Salmonella O48 Serum Resistance is Connected with the Elongation of the Lipopolysaccharide O-Antigen Containing Sialic Acid
Abstract
:1. Introduction
2. Results
2.1. The C3 Concentration in Serum
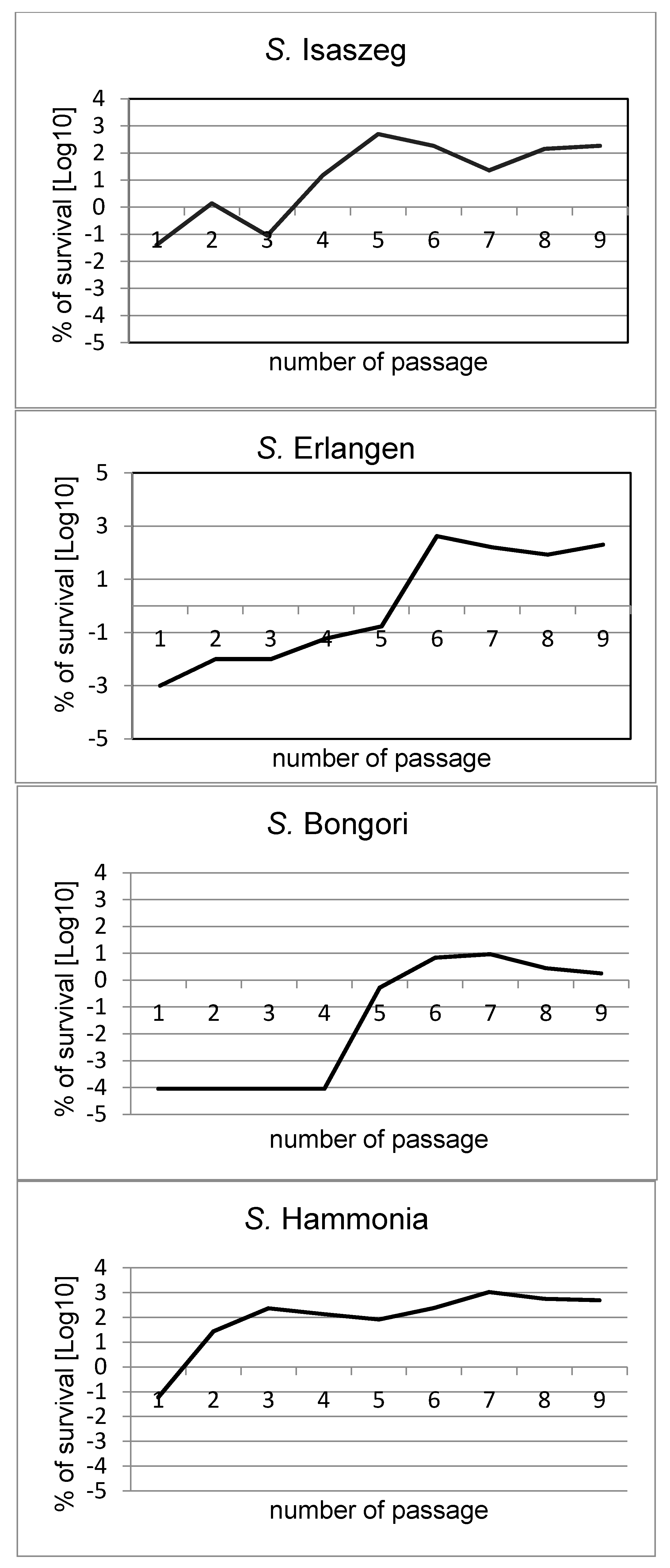
2.2. Passages of Bacteria in NHS (Normal Human Serum)
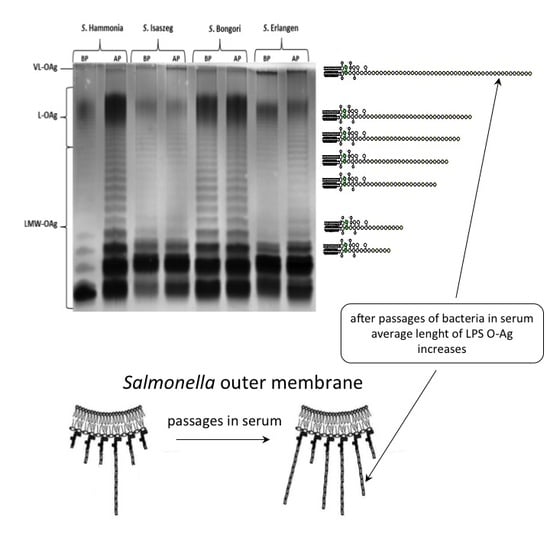
2.3. SDS-PAGE (SDS-Polyacrylamide Gel Electrophoresis) of LPS
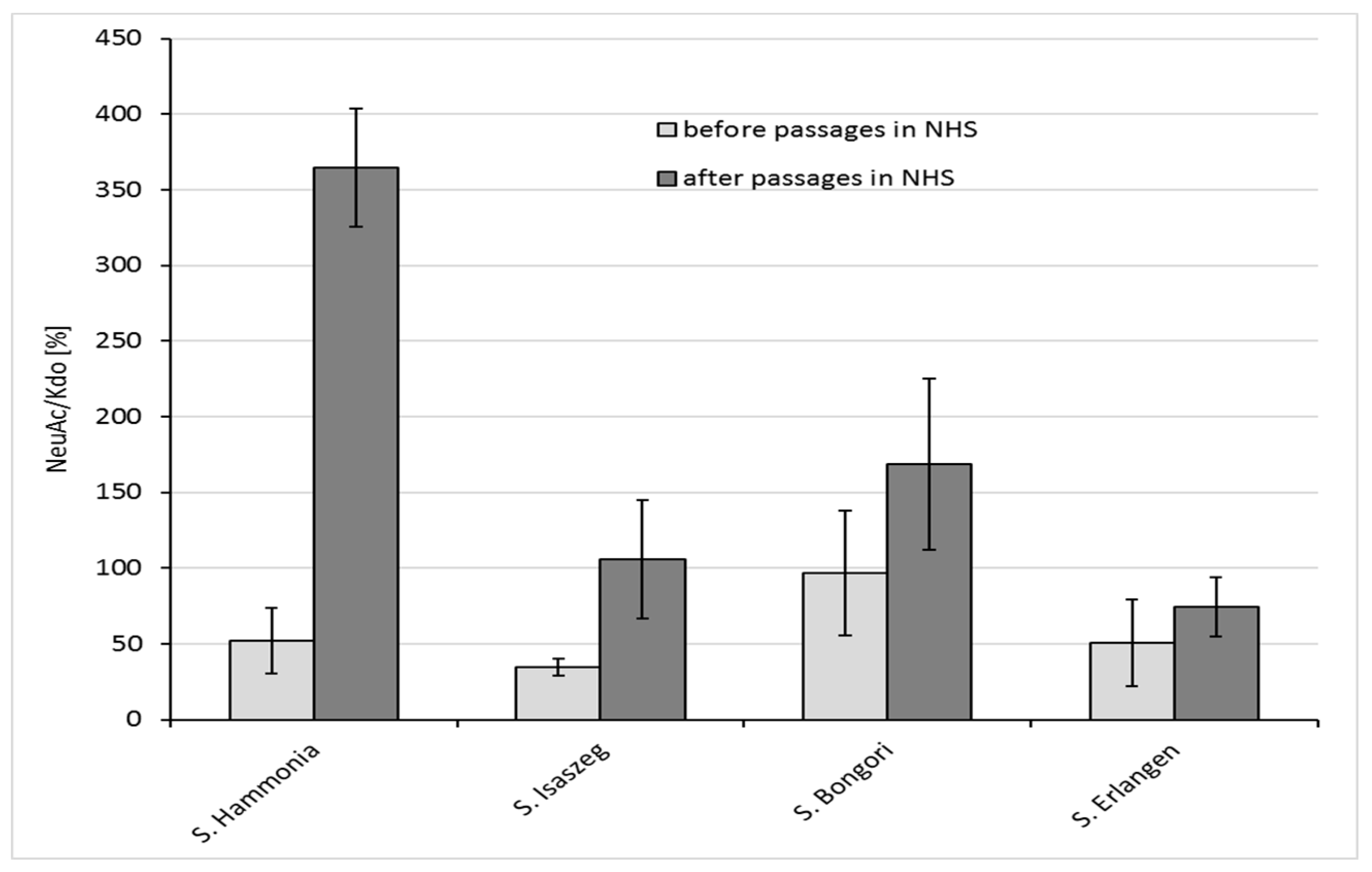
2.4. GLC-MS/MS (Gas Liquid Chromatography-Mass Spectrometry) Analysis
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Media
4.3. Sera
4.4. Bactericidal Assay of NHS
4.5. Passages of Bacterial Cells in NHS
4.6. Isolation of Lipopolysaccharides and Analysis by SDS-PAGE (SDS-Polyacrylamide Gel Electrophoresis)
4.7. Preparation of Samples for GLC-MS/MS Analysis
4.8. GLC-MS/MS Analysis
4.9. Standard Curve for NeuAc Determination by GLC-MS/MS Method
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LPS | lipopolysaccharide |
| VL-OAg | very long O-antigen |
| Kdo | 3-Deoxy-d-manno-octulosonic acid |
| L-OAg | long O-antigen |
| CDC | Center for Disease Control and Prevention |
| NeuAc | sialic acid |
| NTS | non-typhoidal salmonellosis |
| RAS | reptile-associated salmonellosis |
| REPAS | reptile exotic pet associated salmonellosis |
| NHS | normal human serum |
| CFU | colony forming units |
| OMP | outer membrane protein |
| GLC-MS | gas liquid chromatography-mass spectrometry |
| PCM | Polish Collection of Microorganisms |
| SDS-PAGE | SDS-polyacrylamide gel electrophoresis |
References
- Markiewski, M.M.; DeAngelis, R.A.; Lambris, J.D. Complexity of complement activation in sepsis. J. Cell. Mol. Med. 2008, 12, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Haeney, M.R. The role of the complement cascade in sepsis. J. Antimicrob. Chemother. 1998, 41 (Suppl. A), 41–46. [Google Scholar] [CrossRef] [PubMed]
- Heikema, A.P.; Koning, R.I.; Duarte dos Santos Rico, S.; Rempel, H.; Jacobs, B.C.; Endtz, H.P.; van Wamel, W.J.B.; Samsom, J.N. Enhanced, Sialoadhesin-dependent uptake of Guillain-Barre syndrome-associated Campylobacter jejuni strains by human macrophages. Infect. Immun. 2013, 81, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Spinola, S.M.; Li, W.; Fortney, K.R.; Janowicz, D.M.; Zwickl, B.; Katz, B.P.; Munson, R.S. Sialylation of lipooligosaccharides is dispensable for the virulence of Haemophilus ducreyi in humans. Infect. Immun. 2012, 80, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Bax, M.; Kuijf, M.L.; Heikema, A.P.; van Rijs, W.; Bruijns, S.C.; García-Vallejo, J.J.; Crocker, P.R.; Jacobs, B.C.; van Vliet, S.J.; van Kooyk, Y. Campylobacter jejuni lipooligosaccharides modulate dendritic cell-mediated T cell polarization in a sialic acid linkage-dependent manner. Infect. Immun. 2011, 79, 2681–2689. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. International Collaboration on Enteric Disease “Burden of Illness” studies The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis Off. Publ. Infect. Dis. Soc. Am. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Rondini, S.; Micoli, F.; Lanzilao, L.; Gavini, M.; Alfini, R.; Brandt, C.; Clare, S.; Mastroeni, P.; Saul, A.; MacLennan, C.A. Design of glycoconjugate vaccines against invasive African Salmonella enterica serovar Typhimurium. Infect. Immun. 2015, 83, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- LaRock, D.L.; Chaudhary, A.; Miller, S.I. Salmonellae interactions with host processes. Nat. Rev. Microbiol. 2015, 13, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Onsare, R.S.; Micoli, F.; Lanzilao, L.; Alfini, R.; Okoro, C.K.; Muigai, A.W.; Revathi, G.; Saul, A.; Kariuki, S.; MacLennan, C.A.; et al. Relationship between antibody susceptibility and lipopolysaccharide O-antigen characteristics of invasive and gastrointestinal nontyphoidal Salmonellae isolates from Kenya. PLos Negl. Trop. Dis. 2015, 9, e0003573. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.M.; Gunn, J.S. The O-Antigen Capsule of Salmonella enterica serovar Typhimurium Facilitates serum Resistance and surface Expression of FliC. Infect. Immun. 2015, 83, 3946–3959. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.M.; Wang, J.Y.; Galen, J.E.; Simon, R.; Pasetti, M.F.; Gat, O.; Levine, M.M. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect. Immun. 2011, 79, 4175–4185. [Google Scholar] [CrossRef] [PubMed]
- Keestra-Gounder, A.M.; Tsolis, R.M.; Bäumler, A.J. Now you see me, now you don’t: The interaction of Salmonella with innate immune receptors. Nat. Rev. Microbiol. 2015, 13, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Moreno Switt, A.I.; Wiedmann, M. Animal contact as a source of human non-typhoidal salmonellosis. Vet. Res. 2011, 42, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Knirel, Y.A.; Feng, L.; Perepelov, A.V.; Senchenkova, S.N.; Reeves, P.R.; Wang, L. Structural diversity in Salmonella O antigens and its genetic basis. FEMS Microbiol. Rev. 2014, 38, 56–89. [Google Scholar] [CrossRef] [PubMed]
- Parry, C.M.; Thomas, S.; Aspinall, E.J.; Cooke, R.P.D.; Rogerson, S.J.; Harries, A.D.; Beeching, N.J. A retrospective study of secondary bacteraemia in hospitalised adults with community acquired non-typhoidal Salmonella gastroenteritis. BMC Infect. Dis. 2013, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Van Meervenne, E.; Botteldoorn, N.; Lokietek, S.; Vatlet, M.; Cupa, A.; Naranjo, M.; Dierick, K.; Bertrand, S. Turtle-associated Salmonella septicaemia and meningitis in a 2-month-old baby. J. Med. Microbiol. 2009, 58, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Ehlinger, M.; Stanchina, C.; Giacomelli, M.-C.; Gicquel, P.; Karger, C.; Clavert, J.-M. Salmonella enterica subsp. arizonae bone and joints sepsis. A case report and literature review. Orthop. Traumatol. Surg. Res. 2009, 95, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Gyang, A.; Saunders, M. Salmonella Mississippi: A rare cause of second trimester miscarriage. Arch. Gynecol. Obstet. 2008, 277, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Bugla-Płoskońska, G.; Rybka, J.; Futoma-Kołoch, B.; Cisowska, A.; Gamian, A.; Doroszkiewicz, W. Sialic acid-containing lipopolysaccharides of Salmonella O48 strains—potential role in camouflage and susceptibility to the bactericidal effect of normal human serum. Microb. Ecol. 2010, 59, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Pees, M.; Rabsch, W.; Plenz, B.; Fruth, A.; Prager, R.; Simon, S.; Schmidt, V.; Munch, S.; Braun, P. Evidence for the transmission of Salmonella from reptiles to children in Germany, July 2010 to October 2011. Euro Surveill. 2013, 18. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20634 (accessed on 1 August 2017). [CrossRef]
- Friedman, C.R.; Torigian, C.; Shillam, P.J.; Hoffman, R.E.; Heltzel, D.; Beebe, J.L.; Malcolm, G.; DeWitt, W.E.; Hutwagner, L.; Griffin, P.M. An outbreak of salmonellosis among children attending a reptile exhibit at a zoo. J. Pediatr. 1998, 132, 802–807. [Google Scholar] [CrossRef]
- Geue, L.; Löschner, U. Salmonella enterica in reptiles of German and Austrian origin. Vet. Microbiol. 2002, 84, 79–91. [Google Scholar] [CrossRef]
- Mermin, J.; Hoar, B.; Angulo, F.J. Iguanas and Salmonella marina infection in children: A reflection of the increasing incidence of reptile-associated salmonellosis in the United States Pediatrics. Pediatrics 1997, 99, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Cain, C.; Tyre, D.; Ferraro, D. Incidence of Salmonella on Reptiles in the Pet Trade. Rev. Undergrad. Res Agric. Life Sci. 2009, 4, 1. [Google Scholar]
- O’Byrne, A.M.; Mahon, M. Reptile-associated salmonellosis in residents in the south East of Ireland 2005–2007. Eurosurveillance 2008, 13, 1854–1861. [Google Scholar]
- Schröter, M.; Roggentin, P.; Hofmann, J.; Speicher, A.; Laufs, R.; Mack, D. Pet snakes as a reservoir for Salmonella enterica subsp. diarizonae (serogroup IIIb): A prospective study. Appl. Environ. Microbiol. 2004, 70, 613–615. [Google Scholar]
- Warwick, C.; Lambiris, A.J.; Westwood, D.; Steedman, C. Reptile-related salmonellosis. J. R. Soc. Med. 2001, 94, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Gamian, A.; Jones, C.; Lipiński, T.; Korzeniowska-Kowal, A.; Ravenscroft, N. Structure of the sialic acid-containing O-specific polysaccharide from Salmonella enterica serovar Toucra O48 lipopolysaccharide. Eur. J. Biochem. FEBs 2000, 267, 3160–3167. [Google Scholar] [CrossRef]
- Ram, S.; Sharma, A.K.; Simpson, S.D.; Gulati, S.; McQuillen, D.P.; Pangburn, M.K.; Rice, P.A. A Novel sialic Acid Binding site on Factor H Mediates serum Resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 1998, 187, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Ilg, K.; Zandomeneghi, G.; Rugarabamu, G.; Meier, B.H.; Aebi, M. HR-MAs NMR reveals a pH-dependent LPS alteration by de-O-acetylation at abequose in the O-antigen of Salmonella enterica serovar Typhimurium. Carbohydr. Res. 2013, 382, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Schlecht, S.; Wagner, M.; Mayer, H.L. The sialic acid-containing lipopolysaccharides of Salmonella djakarta and Salmonella isaszeg (serogroup O: 48): Chemical characterization and reactivity with a sialic acid-binding lectin from Cepaea hortensis. FEMS Immunol. Med. Microbiol. 1994, 9, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M. Structural Modifications of Bacterial Lipopolysaccharide that Facilitate Gram-Negative Bacteria Evasion of Host Innate Immunity. Front. Immunol. 2013, 4, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munn, C.B.; Ishiguro, E.E.; Kay, W.W.; Trust, T.J. Role of surface components in serum resistance of virulent Aeromonas salmonicida. Infect. Immun. 1982, 36, 1069–1075. [Google Scholar] [PubMed]
- Taylor, P.W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol. Rev. 1983, 47, 46–83. [Google Scholar] [PubMed]
- Bravo, D.; Silva, C.; Carter, J.A.; Hoare, A.; Alvarez, S.A.; Blondel, C.J.; Zaldívar, M.; Valvano, M.A.; Contreras, I. Growth-phase regulation of lipopolysaccharide O-antigen chain length influences serum resistance in serovars of Salmonella. J. Med. Microbiol. 2008, 57, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Vimr, E.R. Unified theory of bacterial sialometabolism: How and why bacteria metabolize host sialic acids. ISRN Microbiol. 2013, 2013, 816713. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.L.; Attridge, S.R.; Morona, R. Regulation of Salmonella typhimurium lipopolysaccharide O antigen chain length is required for virulence; identification of FepE as a second Wzz. Mol. Microbiol. 2003, 47, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.L.; Attridge, S.R.; Morona, R. Inducible serum resistance in Salmonella typhimurium is dependent on wzz (fepE)-regulated very long O antigen chains. Microbes Infect. 2005, 7, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.W.; Keestra, A.M.; Winter, S.E.; Xavier, M.N.; Tsolis, R.M.; Tolstikov, V.; Bäumler, A.J. Very long O-antigen chains enhance fitness during Salmonella-induced colitis by increasing bile resistance. PLoS Pathog. 2012, 8, e1002918. [Google Scholar] [CrossRef] [PubMed]
- Dudek, B.; Krzyżewska, E.; Kapczyńska, K.; Rybka, J.; Pawlak, A.; Korzekwa, K.; Klausa, E.; Bugla-Płoskońska, G. Proteomic Analysis of Outer Membrane Proteins from Salmonella Enteritidis strains with Different sensitivity to Human serum. PLoS ONE 2016, 11, e0164069. [Google Scholar] [CrossRef] [PubMed]
- Bugla-Płoskońska, G.; Korzeniowska-Kowal, A.; Guz-Regner, K. Reptiles as a source of Salmonella O48—Clinically important bacteria for children: The relationship between resistance to normal cord serum and outer membrane protein patterns. Microb. Ecol. 2011, 61, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Futoma-Koloch, B.; Bugla-Ploskonska, G.; Sarowska, J. Searching for Outer Membrane Proteins Typical of serum-sensitive and serum-Resistant Phenotypes of Salmonella. In Salmonella-Distribution, Adaptation, Control Measures and Molecular Technologies; InTech: Rijeka, Croatia, 2012; pp. 265–290. [Google Scholar]
- Skwara, A.; Dudek, B.; Rybka, J.; Rybka, W.; Klausa, E.; Doroszkiewicz, W.; Gamian, A.; Bugla-Płoskońska, G. Zmiany w ilości kwasu sjalowego w lipopolisacharydach pałeczek Salmonella O48 po pasażach w surowicy ludzkiej (in polish). In Proceedings of the Zjazd PTM, Lublin, Poland, 5–8 September 2012. [Google Scholar]
- Yi, E.C.; Hackett, M. Rapid isolation method for lipopolysaccharide and lipid A from gram-negative bacteria. Analyst 2000, 125, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P.; Hancock, R.E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J. Bacteriol. 1983, 155, 831–838. [Google Scholar] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Frasch, C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar]
- Fomsgaard, A.; Freudenberg, M.A.; Galanos, C. Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J. Clin. Microbiol. 1990, 28, 2627–2631. [Google Scholar] [PubMed]
- Rybka, J.; Gamian, A. Determination of endotoxin by the measurement of the acetylated methyl glycoside derivative of Kdo with gas–liquid chromatography-mass spectrometry. J. Microbiol. Methods 2006, 64, 171–184. [Google Scholar] [CrossRef] [PubMed]
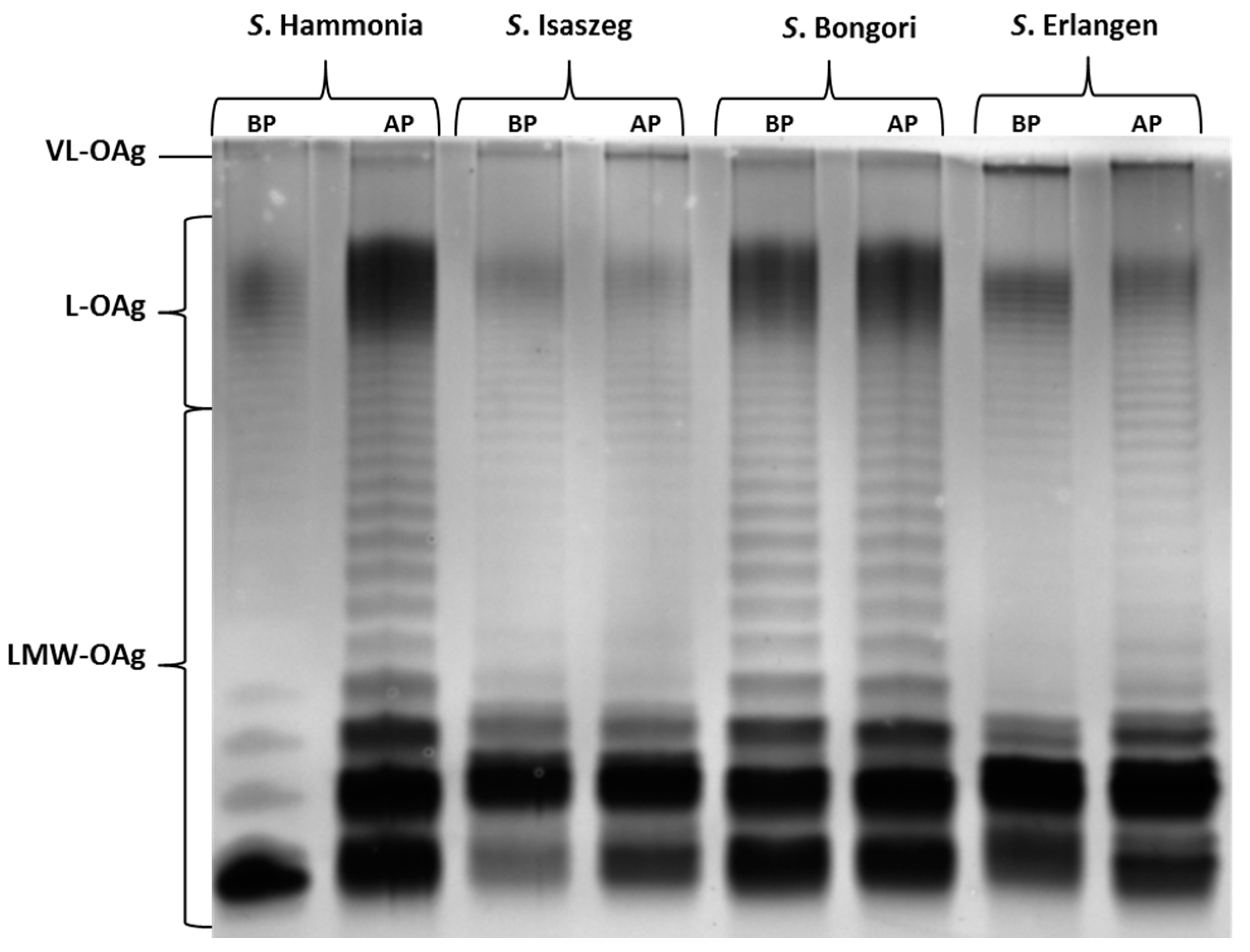

| S. Hammonia | S. Isaszeg | S. Bongori | S. Erlangen | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Passages | CFU/mL * | Survival of Cells at T3 (%) | CFU/mL | Survival of Cells at T3 (%) | CFU/mL | Survival of Cells at T3 (%) | CFU/mL | Survival of Cells at T3 (%) | ||||
| T0 | T3 | T0 | T3 | T0 | T3 | T0 | T3 | |||||
| 1 | 1.7 × 106 | 9.6 × 102 | 0.06 | 5.7 × 106 | 2.0 × 103 | 0.04 | 6.9 × 106 | ≤100 | ≤0.00009 | 5.1 × 106 | 5.5 × 101 | 0.001 |
| 2 | 1.0 × 106 | 2.7 × 105 | 27.00 | 6.5 × 106 | 9.0 × 104 | 1.38 | 3.5 × 106 | ≤100 | ≤0.00009 | 7.1 × 106 | 7.1 × 102 | 0.01 |
| 3 | 2.3 × 106 | 5.4 × 106 | 234.78 | 5.4 × 106 | 5.1 × 103 | 0.09 | 3.4 × 106 | ≤100 | ≤0.00009 | 8.4 × 106 | 9.2 × 102 | 0.01 |
| 4 | 4.7 × 106 | 6.4 × 105 | 136.17 | 4.8 × 106 | 7.2 × 105 | 15.00 | 2.8 × 106 | ≤100 | ≤0.00009 | 4.2 × 106 | 2.6 × 103 | 0.06 |
| 5 | 1.7 × 106 | 1.4 × 106 | 82.35 | 3.2 × 106 | 1.6 × 107 | 500.00 | 1.5 × 106 | 8.0 × 103 | 0.53 | 5.0 × 106 | 8.6 × 103 | 0.17 |
| 6 | 1.0 × 106 | 2.4 × 106 | 240.00 | 3.7 × 106 | 6.8 × 106 | 183.78 | 3.2 × 106 | 2.2 × 105 | 6.88 | 2.0 × 106 | 8.5 × 106 | 425.00 |
| 7 | 1.7 × 106 | 1.8 × 107 | 1058.82 | 3.4 × 106 | 7.8 × 105 | 22.94 | 4.5 × 106 | 4.2 × 105 | 9.33 | 3.2 × 106 | 5.1 × 106 | 159.38 |
| 8 | 1.3 × 106 | 7.3 × 106 | 561.64 | 4.1 × 106 | 5.8 × 106 | 141.46 | 5.0 × 105 | 1.4 × 104 | 2.80 | 4.5 × 106 | 3.8 × 106 | 84.44 |
| 9 | 6.1 × 106 | 3.0 × 107 | 491.80 | 3.7 × 106 | 6.8 × 106 | 183.78 | 5.0 × 106 | 8.1 × 104 | 1.80 | 3.0 × 106 | 6.0 × 106 | 200.00 |
| S. Hammonia | S. Isaszeg | S. Bongori | S. Erlangen | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CFU/mL * | Survival of Cells at T3 (%) | CFU/mL | Survival of Cells at T3 (%) | CFU/mL | Survival of Cells at T3 (%) | CFU/mL | Survival of Cells at T3 (%) | ||||
| T0 | T3 | T0 | T3 | T0 | T3 | T0 | T3 | ||||
| 4.6 × 106 | 5.1 × 107 | 1108.69 | 3.7 × 106 | 2.7 × 107 | 729.73 | 4.2 × 106 | 3.6 × 107 | 857.15 | 3.6 × 106 | 3.1 × 107 | 861.11 |
| Species | Subspecies | Serovar | Somatic (O) Antigen | Source |
|---|---|---|---|---|
| Salmonella bongori | - | Bongori | 48 | PCM * 2547 |
| Salmonella enterica | enterica | Isaszeg | 48 | PCM * 2550 |
| Salmonella enterica | salamae | Erlangen | 48 | PCM * 2533 |
| Salmonella enterica | salamae | Hammonia | 48 | PCM * 2535 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlak, A.; Rybka, J.; Dudek, B.; Krzyżewska, E.; Rybka, W.; Kędziora, A.; Klausa, E.; Bugla-Płoskońska, G. Salmonella O48 Serum Resistance is Connected with the Elongation of the Lipopolysaccharide O-Antigen Containing Sialic Acid. Int. J. Mol. Sci. 2017, 18, 2022. https://doi.org/10.3390/ijms18102022
Pawlak A, Rybka J, Dudek B, Krzyżewska E, Rybka W, Kędziora A, Klausa E, Bugla-Płoskońska G. Salmonella O48 Serum Resistance is Connected with the Elongation of the Lipopolysaccharide O-Antigen Containing Sialic Acid. International Journal of Molecular Sciences. 2017; 18(10):2022. https://doi.org/10.3390/ijms18102022
Chicago/Turabian StylePawlak, Aleksandra, Jacek Rybka, Bartłomiej Dudek, Eva Krzyżewska, Wojciech Rybka, Anna Kędziora, Elżbieta Klausa, and Gabriela Bugla-Płoskońska. 2017. "Salmonella O48 Serum Resistance is Connected with the Elongation of the Lipopolysaccharide O-Antigen Containing Sialic Acid" International Journal of Molecular Sciences 18, no. 10: 2022. https://doi.org/10.3390/ijms18102022