion
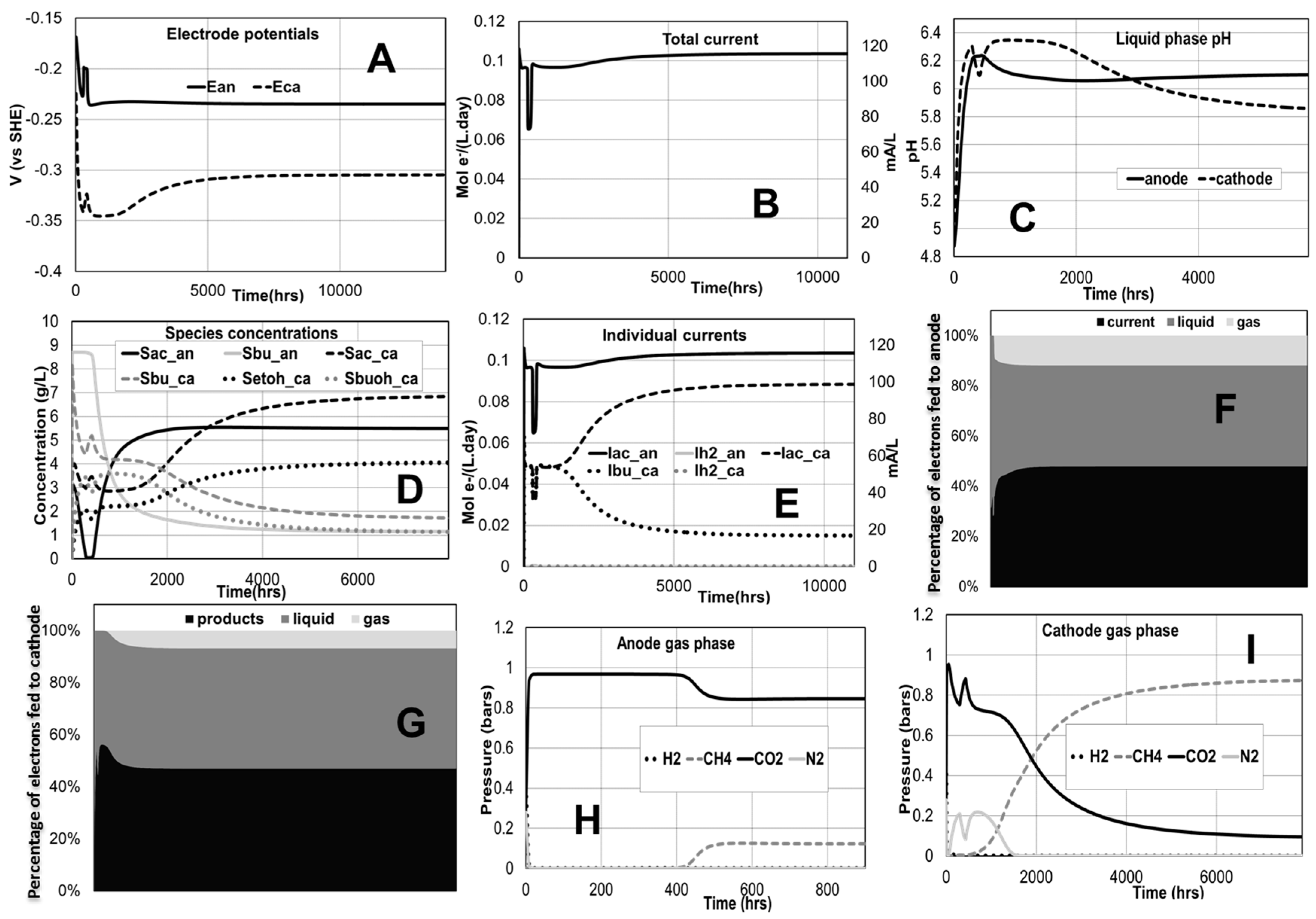
2.1. Ethanol/Butanol Production in an MEC
2.1.1. Effect of Applied Voltage
2.1.2. Effect of Anode-to-Cathode Feed Ratio
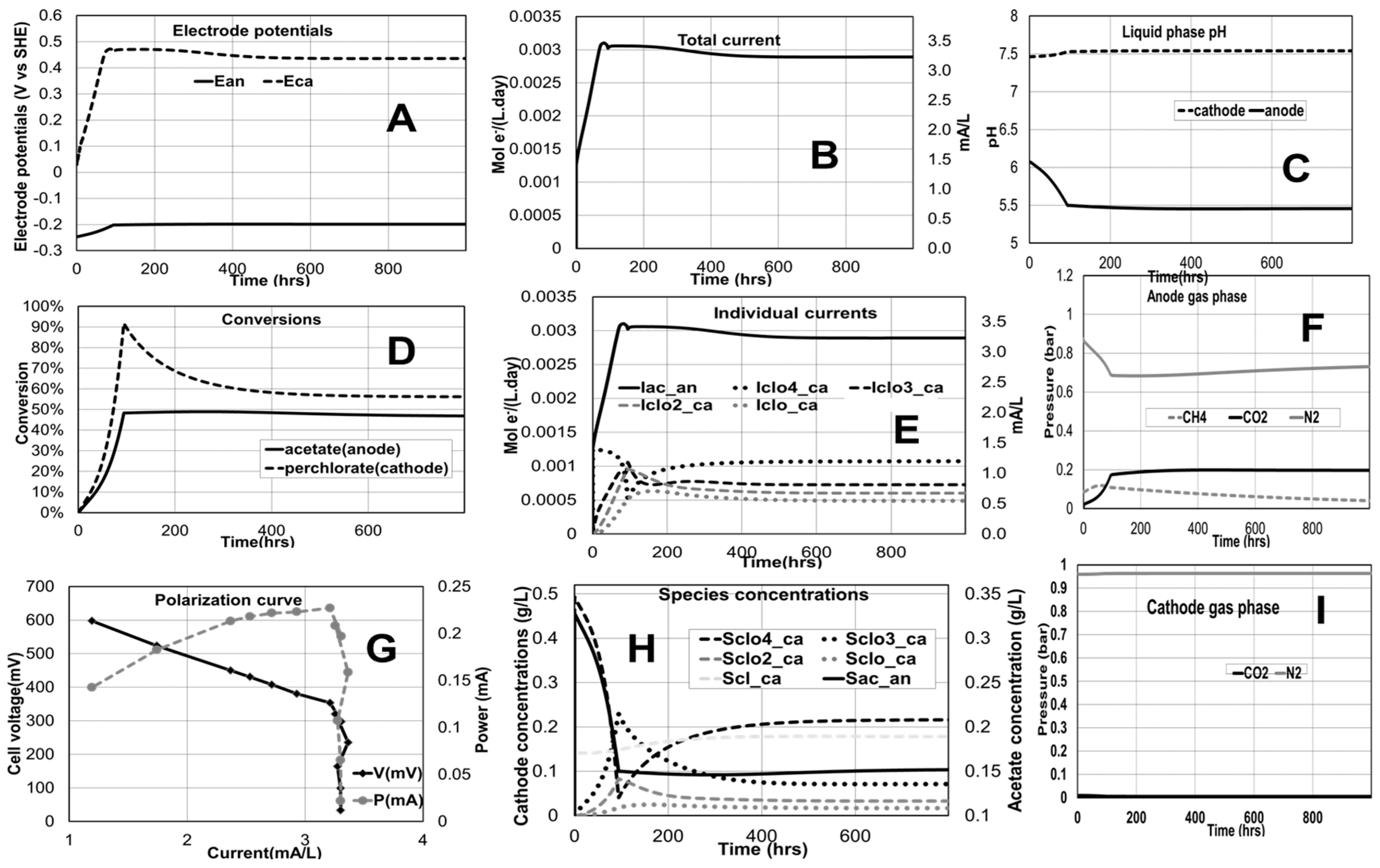
2.2. Perchlorate Remediation in an MFC
Effect of Influent Flow Rates and Concentrations
3. Materials and Methods
3.1. Model Structure Selection
3.2. Generalized Physico-Chemical Framework for Bioprocess Modeling
3.3. Modeling Competing Anaerobic Fermentative Processes
3.4. Electrode Reaction Kinetics
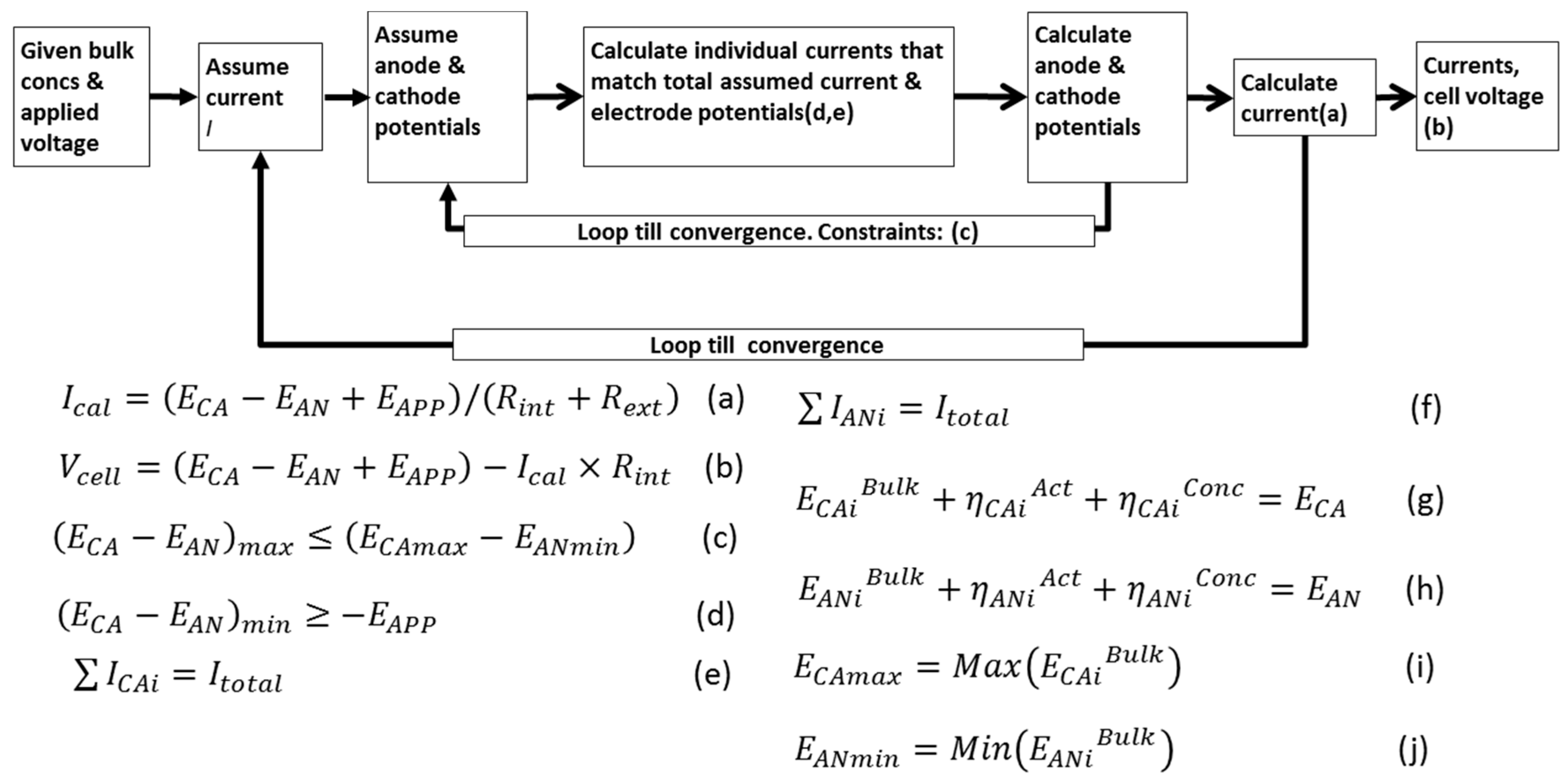
3.5. Electrical Model (Modeling Multiple Electrode Reactions)
- (i).
- In each electrode, the individual current contributions of each electroactive reaction must add up to the observed total current; and
- (ii).
- The electrode potential is unique for all reactions taking place at that electrode. This means that the sum of the reaction potential at the bulk plus the potential losses both by concentration polarization and activation for each reaction must match a unique electrode potential both for the anode and for the cathode.
3.6. Modeling Competition between Electroactive and Non-Electroactive Microbial Species
3.7. Modeling Ionic Flow across Different Types of Membranes
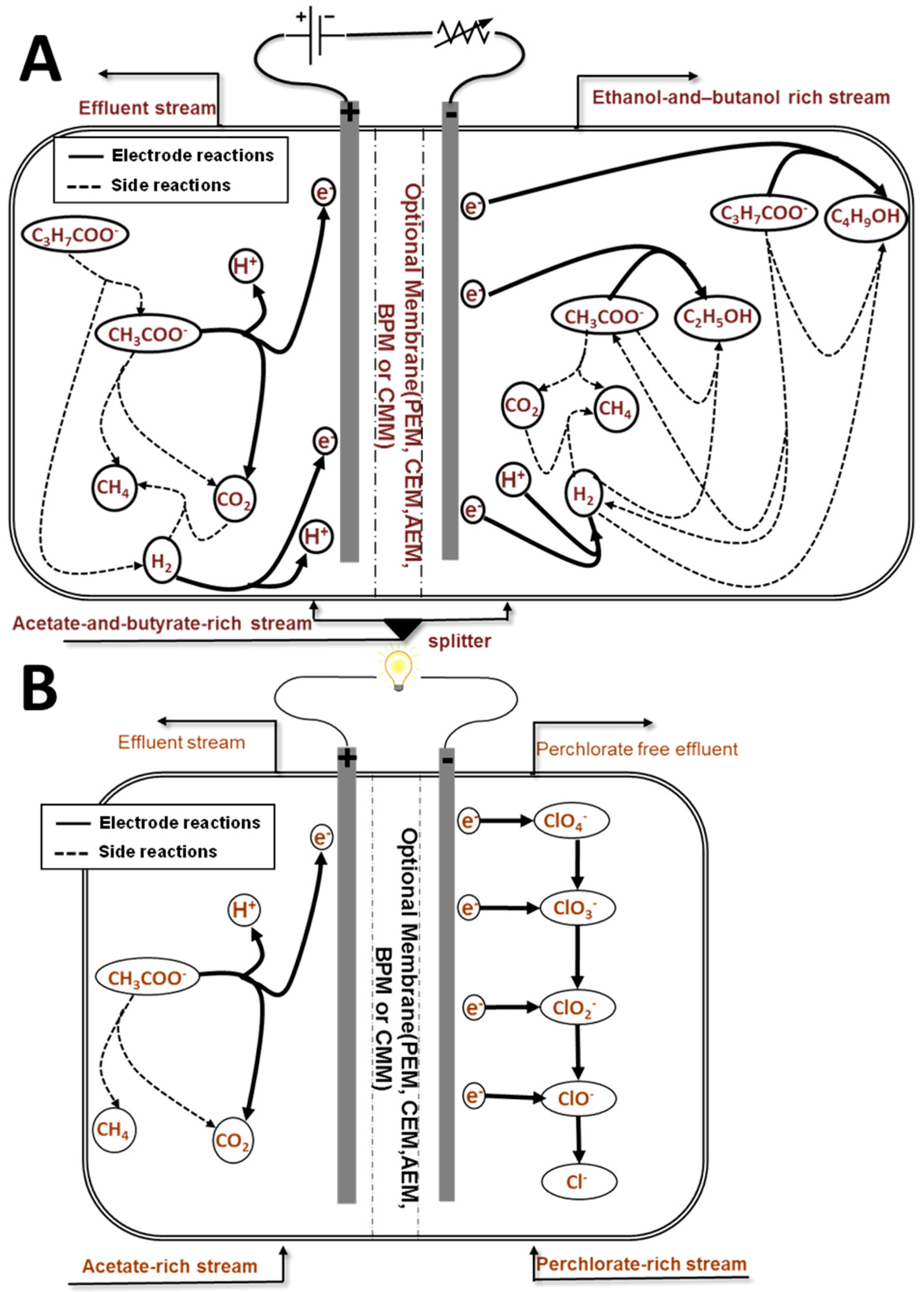
4. Description of Case Studies
4.1. Ethanol/Butanol Production in an MEC
4.2. Perchlorate Remediation in an MFC
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dochain, D.; Vanrolleghem, P.A. Dynamic Modelling and Estimation in Wastewater Treatment Processes; IWA Publishing: London, UK, 2001. [Google Scholar]
- Picioreanu, C.; Head, I.M.; Katuri, K.P.; van Loosdrecht, M.C.M.; Scott, K. A computational model for biofilm-based microbial fuel cells. Water Res. 2007, 41, 2921–2940. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Premier, G. Towards A Mathematical Description of Bioelectrochemical Systems. In Bioelectrochemical Systems: From Extracellular Electron Transfer to Biotechnological Application; Rabaey, K., Angenent, L., Schroder, U., Keller, J., Eds.; IWA Publishing: London, UK, 2010. [Google Scholar]
- Hamelers, H.V.M.; ter Heijne, A.; Stein, N.; Rozendal, R.A.; Buisman, C.J.N. Butler-Volmer-Monod model for describing bio-anode polarization curves. Bioresour. Technol. 2011, 102, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.P.; Srinivasan, B.; Escapa, A.; Tartakovsky, B. Multi-population model of a microbial electrolysis cell. Environ. Sci. Technol. 2011, 45, 5039–5046. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Choo, Y.F.; Kim, B.H.; Wu, P. Modelling and simulation of two-chamber microbial fuel cell. J. Power Sour. 2010, 195, 79–89. [Google Scholar] [CrossRef]
- Merkey, B.; Chopp, D. The performance of a microbial fuel cell depends strongly on anode geometry: A multidimensional modeling study. Bull. Math. Biol. 2012, 74, 834–857. [Google Scholar] [CrossRef] [PubMed]
- Marcus, A.K.; Torres, C.I.; Rittmann, B.E. Analysis of a microbial electrochemical cell using the proton condition in biofilm (PCBIOFILM) model. Bioresour. Technol. 2011, 102, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martínez, V.M.; Salar-Garcíaa, M.J.; de los Ríosb, A.P.; Hernández-Fernándeza, F.J.; Egeac, J.A.; Lozanoa, L.J. Developments in microbial fuel cell modeling. Chem. Eng. J. 2015, 271, 50–60. [Google Scholar] [CrossRef]
- Yahya, A.M.; Hussain, M.A.; Wahab, A.; Khairi, A. Modeling, optimization, and control of microbial electrolysis cells in a fed-batch reactor for production of renewable biohydrogen gas. Int. J. Energy Res. 2015, 39, 557–572. [Google Scholar] [CrossRef]
- Alavijeh, M.K.; Yaghmaei, S.; Mardanpour, M.M. A combined model for large scale batch culture MFC-digester with various wastewaters through different populations. Bioelectrochemistry 2015, 106, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Cocom, L.; Guerrero-Álvarez, A.; Domínguez-Maldonado, J.; Ávila-Vales, E.; Alzate-Gaviria, L. Mathematical model for a continuous hydrogen production system: Stirred fermenter connected to a biocatalyzed electrolysis cell. Biomass Bioenerg. 2013, 48, 90–99. [Google Scholar] [CrossRef]
- Alavijeh, M.K.; Mardanpour, M.M.; Yaghmaei, S. One-dimensional conduction-based modeling of bioenergy production in a microbial fuel cell engaged with multi-population biocatalysts. Electrochim. Acta 2015, 184, 151–163. [Google Scholar] [CrossRef]
- Recio-Garrido, D.; Perrier, M.; Tartakovsky, B. Modeling, optimization and control of bioelectrochemical systems. Chem. Eng. J. 2016, 289, 180–190. [Google Scholar] [CrossRef]
- Boghani, H.C.; Michie, I.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Control of microbial fuel cell voltage using a gain scheduling control strategy. J. Power Sour. 2016, 322, 106–115. [Google Scholar] [CrossRef]
- Molognoni, D.; Sebastià, P.; Dolors Balaguer, M.; Capodaglio, A.G.; Callegari, A.; Colprim, J. Multiparametric control for enhanced biofilm selection in microbial fuel cells. J. Chem. Technol. Biotechnol. 2016, 91, 1720–1727. [Google Scholar] [CrossRef]
- Oh, S.T.; Kim, J.R.; Premier, G.C.; Lee, T.H.; Kim, C.; Sloan, W.T. Sustainable wastewater treatment: How might microbial fuel cells contribute. Biotechnol. Adv. 2010, 28, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.P. Dynamic Modelling and Optimization of Microbial Fuel Cells and Microbial Electrolysis Cells Ecole Polytechnic. Ph.D. Thesis, École Polytechnique de Montréal, Montreal, QC, Canada, 2011. [Google Scholar]
- Kato Marcus, A.; Torres, C.I.; Rittmann, B.E. Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol. Bioeng. 2007, 98, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Steinbusch, K.J.; Hamelers, H.V.; Buisman, C.J. Alcohol production through volatile fatty acids reduction with hydrogen as electron donor by mixed cultures. Water Res. 2008, 42, 4059–4066. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cheng, S.; Logan, B.E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Steinbusch, K.J.; Hamelers, H.V.; Schaap, J.D.; Kampman, C.; Buisman, C.J. Bioelectrochemical ethanol production through mediated acetate reduction by mixed cultures. Environ. Sci. Technol. 2009, 44, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Harma, M.; Aryal, N.; Sarma, P.M.; Vanbroekhoven, K.; Lal, B.; Dominguez-Benetton, X.; Pant, D. Bioelectrocatalyzed reduction of acetic and butyric acids via direct electron transfer by a mixed culture of sulfate-reducers drives electrosynthesis of alcohols and acetone. Chem. Commun. 2013, 49, 6495–6497. [Google Scholar]
- Lakaniemi, A.-M.; Tuovinen, O.; Puhakka, J. Production of electricity and butanol from microalgal biomass in microbial fuel cells. BioEnerg. Res. 2012, 5, 481–491. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Logan, B.E. Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl. Microbiol. Biotechnol. 2008, 80, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.S.; Clauwaert, P.; Green, S.J.; Verstraete, W.; Nerenberg, R. Bioelectrochemical perchlorate reduction in a microbial fuel cell. Environ. Sci. Technol. 2010, 44, 4685–4691. [Google Scholar] [CrossRef] [PubMed]
- Nor, S.J.; Lee, S.H.; Cho, K.S.; Cha, D.K.; Lee, K.I.; Ryu, H.W. Microbial treatment of high-strength perchlorate wastewater. Bioresour. Technol. 2011, 102, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Benziger, J.B.; Satterfield, M.B.; Hogarth, W.H.J.; Nehlsen, J.P.; Kevrekidis, I.G. The power performance curve for engineering analysis of fuel cells. J. Power Sources 2006, 155, 272–285. [Google Scholar] [CrossRef]
- Jafary, T.; Ghoreyshi, A.A.; Najafpour, G.D.; Fatemi, S.; Rahimnejad, M. Investigation on performance of microbial fuel cells based on carbon sources and kinetic models. Int. J. Energ. Res. 2013, 37, 1539–1549. [Google Scholar] [CrossRef]
- González-Cabaleiro, R.; Lema, J.M.; Rodríguez, J. Generalised acid-base calculation method for aqueous systems. In Proceedings of the IWA 13th World Congress on Anaerobic Digestion, Santiago de Compostella, Spain, 25 June 2013.
- Oyetunde, T.; González-Cabaleiro, R.; Ahmad, F.; Rodríguez, J. A Generalized Excel/C-compatible Simulink-based implementation architecture for fast model development and simulations. In Proceedings of the 11th IWA Conference on Instrumentation Control and Automation, Narbonne, France, 18–20 September 2013; IWA Publishing: London, UK, 2013. [Google Scholar]
- Rodríguez, J.; Premier, G.; Dinsdale, R.; Guwy, A. An implementation framework for wastewater treatment models requiring a minimum programming expertise. Water Sci. Technol. 2009, 59, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T. Anaerobic Digestion Model No. 1; IWA Publishing: London, UK, 2001. [Google Scholar]
- Capodaglio, A.G.; Molognoni, D.; Callegari, A. Formulation and preliminary application of an integrated model of microbial fuel cell processes. In Proceedings of the 29th European Conference on Modelling and Simulation ECMS, Albena, Bulgaria, 26–29 May 2015.
- Giles, G.E.; Gray, L.J.; Bullock, I.V.J.S.; van Berkel, G.J. Numerical simulation of anodic reactions. Fundamental Aspects of Electrochemical Deposition and Dissolution. In Proceedings of the International Symposium; The Electrochemical Society: Pennington, NJ, USA, 2000; p. 335. [Google Scholar]
- Pinto, R.P.; Srinivasan, B.; Manuel, M.F.; Tartakovsky, B. A two-population bio-electrochemical model of a microbial fuel cell. Bioresour. Technol. 2010, 101, 5256–5265. [Google Scholar] [CrossRef] [PubMed]
- Harnisch, F.; Warmbier, R.; Schneider, R.; Schröder, U. Modeling the ion transfer and polarization of ion exchange membranes in bioelectrochemical systems. Bioelectrochemistry 2009, 75, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Harnisch, F.; Schröder, U.; Scholz, F. The suitability of monopolar and bipolar ion exchange membranes as separators for biological fuel cells. Environ. Sci. Technol. 2008, 42, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.E.; Logan, B. Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Appl. Microbiol. Biotechnol. 2006, 70, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.; Molenkamp, R.J.; Buisman, C.J. Performance of single chamber biocatalyzed electrolysis with different types of ion exchange membranes. Water Res. 2007, 41, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.; Buisman, C.J. Effects of membrane cation transport on pH and microbial fuel cell performance. Environ. Sci. Technol. 2006, 40, 5206–5211. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.; Sleutels, T.; Hamelers, H.; Buisman, C. Effect of the type of ion exchange membrane on performance, ion transport, and pH in biocatalyzed electrolysis of wastewater. Water Sci. Technol. 2008, 57, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Cheng, S.; Oh, S.E.; Logan, B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Girguis, P.; Nielsen, L.K. Metabolic and practical considerations on microbial electrosynthesis. Curr. Opin. Biotechnol. 2011, 22, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Hatzinger, P.B. Perchlorate biodegradation for water treatment. Environ. Sci. Technol. 2005, 39, 239A–247A. [Google Scholar] [CrossRef] [PubMed]
- Son, A.; Lee, J.; Chiu, P.C.; Kim, B.J.; Cha, D.K. Microbial reduction of perchlorate with zero-valent iron. Water Res. 2006, 40, 2027–2032. [Google Scholar] [CrossRef] [PubMed]
- Mieseler, M.; Atiyeh, M.; Hernandez, H.; Ahmad, F. Direct enrichment of perchlorate-reducing microbial community for efficient electroactive perchlorate reduction in biocathodes. J. Ind. Microbiol. Biotechnol. 2013, 40, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Thrash, J.C.; Van Trump, J.I.; Weber, K.A.; Miller, E.; Achenbach, L.A.; Coates, J.D. Electrochemical stimulation of microbial perchlorate reduction. Environ. Sci. Technol. 2007, 41, 1740–1746. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyetunde, T.; Sarma, P.M.; Ahmad, F.; Rodríguez, J. A Multiple Reaction Modelling Framework for Microbial Electrochemical Technologies. Int. J. Mol. Sci. 2017, 18, 86. https://doi.org/10.3390/ijms18010086
Oyetunde T, Sarma PM, Ahmad F, Rodríguez J. A Multiple Reaction Modelling Framework for Microbial Electrochemical Technologies. International Journal of Molecular Sciences. 2017; 18(1):86. https://doi.org/10.3390/ijms18010086
Chicago/Turabian StyleOyetunde, Tolutola, Priyangshu M. Sarma, Farrukh Ahmad, and Jorge Rodríguez. 2017. "A Multiple Reaction Modelling Framework for Microbial Electrochemical Technologies" International Journal of Molecular Sciences 18, no. 1: 86. https://doi.org/10.3390/ijms18010086





