Anti-Neuroblastoma Properties of a Recombinant Sunflower Lectin
Abstract
:1. Introduction
2. Results and Discussion
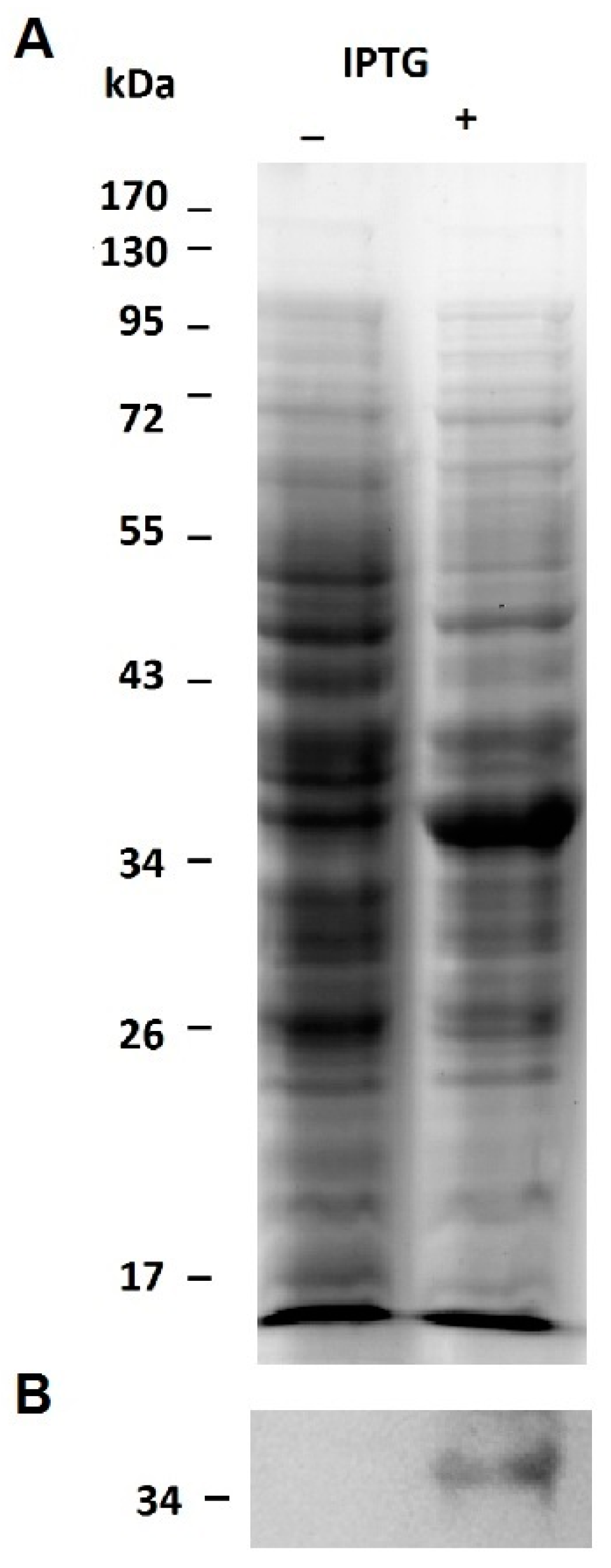
2.1. Expression of the Recombinant Jacalin-Like Lectin Helja
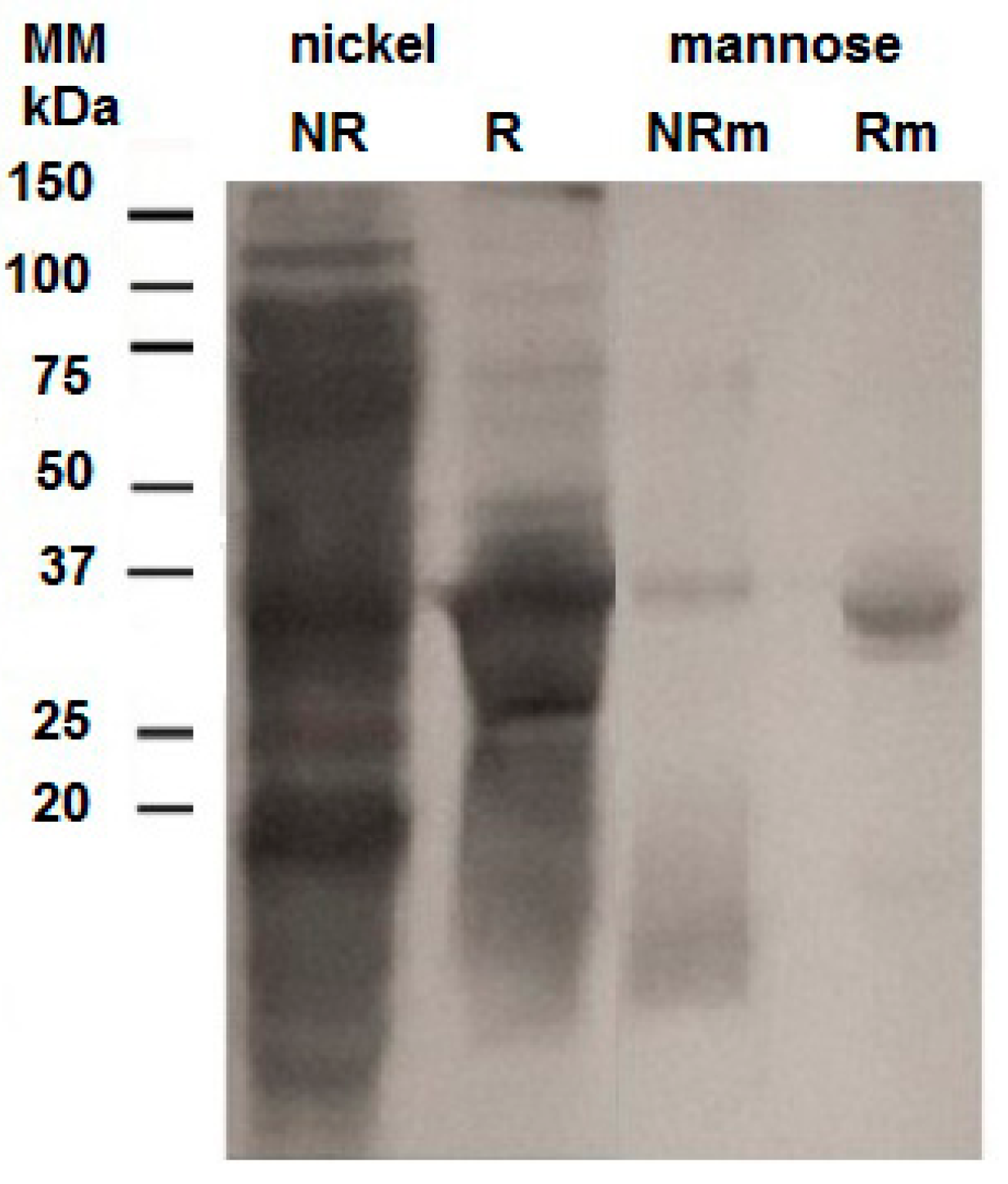
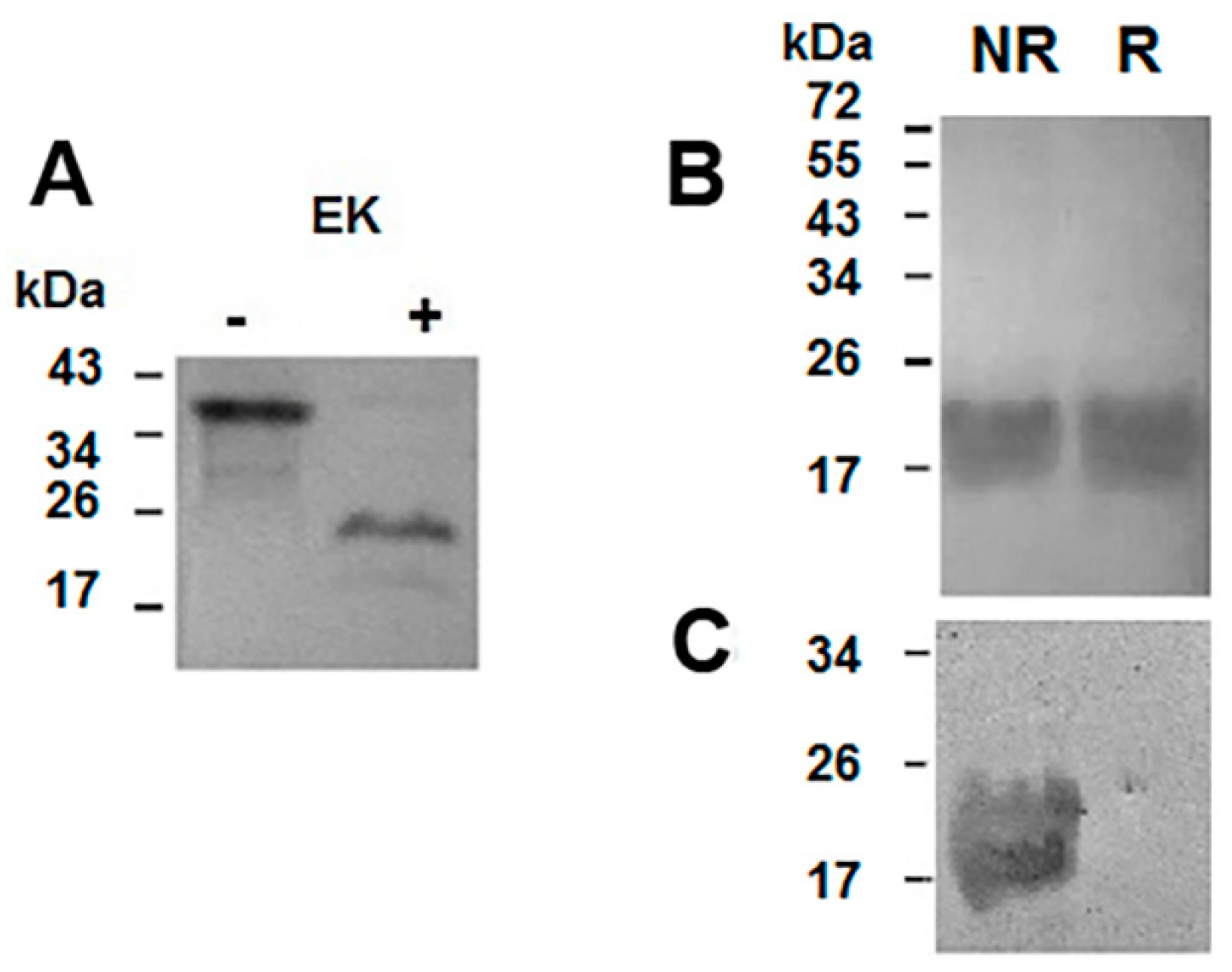
2.2. Purification of the Recombinant Helja
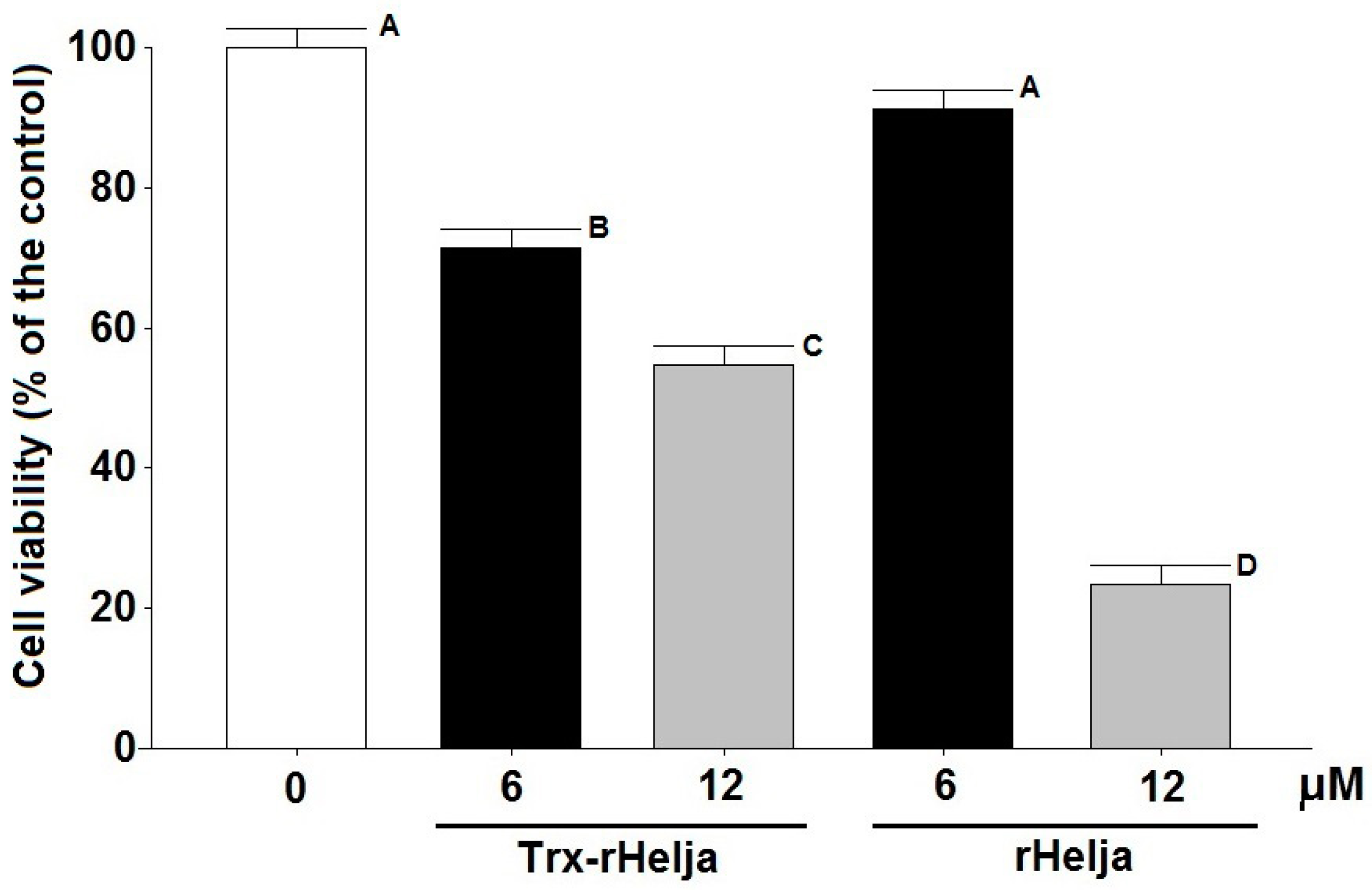
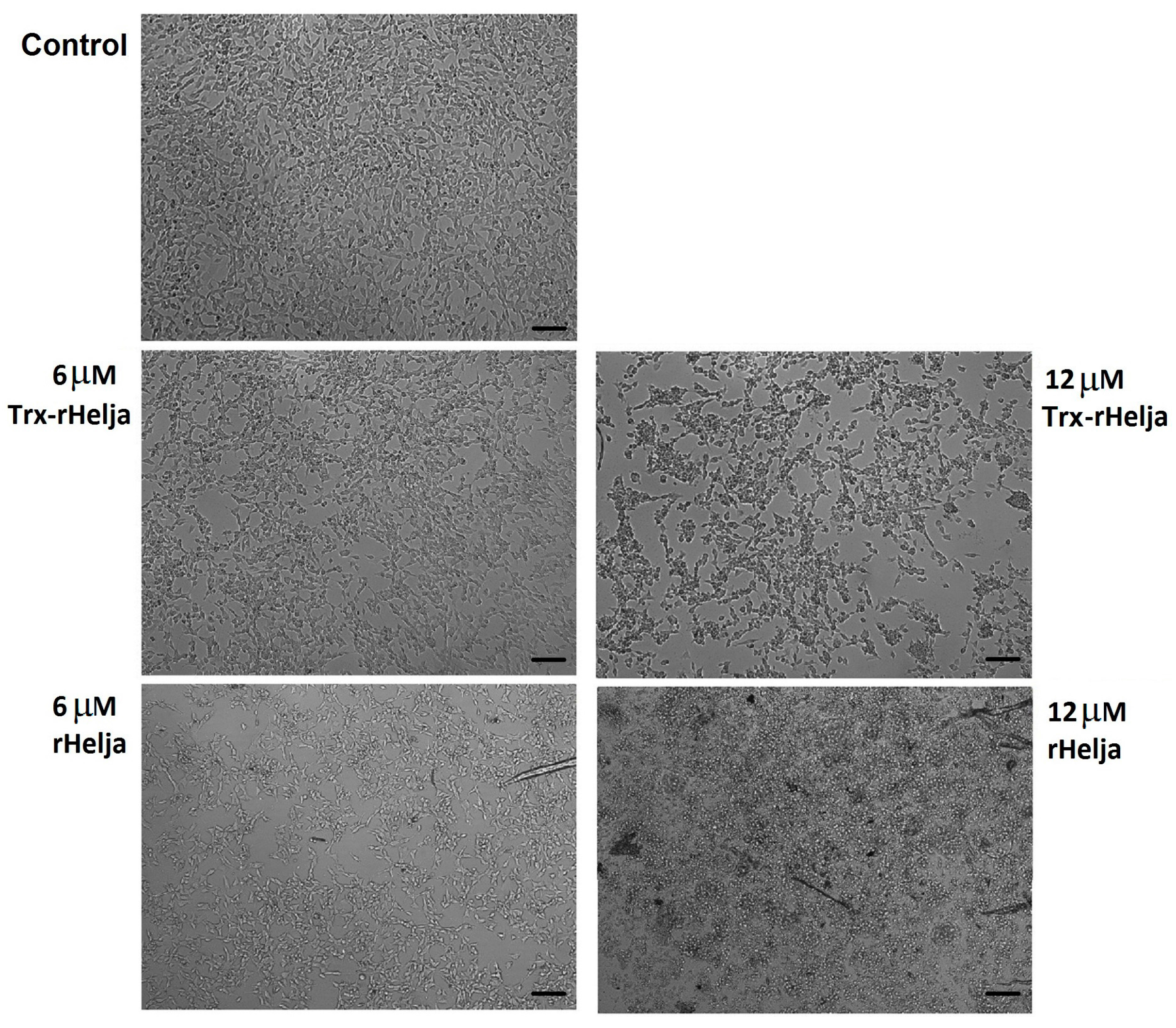
2.3. Helja Activity on Human Neuroblastomas
3. Material and Methods
3.1. Biological Material
3.2. Construction of the Recombinant Expression Vector
3.3. Transformation, Positive Colony Screening and Sequencing
3.4. Expression of the Recombinant Lectin Helja in E. coli
3.5. Purification of the Recombinant Lectin in Ni2+-Sepharose and Mannose-Agarose Matrixes
3.6. Digestion of the Recombinant Protein
3.7. Protein Analysis and Western Blotting
3.8. Neuroblastoma Cell Culture
3.9. Cell Viability Assay
3.10. Microscopic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
Abbreviations
| CDS | coding sequence |
| Con A | concanavalin A |
| EM | extracellular matrix |
| Helja | Helianthus annuus jacalin |
| IPTG | isopropyl-β-d-thiogalactopyranoside |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| ORF | open reading frame |
| PBS | phosphate buffered saline |
| PCR | polymerase chain reaction |
| ROS | reactive oxygen species |
References
- Sharon, N. Lectins: Past, present and future. Biochem. Soc. Trans. 2008, 36, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, K.; van Damme, E.J.M. Protein-carbohydrate interactions as part of plant defense and animal immunity. Molecules 2015, 20, 9029–9053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Koyama, Y.; Isemura, M.; Nakamura, Y. Plant lectins in therapeutic and diagnostic cancer research. Int. J. Plant Biol. Res. 2015, 3, 1030–1035. [Google Scholar]
- Christiansen, M.N.; Chik, J.; Lee, L.; Anugraham, M.; Abrahams, J.L.; Packer, N.H. Cell surface protein glycosylation in cancer. Proteomics 2014, 14, 525–546. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, E.; Galili, U.; Raz, A. On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer Metastasis Rev. 2001, 20, 245–277. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, V.; Mohamed Adil, A.A.; Ahmed, N.; Jamal, S. Lectins-the promising cancer therapeutics. Oncobiol. Targets 2014, 1, 12–15. [Google Scholar]
- Yau, T.; Dan, X.; Ng, C.C.; Ng, T.B. Lectins with potential for anti-cancer therapy. Molecules 2015, 20, 3791–3810. [Google Scholar] [CrossRef] [PubMed]
- Gemeiner, P.; Mislovičová, D.; Tkáč, J.; Švitel, J.; Pätoprstý, V.; Hrabárová, E.; Kogan, G.; Kožár, T. Lectinomics II. A highway to biomedical/clinical diagnostics. Biotechnol. Adv. 2009, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, C.; Bian, H.; Min, M.; Chen, L.; Bao, J. Antiproliferative activity and apoptosis-inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch. Biochem. Biophys. 2009, 482, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, B.; Min, M.W.; Bian, H.J.; Chen, L.F.; Liu, Q.; Bao, J.K. Induction of apoptosis by Polygonatum odoratum lectin and its molecular mechanisms in murine fibrosarcoma L929 cells. Biochim. Biophys. Acta 2009, 1790, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Komath, S.S.; Kavitha, M.; Swamy, M.J. Beyond carbohydrate binding: New directions in plant lectin research. Org. Biomol. Chem. 2006, 4, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Ng, T.B. Lectins: Production and practical applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regente, M.; Taveira, G.B.; Pinedo, M.; Elizalde, M.M.; Ticchi, A.J.; Diz, M.S.; Carvalho, A.O.; de la Canal, L.; Gomes, V.M. A sunflower lectin with antifungal properties and putative medical mycology applications. Curr. Microbiol. 2014, 69, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.D.; Winter, H.C.; Goldstein, I.J.; Markovitz, D.M. A lectin isolated from bananas is a potent inhibitor of HIV replication. J. Biol. Chem. 2010, 285, 8645–8655. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, A.V.; Gorudko, I.V.; Gabius, H.J. Lectins from medicinal plants: Bioeffectors with diverse activities. In Recent Advances in Phytochemistry; Jetter, R., Ed.; Springer International Publishing: Cham, Switzerland, 2014; VOlume 44, pp. 43–56. [Google Scholar]
- Pinedo, M.; Regente, M.; Elizalde, M.; Quiroga, I.Y.; Pagnussat, L.A.; Jorrín-Novo, J.; Maldonado, A.; de la Canal, L. A non-classically secreted lectin occurs in the apoplast of sunflower seedlings. Protein Pept. Lett. 2012, 19, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; Elizalde, M.; de la Canal, L. Apoplastic exosome-like vesicles: A new way of protein secretion in plants? Plant Signal. Behav. 2012, 7, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.G.; Ross, J.A.; Wall, D.A.; Bleyer, W.A.; Severson, R.K.; Robison, L.L. Infant cancer in the U.S.: Histology-specific incidence and trends, 1973 to 1992. J. Pediatr. Hematol. Oncol. 1997, 19, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Bleckmann, C.; Geyer, H.; Lieberoth, A.; Splittstoesser, F.; Liu, Y.; Feizi, T.; Schachner, M.; Kleene, R.; Reinhold, V.; Geyer, R. O-glycosylation pattern of CD24 from mouse brain. Biol. Chem. 2009, 390, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Poncet, C.; Frances, V.; Gristina, R.; Scheiner, C.; Pellisier, J.F.; Figarella-Branger, D. CD24, a glycosyl phosphatidyl inositol-anchored molecule, is transiently expressed during the development of human central nervous system and is a marker of human neural cell lineage tumors. Acta Neuropathol. 1996, 91, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Valentiner, U.; Mühlenhoff, M.; Lehmann, U.; Hildebrant, H.; Schumacher, U. Expression of the neural cell adhesion molecule and polysialic acid in human neuroblastoma cell lines. Int. J. Oncol. 2011, 39, 417–424. [Google Scholar] [CrossRef] [PubMed]
- LaVallie, E.R.; DiBlasio, E.A.; Kovacic, S.; Grant, K.L.; Schendel, P.F.; McCoy, J.M.A. Thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Biotechnology 1993, 11, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, M.; Orts, F.; Carvalho, A.O.; Regente, M.; Soares, J.R.; Gomes, V.M.; de la Canal, L. Molecular characterization of Helja, an extracellular jacalin-related protein from Helianthus annuus: Insights into the relationship of this protein with unconventionally secreted lectins. J. Plant Physiol. 2015, 183, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liao, X.; Chen, G.; Yap, Y.; Zhang, X. Cloning, Expression and Purification of Microcystis viridis Lectin in Escherichia coli. Mol. Biotechnol. 2011, 47, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, T.; Nomura, K.; Yagi, F. Cloning and expression of a mannose-binding Jacalin-Related lectin from leaves of Japanese Cycad (Cycas revoluta Thunb.). Biosci. Biotechnol. Biochem. 2006, 70, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Costa, S.; Teixeira, J.A.; Domingues, L. cDNA cloning and functional expression of the alpha-d-galactose-binding lectin frutalin in Escherichia coli. Mol. Biotechnol. 2009, 43, 212–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahasrabuddhe, A.A.; Gaikwad, S.M.; Krishnasastry, M.V.; Khan, M.I. Studies on recombinant single chain Jacalin lectin reveal reduced affinity for saccharides despite normal folding like native Jacalin. Protein Sci. 2004, 13, 3264–3273. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Nicolau, A.; Teixeira, J.A.; Domingues, L. Cytotoxic effects of native and recombinant frutalin, a plant galactose-binding lectin, on HeLa cervical cancer cells. J. Biomed. Biotechnol. 2011, 2011, 568932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, A.H.; Wong, J.H.; Ng, T.B. Musa acuminata (Del Monte banana) lectin is a fructose-binding lectin with cytokine-inducing activity. Phytomedicine 2009, 16, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Vetri, V.; Carrotta, R.; Picone, P.; di Carlo, M.; Militello, V. Concanavalin A aggregation and toxicity on cell cultures. Biochim. Biophys. Acta 2010, 1804, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.L.; Kuzmanoff, K.L.; Ling-Indeck, L.; Pezzuto, J.M. Betulinic acid induces apoptosis in human neuroblastoma cell lines. Eur. J. Cancer 1997, 33, 2007–2010. [Google Scholar] [CrossRef]
- Gu, J.; Isaji, T.; Xu, Q.; Kariya, Y.; Gu, W.; Fukuda, T.; Du, Y. Potential roles of N-glycosylation in cell adhesion. Glycoconj. J. 2012, 29, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Notter, M.F.I.; Leary, J.F. Surface glycoproteins of differentiating neuroblastoma cells analyzed by lectin binding and flow cytometry. Cytometry 1987, 5, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Rao, M.V.; Tweardy, D.J.; Prakash, M.; Galili, U.; Gorelik, E. Lectin-induced apoptosis of tumour cells. Glycobiology 1993, 3, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; So, H.S.; Lee, K.M.; Park, J.S.; Lee, J.H.; Moon, S.K.; Ryu, D.G.; Chung, S.Y.; Jung, B.H.; Kim, Y.K.; et al. Activation of caspases cascades in Korean mistetloe (Viscum album varcoloratum) lectin-II-induced apoptosis of human myeloleukemic U937 cells. Gen. Pharmacol. 2000, 34, 349–355. [Google Scholar] [CrossRef]
- Suen, Y.K.; Fung, K.P.; Choy, Y.M.; Lee, C.Y.; Chan, C.W.; Kong, S.K. Concanavalin A induced apoptosis in murine macrophage PU5-1.8 cells through clustering of mitochondria and release of cytochrome c. Apoptosis 2000, 5, 369–737. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Ng, T.B. First report of a haemagglutinin-induced apoptotic pathway in breast cancer cells. Biosci. Rep. 2010, 30, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Faheina-Martins, G.V.; da Silveira, A.L.; Cavalcanti, B.C.; Ramos, M.V.; Moraes, M.O.; Pessoa, C.; Araújo, D.A. Antiproliferative effects of lectins from Canavalia ensiformis and Canavalia brasiliensis in human leukemia cell lines. Toxicol. In Vitro 2012, 26, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Nojima, H.; Okayama, H. High efficiency transformation of Escherichia coli with plasmids. Gene 1990, 96, 23–28. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assambly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.J.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 50, 76–85. [Google Scholar] [CrossRef]
- Mossmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinedo, M.; Genoula, M.; Silveyra, M.X.; De Oliveira Carvalho, A.; Regente, M.; Del Río, M.; Ribeiro Soares, J.; Moreira Gomes, V.; De La Canal, L. Anti-Neuroblastoma Properties of a Recombinant Sunflower Lectin. Int. J. Mol. Sci. 2017, 18, 92. https://doi.org/10.3390/ijms18010092
Pinedo M, Genoula M, Silveyra MX, De Oliveira Carvalho A, Regente M, Del Río M, Ribeiro Soares J, Moreira Gomes V, De La Canal L. Anti-Neuroblastoma Properties of a Recombinant Sunflower Lectin. International Journal of Molecular Sciences. 2017; 18(1):92. https://doi.org/10.3390/ijms18010092
Chicago/Turabian StylePinedo, Marcela, Melanie Genoula, María Ximena Silveyra, André De Oliveira Carvalho, Mariana Regente, Marianela Del Río, Júlia Ribeiro Soares, Valdirene Moreira Gomes, and Laura De La Canal. 2017. "Anti-Neuroblastoma Properties of a Recombinant Sunflower Lectin" International Journal of Molecular Sciences 18, no. 1: 92. https://doi.org/10.3390/ijms18010092





