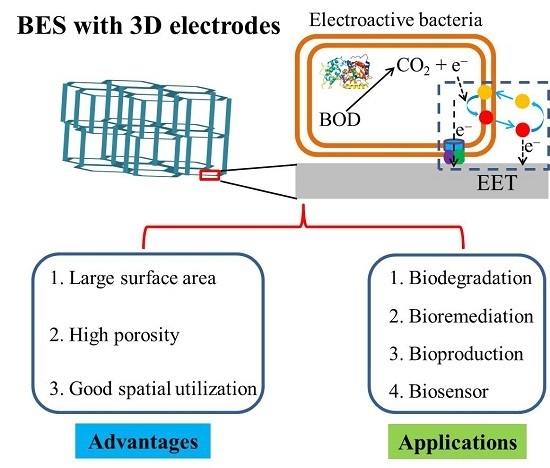
Three-Dimensional Electrodes for High-Performance Bioelectrochemical Systems
Abstract
:1. Introduction
2. Building 3D Electrodes for High-Performance BES
2.1. Conventional 3D Electrodes: Packed Bed and Brush Electrode
2.2. 3D Matrix Fabricated on a 2D Electrode
2.2.1. Physical Deposition and Self-Assembly
2.2.2. In Situ Growth
2.3. Monolithic 3D Electrode from 3D Porous Template
2.3.1. 3D Electrodes Fabricated from Conductive Porous Templates
2.3.2. 3D Electrodes Fabricated from Non-Conductive Porous Template
2.3.3. 3D Electrodes Fabricated with a Sacrificial Porous Template
2.3.4. 3D Electrodes Fabricated from Natural Porous Template
2.4. 3D Bioelectrode with Hybridized Biofilm
2.4.1. Bacteria Immobilization
2.4.2. Self-Assembled Hybrid Biofilm
3. Challenges and Perspectives
Acknowledgments
Conflicts of Interest
References
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. B Biol. Sci. 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Harnisch, F.; Schroeder, U. From MFC to MXC: Chemical and biological cathodes and their potential for microbial bioelectrochemical systems. Chem. Soc. Rev. 2010, 39, 4433–4448. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Mecheri, B.; Garavaglia, V.; Licoccia, S.; Di Nardo, P.; Traversa, E. Engineering materials and biology to boost performance of microbial fuel cells: A critical review. Energy Environ. Sci. 2008, 1, 417–429. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.T.; Kim, J.R.; Premier, G.C.; Lee, T.H.; Kim, C.; Sloan, W.T. Sustainable wastewater treatment: How might microbial fuel cells contribute. Biotechnol. Adv. 2010, 28, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, Y.Y.; Bi, Z.; Cao, Y.Q. Simultaneous decolorization of azo dye and bioelectricity generation using a microfiltration membrane air-cathode single-chamber microbial fuel cell. Bioresour. Technol. 2009, 100, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Boon, N.; Marzorati, M.; Verstraete, W. Enhanced removal of 1,2-dichloroethane by anodophilic microbial consortia. Water Res. 2009, 43, 2936–2946. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Catervi, A.; Majone, M.; Panero, S.; Reale, P.; Rossetti, S. Electron transfer from a solid-state electrode assisted by methyl viologen sustains efficient microbial reductive dechlorination of tce. Environ. Sci. Technol. 2007, 41, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- And, K.B.G.; Lovley, D.R. Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environ. Sci. Technol. 2005, 39, 8943–8947. [Google Scholar]
- Clauwaert, P.; Rabaey, K.; Aelterman, P.; De, S.L.; Pham, T.H.; Boeckx, P.; Boon, N.; Verstraete, W. Biological denitrification in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.X.; Xia, H.; Peng, L.; Kang, X.; Zhou, Y.J.; Zhang, X.Y.; Logan, B.E. A new method for water desalination using microbial desalination cells. Environ. Sci. Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Kalleary, S.; Abbas, F.M.; Ganesan, A.; Meenatchisundaram, S.; Srinivasan, B.; Packirisamy, A.S.B.; Kesavan, R.K.; Muthusamy, S. Biodegradation and bioelectricity generation by microbial desalination cell. Int. Biodeterior. Biodegrad. 2014, 92, 20–25. [Google Scholar] [CrossRef]
- Meng, F.; Jiang, J.; Zhao, Q.; Wang, K.; Zhang, G.; Fan, Q.; Wei, L.; Jing, D.; Zhen, Z. Bioelectrochemical desalination and electricity generation in microbial desalination cell with dewatered sludge as fuel. Bioresour. Technol. 2014, 157, 120–126. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Zhang, H.; Wen, Q.; Chen, Z.; Du, M. Desalination combined with copper(II) removal in a novel microbial desalination cell. Desalination 2014, 346, 115–121. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Sustainable and efficient biohydrogen production via electrohydrogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 18871–18873. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Hydrogen production with a microbial biocathode. Environ. Sci. Technol. 2008, 42, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Leone, E.; Keller, J.; Rabaey, K. Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochem. Commun. 2009, 11, 1752–1755. [Google Scholar] [CrossRef]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, F.; Liu, J.; Zhang, X.; Huang, X.; Logan, B.E. Methane production in microbial reverse-electrodialysis methanogenesis cells (MRMCs) using thermolytic solutions. Environ. Sci. Technol. 2014, 48, 8911–8918. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.A.; Xing, D.F.; Call, D.F.; Logan, B.E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef] [PubMed]
- Jourdin, L.; Grieger, T.; Monetti, J.; Flexer, V.; Freguia, S.; Lu, Y.; Chen, J.; Romano, M.; Wallace, G.G.; Keller, J. High acetic acid production rate obtained by microbial electrosynthesis from carbon dioxide. Environ. Sci. Technol. 2015, 49, 13566–13574. [Google Scholar] [CrossRef] [PubMed]
- Gildemyn, S.; Verbeeck, K.; Slabbinck, R.; Andersen, S.J.; Prevoteau, A.; Rabaey, K. Integrated production, extraction, and concentration of acetic acid from CO2 through microbial electrosynthesis. Environ. Sci. Technol. Lett. 2015, 2, 325–328. [Google Scholar] [CrossRef]
- Wang, Q.N.; Dong, H.; Yu, H.; Yu, H.B. Enhanced performance of gas diffusion electrode for electrochemical reduction of carbon dioxide to formate by adding polytetrafluoroethylene into catalyst layer. J. Power Sources 2015, 279, 1–5. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Zhang, Y.; Chang, Y.Y.; Li, Z.S. Conversion of a substrate carbon source to formic acid for carbon dioxide emission reduction utilizing series-stacked microbial fuel cells. J. Power Sources 2012, 217, 59–64. [Google Scholar] [CrossRef]
- Pasco, N.F.; Weld, R.J.; Hay, J.M.; Gooneratne, R. Development and applications of whole cell biosensors for ecotoxicity testing. Anal. Bioanal. Chem. 2011, 400, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Stein, N.E.; Keesman, K.J.; Hamelers, H.V.M.; van Straten, G. Kinetic models for detection of toxicity in a microbial fuel cell based biosensor. Biosens. Bioelectron. 2011, 26, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, N.S.J.; Zhou, Q.X. Concentration responses of toxicity sensor with shewanella oneidensis mr-1 growing in bioelectrochemical systems. Biosens. Bioelectron. 2013, 43, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Thomson, A.R.; Schneider, K.; Cameron, P.J.; Ieropoulos, I. A small-scale air-cathode microbial fuel cell for on-line monitoring of water quality. Biosens. Bioelectron. 2014, 62, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.F.; Angelidaki, I. A simple and rapid method for monitoring dissolved oxygen in water with a submersible microbial fuel cell (SBMFC). Biosens. Bioelectron. 2012, 38, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Angelidaki, I. Submersible microbial fuel cell sensor for monitoring microbial activity and bod in groundwater: Focusing on impact of anodic biofilm on sensor applicability. Biotechnol. Bioeng. 2011, 108, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Jang, J.K.; Pham, T.H.; Moon, H.; Chang, I.S.; Kim, B.H. A microbial fuel cell with improved cathode reaction as a low biochemical oxygen demand sensor. Biotechnol. Lett. 2003, 25, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Modin, O.; Wilen, B.M. A novel bioelectrochemical bod sensor operating with voltage input. Water Res. 2012, 46, 6113–6120. [Google Scholar] [CrossRef] [PubMed]
- Atci, E.; Babauta, J.T.; Sultana, S.T.; Beyenal, H. Microbiosensor for the detection of acetate in electrode-respiring biofilms. Biosens. Bioelectron. 2016, 81, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Si, R.W.; Zhai, D.D.; Liao, Z.H.; Gao, L.; Yong, Y.C. A whole-cell electrochemical biosensing system based on bacterial inward electron flow for fumarate quantification. Biosens. Bioelectron. 2015, 68, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; You, S.J.; Wang, J.Y. Carbon nanotubes as electrode modifier promoting direct electron transfer from shewanella oneidensis. Biosens. Bioelectron. 2010, 25, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Guo, S.; Liu, Z.; Zhou, M.; Li, D.; Liu, L.; Li, G.; Wang, E.; Dong, S. To boost c-type cytochrome wire efficiency of electrogenic bacteria with Fe3O4/Au nanocomposites. Chem. Commun. 2010, 46, 7172–7174. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, Y.-Y.; Deng, X.-P.; Ng, C.K.; Cao, B.; Wang, J.-Y.; Rice, S.A.; Kjelleberg, S.; Song, H. Enhanced shewanella biofilm promotes bioelectricity generation. Biotechnol. Bioeng. 2015, 112, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Leang, C.; Malvankar, N.S.; Franks, A.E.; Nevin, K.P.; Lovley, D.R. Engineering geobacter sulfurreducens to produce a highly cohesive conductive matrix with enhanced capacity for current production. Energy Environ. Sci. 2013, 6, 1901–1908. [Google Scholar] [CrossRef]
- Yong, Y.C.; Cai, Z.; Yu, Y.Y.; Chen, P.; Jiang, R.R.; Cao, B.; Sun, J.Z.; Wang, J.Y.; Song, H. Increase of riboflavin biosynthesis underlies enhancement of extracellular electron transfer of shewanella in alkaline microbial fuel cells. Bioresour. Technol. 2013, 130, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, Y.-Y.; Chen, T.; Chen, W.N. A synthetic microbial consortium of shewanella and bacillus for enhanced generation of bioelectricity. Biotechnol. Bioeng. 2016. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I.; Marcus, A.K.; Rittmann, B.E. Proton transport inside the biofilm limits electrical current generation by anode-respiring bacteria. Biotechnol. Bioeng. 2008, 100, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.M.; Lv, M.L.; Zhu, Y.; Jiang, L.; Liu, H. Frontispiece: Wettability-regulated extracellular electron transfer from the living organism of shewanella loihica pv-4. Angew. Chem. Int. Ed. 2015, 54, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I.; Marcus, A.K.; Parameswaran, P.; Rittmann, B.E. Kinetic experiments for evaluating the nernst-monod model for anode-respiring bacteria (ARB) in a biofilm anode. Environ. Sci. Technol. 2008, 42, 6593–6597. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Effect of mass and charge transport speed and direction in porous anodes on microbial electrolysis cell performance. Bioresour. Technol. 2011, 102, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air-cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Lai, B.; Tang, X.; Li, H.; Du, Z.; Liu, X.; Zhang, Q. Power production enhancement with a polyaniline modified anode in microbial fuel cells. Biotechnol. Bioeng. 2011, 28, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Le, M.; Li, F.; Mai, H.; Lang, X.; Fan, S. A polypyrrole/anthraquinone-2,6-disulphonic disodium salt (ppy/aqds)-modified anode to improve performance of microbial fuel cells. Biosens. Bioelectron. 2010, 25, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Y.; Chen, S. Improved microbial electrocatalysis with neutral red immobilized electrode. J. Power Sources 2011, 196, 164–168. [Google Scholar] [CrossRef]
- Xie, X.; Criddle, C.; Cui, Y. Design and fabrication of bioelectrodes for microbial bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 94–113. [Google Scholar] [CrossRef]
- Rabaey, K.; Clauwaert, P.; Aelterman, P.; Verstraete, W. Tubular microbial fuel cells for efficient electricity generation. Environ. Sci. Technol. 2005, 39, 8077–8082. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wagner, N.; Minteer, S.D.; Angenent, L.T. An upflow microbial fuel cell with an interior cathode: Assessment of the internal resistance by impedance spectroscopy. Environ. Sci. Technol. 2006, 40, 5212–5217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Watanabe, K.; Nakamura, R.; Mori, S.; Liu, H.; Ishii, K.; Hashimoto, K. Three-dimensional conductive nanowire networks for maximizing anode performance in microbial fuel cells. Chem. Eur. J. 2010, 16, 4982–4985. [Google Scholar] [CrossRef] [PubMed]
- Erbay, C.; Yang, G.; Figueiredo, P.D.; Sadr, R.; Yu, C.; Han, A. Three-dimensional porous carbon nanotube sponges for high-performance anodes of microbial fuel cells. J. Power Sources 2015, 298, 177–183. [Google Scholar] [CrossRef]
- Aelterman, P.; Versichele, M.; Marzorati, M.; Boon, N.; Verstraete, W. Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour. Technol. 2008, 99, 8895–8902. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Li, B. Granular activated carbon single-chamber microbial fuel cells (GAC-SCMFCs): A design suitable for large-scale wastewater treatment processes. Biochem. Eng. J. 2009, 47, 31–37. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Scott, K.; Curtis, T.P.; Head, I.M. Effect of increasing anode surface area on the performance of a single chamber microbial fuel cell. Chem. Eng. J. 2010, 156, 40–48. [Google Scholar] [CrossRef]
- Wei, F.; Liang, P.; Cao, X.; Huang, X. Use of inexpensive semicoke and activated carbon as biocathode in microbial fuel cells. Bioresour. Technol. 2011, 102, 10431–10435. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhang, J.; Liu, B.H.; Du, G.C.; Chen, J. Biodegradation of wool waste and keratinase production in scale-up fermenter with different strategies by stenotrophomonas maltophilia BBE11–1. Bioresour. Technol. 2013, 140, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, H.; Zhou, X.; Liang, P.; Zhang, X.; Jiang, Y.; Huang, X. A novel pilot-scale stacked microbial fuel cell for efficient electricity generation and wastewater treatment. Water Res. 2016, 98, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Lanas, V.; Ahn, Y.; Logan, B.E. Effects of carbon brush anode size and loading on microbial fuel cell performance in batch and continuous mode. J. Power Sources 2014, 247, 228–234. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, J.; Li, J.; Ye, D.; Zhu, X.; Zhang, B. Increased performance of a tubular microbial fuel cell with a rotating carbon-brush anode. Biosens. Bioelectron. 2015, 63, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, Z.; Zhang, P. A new method for fabrication of graphene/polyaniline nanocomplex modified microbial fuel cell anodes. J. Power Sources 2013, 224, 139–144. [Google Scholar] [CrossRef]
- Mehdinia, A.; Ziaei, E.; Jabbari, A. Facile microwave-assisted synthesized reduced graphene oxide/tin oxide nanocomposite and using as anode material of microbial fuel cell toimprove power generation. Int. J. Hydrog. Energy 2014, 39, 10724–10730. [Google Scholar] [CrossRef]
- Fu, Y.; Yu, J.; Zhang, Y.; Meng, Y. Graphite coated with manganese oxide/multiwall carbon nanotubes composites as anodes in marine benthic microbial fuel cells. Appl. Surf. Sci. 2014, 317, 84–89. [Google Scholar] [CrossRef]
- Sharma, T.; Reddy, A.L.M.; Chandra, T.S.; Ramaprabhu, S. Development of carbon nanotubes and nanofluids based microbial fuel cell. Int. J. Hydrog. Energy 2008, 33, 6749–6754. [Google Scholar] [CrossRef]
- Mehdinia, A.; Ziaei, E.; Jabbari, A. Multi-walled carbon nanotube/SnO2 nanocomposite: A novel anode material for microbial fuel cells. Electrochim. Acta 2014, 130, 512–518. [Google Scholar] [CrossRef]
- Wei, G.; Cui, Y.; Hong, S.; Sun, J. Layer-by-layer construction of graphene-based microbial fuel cell for improved power generation and methyl orange removal. Bioprocess Biosyst. Eng. 2014, 37, 1749–1758. [Google Scholar]
- Liu, X.-W.; Sun, X.-F.; Huang, Y.-X.; Sheng, G.-P.; Wang, S.-G.; Yu, H.-Q. Carbon nanotube/chitosan nanocomposite as a biocompatible biocathode material to enhance the electricity generation of a microbial fuel cell. Energy Environ. Sci. 2011, 4, 1422–1427. [Google Scholar] [CrossRef]
- Liu, X.W.; Huang, Y.X.; Sun, X.F.; Sheng, G.P.; Zhao, F.; Wang, S.G.; Yu, H.Q. Conductive carbon nanotube hydrogel as a bioanode for enhanced microbial electrocatalysis. ACS Appl. Mater. Int. 2014, 6, 351–418. [Google Scholar] [CrossRef] [PubMed]
- Lamp, J.L.; Guest, J.S.; Naha, S.; Radavich, K.A.; Love, N.G.; Ellis, M.W.; Puri, I.K. Flame synthesis of carbon nanostructures on stainless steel anodes for use in microbial fuel cells. J. Power Sources 2011, 196, 5829–5834. [Google Scholar] [CrossRef]
- Mink, J.E.; Rojas, J.P.; Logan, B.E.; Hussain, M.M. Vertically grown multiwalled carbon nanotube anode and nickel silicide integrated high performance microsized (1.25 μL) microbial fuel cell. Nano Lett. 2012, 12, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Erbay, C.; Pu, X.; Choi, W.; Choi, M.-J.; Ryu, Y.; Hou, H.; Lin, F.; de Figueiredo, P.; Yu, C.; Han, A. Control of geometrical properties of carbon nanotube electrodes towards high-performance microbial fuel cells. J. Power Sources 2015, 280, 347–354. [Google Scholar] [CrossRef]
- Gong, X.B.; You, S.J.; Yuan, Y.; Zhang, J.N.; Sun, K.; Ren, N.Q. Three-dimensional pseudocapacitive interface for enhanced power production in a microbial fuel cell. ChemElectroChem 2015, 2, 1307–1313. [Google Scholar] [CrossRef]
- Tang, J.; Chen, S.; Yong, Y.; Cai, X.; Zhou, S. In situ formation of graphene layers on graphite surfaces for efficient anodes of microbial fuel cells. Biosens. Bioelectron. 2015, 71, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-Z.; Yu, Y.-Y.; Xie, R.-R.; Zhang, C.-L.; Yang, Y.; Zhai, D.-D.; Yang, G.; Liu, L.; Yong, Y.-C. In-situ growth of graphene/polyaniline for synergistic improvement of extracellular electron transfer in bioelectrochemical systems. Biotechnol. Bioeng. 2017, 87, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-F.; Du, L.; Guo, P.-B.; Zhu, B.; Luong, J.H.T. Controlled modification of carbon nanotubes and polyaniline on macroporous graphite felt for high-performance microbial fuel cell anode. J. Power Sources 2015, 283, 46–53. [Google Scholar] [CrossRef]
- Lepage, G.; Albernaz, F.O.; Perrier, G.; Merlin, G. Characterization of a microbial fuel cell with reticulated carbon foam electrodes. Bioresour. Technol. 2012, 124, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Flexer, V.; Chen, J.; Donose, B.C.; Sherrell, P.; Wallace, G.G.; Keller, J. The nanostructure of three-dimensional scaffolds enhances the current density of microbial bioelectrochemical systems. Energy Environ. Sci. 2013, 6, 1291–1298. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Z.; Yang, S.; Zhou, Y. Three-dimensional macroporous anodes based on stainless steel fiber felt for high-performance microbial fuel cells. J. Power Sources 2014, 258, 204–209. [Google Scholar] [CrossRef]
- Qiao, Y.; Bao, S.J.; Li, C.M.; Cui, X.Q.; Lu, Z.S.; Guo, J. Nanostructured polyaniline/titanium dioxide composite anode for microbial fuel cells. ACS Nano 2008, 2, 113–119. [Google Scholar] [PubMed]
- Wang, H.; Wang, G.; Ling, Y.; Qian, F.; Song, Y.; Lu, X.; Chen, S.; Tong, Y.; Li, Y. High power density microbial fuel cell with flexible 3d graphene-nickel foam as anode. Nanoscale 2013, 5, 10283–10290. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, L.; Pasta, M.; Wells, G.F.; Kong, D.; Criddle, C.S.; Cui, Y. Three-dimensional carbon nanotube-textile anode for high-performance microbial fuel cells. Nano Lett. 2011, 11, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ye, M.; Hu, L.B.; Liu, N.; Mcdonough, J.R.; Wei, C.; Alshareef, H.N.; Criddle, C.S.; Cui, Y. Carbon nanotube-coated macroporous sponge for microbial fuel cell electrodes. Energy Environ. Sci. 2012, 5, 5265–5270. [Google Scholar] [CrossRef]
- Xing, X.; Yu, G.H.; Liu, N.; Bao, Z.N.; Criddle, C.S.; Yi, C. Graphene–sponges as high-performance low-cost anodes for microbial fuel cells. Energy Environ. Sci. 2012, 5, 6862–6866. [Google Scholar]
- Liu, X.; Du, X.; Xia, W.; Li, N.; Ping, X.; Yi, D. Improved microbial fuel cell performance by encapsulating microbial cells with a nickel-coated sponge. Biosens. Bioelectron. 2012, 41, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.C.; Dong, X.C.; Chanpark, M.B.; Song, H.; Chen, P. Macroporous and monolithic anode based on polyaniline hybridized three-dimensional graphene for high-performance microbial fuel cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, G.; Liu, Q.; Harnisch, F.; Zhou, Y.; Chen, Y.; Hanif, M.; Wang, S.; Peng, X.; Hou, H. Layered corrugated electrode macrostructures boost microbial bioelectrocatalysis. Energy Environ. Sci. 2012, 5, 9769–9772. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Z.; Zheng, S.; Chen, S.; Zhao, F. Carbonized textile with free-standing threads as an efficient anode material for bioelectrochemical systems. J. Power Sources 2015, 287, 269–275. [Google Scholar] [CrossRef]
- Chen, X.; Dan, C.; Wang, X.; Wang, X.; Li, W. Porous carbon with defined pore size as anode of microbial fuel cell. Biosens. Bioelectron. 2015, 69, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhou, M.; Yang, H.; Ren, G.; Zhao, Y. Titanium dioxide nanoparticles modified three dimensional ordered macroporous carbon for improved energy output in microbial fuel cells. Electrochim. Acta 2016, 190, 463–470. [Google Scholar] [CrossRef]
- Chen, S.; Hou, H.; Harnisch, F.; Patil, S.A.; Carmonamartinez, A.A.; Agarwal, S.; Zhang, Y.; Sinharay, S.; Yarin, A.L.; Greiner, A. Electrospun and solution blown three-dimensional carbon fiber nonwovens for application as electrodes in microbial fuel cells. Energy Environ. Sci. 2011, 4, 1417–1421. [Google Scholar] [CrossRef]
- Chen, S.; He, G.; Agarwal, S.; Greiner, A.; Hou, H.; Schröder, U. Electrospun carbon fiber mat with layered architecture for anode in microbial fuel cells. Electrochem. Commun. 2011, 13, 1026–1029. [Google Scholar] [CrossRef]
- Katuri, K.; Ferrer, M.L.; Gutiérrez, M.C.; Jiménez, R.; Monte, F.D.; Leech, D. Three-dimensional microchanelled electrodes in flow-through configuration for bioanode formation and current generation. Energy Environ. Sci. 2011, 4, 4201–4210. [Google Scholar] [CrossRef]
- He, Z.; Liu, J.; Qiao, Y.; Li, C.M.; Tan, T.T. Architecture engineering of hierarchically porous chitosan/vacuum-stripped graphene scaffold as bioanode for high performance microbial fuel cell. Nano Lett. 2012, 12, 4738–4741. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Huang, Y.X.; Li, D.B.; Yu, H.Q.; Yan, L. Preparation of a macroporous flexible three dimensional graphene sponge using an ice-template as the anode material for microbial fuel cells. RSC Adv. 2014, 4, 21619–21624. [Google Scholar] [CrossRef]
- Chen, S.; He, G.; Hu, X.; Xie, M.; Wang, S.; Zeng, D.; Hou, H.; Schröder, U. A three-dimensionally ordered macroporous carbon derived from a natural resource as anode for microbial bioelectrochemical systems. ChemSusChem 2012, 5, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Q.; He, G.; Zhou, Y.; Hanif, M.; Peng, X.; Wang, S.; Hou, H. Reticulated carbon foam derived from a sponge-like natural product as a high-performance anode in microbial fuel cells. J. Mater. Chem. 2012, 22, 18609–18613. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, S.; Liu, Y.; Tang, J. Nanostructured macroporous bioanode based on polyaniline-modified natural loofah sponge for high-performance microbial fuel cells. Environ. Sci. Technol. 2013, 47, 14525–14532. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yuan, Y.; Liu, T.; Zhou, S. High-capacity carbon-coated titanium dioxide core–shell nanoparticles modified three dimensional anodes for improved energy output in microbial fuel cells. J. Power Sources 2015, 274, 170–176. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Wang, B.; Xuan, J.; Wong, J.W.C.; Lee, P.K.H.; Leung, M.K.H. Interfacial electron transfer and bioelectrocatalysis of carbonized plant material as effective anode of microbial fuel cell. Electrochim. Acta 2015, 157, 314–323. [Google Scholar] [CrossRef]
- Cho, J.S.; Park, J.Y.; Yoo, Y.J. Novel 3-dimensional bioelectrode for mediatorless bioelectrochemical denitrification. Biotechnol. Lett. 2008, 30, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhou, S.; Xu, N.; Zhuang, L. Microorganism-immobilized carbon nanoparticle anode for microbial fuel cells based on direct electron transfer. Appl. Microbiol. Biotechnol. 2011, 89, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Chen, H.L.; Yong, Y.C.; Kim, D.H.; Song, H. Conductive artificial biofilm dramatically enhances bioelectricity production in shewanella-inoculated microbial fuel cells. Chem. Commun. 2011, 47, 12825–12827. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Nishio, K.; Konno, T.; Ishihara, K. The effect of the encapsulation of bacteria in redox phospholipid polymer hydrogels on electron transfer efficiency in living cell-based devices. Biomaterials 2012, 33, 8221–8227. [Google Scholar] [CrossRef] [PubMed]
- Luckarift, H.R.; Sizemore, S.R.; Farrington, K.E.; Roy, J.; Lau, C.; Atanassov, P.B.; Johnson, G.R. Facile fabrication of scalable, hierarchically structured polymer/carbon architectures for bioelectrodes. ACS Appl. Mater. Int. 2012, 4, 2082–2087. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Kai, F.; Okamoto, A.; Newton, G.J.; Hashimoto, K. Self-constructed electrically conductive bacterial networks. Angew. Chem. Int. Ed. 2009, 48, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Park, I.H.; Christy, M.; Kim, P.; Nahm, K.S. Enhanced electrical contact of microbes using Fe3O4/CNT nanocomposite anode in mediator-less microbial fuel cell. Biosens. Bioelectron. 2014, 58, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Zhou, S.; Bo, Z.; Li, Z.; Wang, Y. Microbially-reduced graphene scaffolds to facilitate extracellular electron transfer in microbial fuel cells. Bioresour. Technol. 2012, 116, 453–458. [Google Scholar]
- Yong, Y.C.; Yu, Y.Y.; Zhang, X.; Song, H. Highly active bidirectional electron transfer by a self-assembled electroactive reduced-graphene-oxide-hybridized biofilm. Angew. Chem. Int. Ed. 2014, 53, 4480–4483. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Qu, Y.; He, W.; Du, Y.; Liu, J.; Han, X.; Feng, Y. A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresour. Technol. 2015, 195, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Wang, H.; Xia, X.; Huang, X.; Mo, Y.; Cao, X.; Fan, M. Carbon nanotube powders as electrode modifier to enhance the activity of anodic biofilm in microbial fuel cells. Biosens. Bioelectron. 2011, 26, 3000–3004. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-Y.; Guo, C.X.; Yong, Y.-C.; Li, C.M.; Song, H. Nitrogen doped carbon nanoparticles enhanced extracellular electron transfer for high-performance microbial fuel cells anode. Chemosphere 2015, 140, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; McCarthy, K.; Nevin, K.P.; Johnson, J.P.; Rotello, V.M.; Lovley, D.R. Electricity generation by geobacter sulfurreducens attached to gold electrodes. Langmuir 2008, 24, 4376–4379. [Google Scholar] [CrossRef] [PubMed]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schroeder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef]
- Friedrich, J.M.; Ponce-De-León, C.; Reade, G.W.; Walsh, F.C. Reticulated vitreous carbon as an electrode material. J. Electroanal. Chem. 2004, 561, 203–217. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, Q.; Chen, J.; Wang, B.; Wang, Y.; Liu, K.; Li, M.; Jiang, H.; Lu, Z.; Wang, D. Hierarchically three-dimensional nanofiber based textile with high conductivity and biocompatibility as a microbial fuel cell anode. Environ. Sci. Technol. 2016, 50, 7889–7895. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Rodriguez, J.; Blackall, L.L.; Keller, J.; Gross, P.; Batstone, D.; Verstraete, W.; Nealson, K.H. Microbial ecology meets electrochemistry: Electricity-driven and driving communities. ISME J. 2007, 1, 9–18. [Google Scholar] [CrossRef] [PubMed]











| BES Type # | Inoculum | 3-D Strategy $ | Electrode Configuration & | ja (A/m2) | Pa (W/m2) | Pb (W/m3) | Pc (W/m3) | Reference |
|---|---|---|---|---|---|---|---|---|
| S-MFC/air cathode | MFC effluent | 1 | Graphite granules | * | 0.6 | 48 | 102.1 | [55] |
| D-MFC/ferricyanide | Anaerobic sludge | 1 | Granule activated carbon | * | * | 11.9 | 20.2 | [57] |
| D-MFC/ferricyanide | MFC effluent | 1 | Graphite granules | * | * | 257 | * | [60] |
| S-MFC/Pt-air cathode | Wastewater | 1 | Granule activated carbon | * | 0.245 | 7.2 | * | [61] |
| S-MFC/Pt-air cathode | Anaerobic sludge | 1 | Irregular graphite granules | 1.5 | 0.08 | 2.0 | 2.7 | [62] |
| D-MFC/biocathode | MFC effluent | 1 | Granule activated carbon | 0.91 | 0.194 | 9.72 | 16.2 | [63] |
| S-MFC/Pt-air cathode | MFC effluent | 1 | Granule activated carbon | * | 0.813 | 81.2 | * | [64] |
| D-MFC/biocathode | MDC effluent | 1 | Granule activated carbon | 4.44 | 1.05 | 21.2 | 36.0 | [65] |
| S-MFC/Pt-air cathode | MFC effluent | 1 | Treated carbon brush | 8.4 | 1.37 | 34.7 | 71.4 | [50] |
| S-MFC/Pt-air cathode | MFC effluent | 1 | Carbon brush | 10 | 1.24 | 24.9 | 42.1 | [66] |
| D-MFC/ferricyanide | MFC effluent | 1 | Carbon brush | 9.45 | 2.1 | 210 | 373 | [67] |
| D-MFC/ferricyanide | Anaerobic sludge | 2 | rGO/PANI modified CC | 3.4 | 1.39 | 11.2 | * | [68] |
| D-MFC/ferricyanide | Escherichia coli | 2 | rGO/SnO2 modified CC | 3.4 | 1.62 | * | * | [69] |
| Marine benthic MFC | * | 2 | MWCNTs/MnO2 modified GP | 0.75 | 0.11 | * | * | [70] |
| D-MFC/ferricyanide | Escherichia coli | 2 | MWCNTs/Pt NP modified CP | * | 2.45 | * | * | [71] |
| D-MFC/ferricyanide | Escherichia coli | 2 | MWCNTs/SnO2 coated GCE | 3.5 | 1.421 | * | * | [72] |
| D-MFC/ferricyanide | Anaerobic sludge | 2 | PEI/graphene modified CP | 1.7 | 0.368 | 3.9 | * | [73] |
| D-MFC/biocathode | Anaerobic sludge | 2 | CNT/chitosan modified CP | 1.6 | 0.189 | * | * | [74] |
| S-MFC/Pt-air cathode | Anaerobic sludge | 2 | CNT/chitosan modified CP | 0.8 | 0.132 | * | * | [75] |
| S-MFC/Pt-air cathode | Anaerobic digester | 2 | CNT in-situ growth on SSM | * | 1.87 | 8.5 | * | [76] |
| D-MFC/ferricyanide | Wastewater | 2 | CNT in-situ growth | 0.197 | 0.0196 | 396 | * | [77] |
| D-MFC/ferricyanide | Wastewater | 2 | CNT sponge | 8 | 2.82 | 14.1 | 943 | [59] |
| D-MFC/ferricyanide | Anaerobic sludge | 2 | CNT in-situ growth on SSM | 6.5 | 3.36 | 6.72 | * | [78] |
| D-MFC/air | Active sludge | 2 | CP modified with MnO2 | 3 | 0.596 | 14.9 | * | [79] |
| S-MFC/Pt-air cathode | MFC anode effluent | 2 | Graphene modified GP | 9.45 | 2.36 | 16.5 | 472 | [80] |
| D-MFC/ferricyanide | Active sludge | 2 | Graphene/PANI modified GP | 10.5 | 4.44 | 29.6 | 2220 | [81] |
| D-MFC/ferricyanide | Shewanella oneidensis | 3 | PANI/CNT modified CF | 1.9 | 0.257 | 1.32 | * | [82] |
| D-MFC/air | MFC effluent | 3 | Commercial RVC | 1.04 | 0.11 | 1.72 | 39.4 | [83] |
| Half-cell MFC, +0 V | MFC effluent | 3 | CNT modified RVC | 68 | * | * | * | [84] |
| D-MFC/ferricyanide | MFC effluent | 3 | CNT graphene modified SSM | 8.1 | 2.14 | 7.7 | 2140 | [85] |
| D-MFC/ferricyanide | Escherichia coli | 3 | PANI/TiO2 coated nickel foam | 8 | 1.49 | 0.99 | * | [86] |
| D-MFC/ferricyanide | Shewanella oneidensis | 3 | Nickel foam coated with rGO | 3 | 0.663 | 27 | 663 | [87] |
| D-MFC/Pt-air | Domestic wastewater | 3 | Textile coated with CNT | 7.2 | 1.1 | 0.599 | * | [88] |
| D-MFC/Pt-air | Domestic wastewater | 3 | Sponge coated with CNT | 21.3 | 1.99 | 1.32 | 995 | [89] |
| D-MFC | MFC effluent | 3 | Graphene coated sponge | 10.7 | 1.57 | * | 394 | [90] |
| D-MFC/ferricyanide | Wastewater | 3 | Nickel coated sponge | 4.3 | 0.993 | 5.53 | * | [91] |
| D-MFC/ferricyanide | Shewanella oneidensis | 3 | Monolithic graphene electrode | 4.5 | 0.77 | 0.512 | 768 | [92] |
| Half-cell MFC, +0.2 V | MFC effluent | 3 | Layered corrugated carbon | 390 | * | * | * | [93] |
| Half-cell MFC, +0.3 V | Anaerobic sludge | 3 | Towel carbonization | 8 | * | * | * | [94] |
| S-MFC/Pt-air cathode | Escherichia coli | 3 | Porous carbon | 13.5 | 1.6 | 14.5 | * | [95] |
| S-MFC/Pt-air cathode | Anaerobic sludge | 3 | TiO2 modified porous carbon | 3.69 | 0.973 | 48.6 | * | [96] |
| Half-cell MFC, +0.2 V | MFC effluent | 3 | Carbonized polymer matrix | 30 | * | * | * | [97] |
| Half-cell MFC, +0.2 V | MFC effluent | 3 | Carbonized polymer matrix | 20 | * | * | * | [98] |
| D-MFC/air | Geobacter sulfurreducens | 3 | Chitosan/CNT scaffold | 19 | 2.87 | 2.23 | 2000 | [99] |
| D-MFC/ferricyanide | Pseudomonas. aeruginosa | 3 | Chitosan/graphene scaffold | 5.25 | 1.53 | * | * | [100] |
| D-MFC/ferricyanide | Anaerobic sludge | 3 | Graphene sponge | * | 0.71 | * | 427 | [101] |
| Half-cell MFC, +0.2 V | MFC effluent | 3 | Corp plant carbonization | 32.5 | * | * | * | [102] |
| Half-cell MFC, +0.2 V | MFC effluent | 3 | Pomelo peel carbonization | 51.9 | * | * | * | [103] |
| S-MFC/Pt-air cathode | MFC effluent | 3 | PANI modified LSC | 12.4 | 2.54 | 27.2 | 509 | [104] |
| S-MFC/Pt-air cathode | MFC effluent | 3 | TiO2 modified LSC | 15 | 2.59 | 27.7 | 518 | [105] |
| Half-cell MEC, +0.2 V | Anaerobic digester | 3 | Carbonized plant | 31.2 | * | * | * | [106] |
| S-MFC/denitrification | Ochrobactrum anthropi | 4 | Bacteria/copper powder | * | * | * | * | [107] |
| S-MFC/Pt-air cathode | Mixed culture | 4 | Bacteria/CNP paste | 9.2 | 1.94 | * | * | [108] |
| D-MFC/ferricyanide | Shewanella oneidensis | 4 | Bacteria/graphite/PPy matrix | 0.8 | 0.207 | * | * | [109] |
| Half-cell MFC, +0.2 V | Shewanella oneidensis | 4 | PMBVF/PVA/bacteria hydrogel | 0.082 | * | * | * | [110] |
| Half-cell MFC, −0.15 V | Shewanella oneidensis | 4 | EAB on polymer/GF scaffold | 0.072 | * | 0.17 | 4.38 | [111] |
| Half-cell MFC, +0.2 V | Shewanella oneidensis | 4 | Fe2O3/bacteria hybrid biofilm | 0.23 | * | * | * | [112] |
| D-MFC/ferricyanide | Escherichia coli | 4 | Fe3O4/CNT/bacteria hybrid biofilm | 1.9 | 0.83 | * | * | [113] |
| S-MFC/Pt-air cathode | Anaerobic sludge | 4 | rGO/bacteria hybrid biofilm | 8.9 | 1.9 | 47.7 | * | [114] |
| D-MFC/ferricyanide | Shewanella oneidensis | 4 | rGO/bacteria hybrid biofilm | 5.2 | 0.843 | * | * | [115] |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.-Y.; Zhai, D.-D.; Si, R.-W.; Sun, J.-Z.; Liu, X.; Yong, Y.-C. Three-Dimensional Electrodes for High-Performance Bioelectrochemical Systems. Int. J. Mol. Sci. 2017, 18, 90. https://doi.org/10.3390/ijms18010090
Yu Y-Y, Zhai D-D, Si R-W, Sun J-Z, Liu X, Yong Y-C. Three-Dimensional Electrodes for High-Performance Bioelectrochemical Systems. International Journal of Molecular Sciences. 2017; 18(1):90. https://doi.org/10.3390/ijms18010090
Chicago/Turabian StyleYu, Yang-Yang, Dan-Dan Zhai, Rong-Wei Si, Jian-Zhong Sun, Xiang Liu, and Yang-Chun Yong. 2017. "Three-Dimensional Electrodes for High-Performance Bioelectrochemical Systems" International Journal of Molecular Sciences 18, no. 1: 90. https://doi.org/10.3390/ijms18010090