Sphingosine 1-Phosphate Signaling and Its Pharmacological Modulation in Allogeneic Hematopoietic Stem Cell Transplantation
Abstract
:1. Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) for the Treatment of Hematological Malignances: Overview
2. Sphingosine 1-Phosphate (S1P) and the S1P Receptors (S1PRs)
3. Pharmacological S1PR Modulation
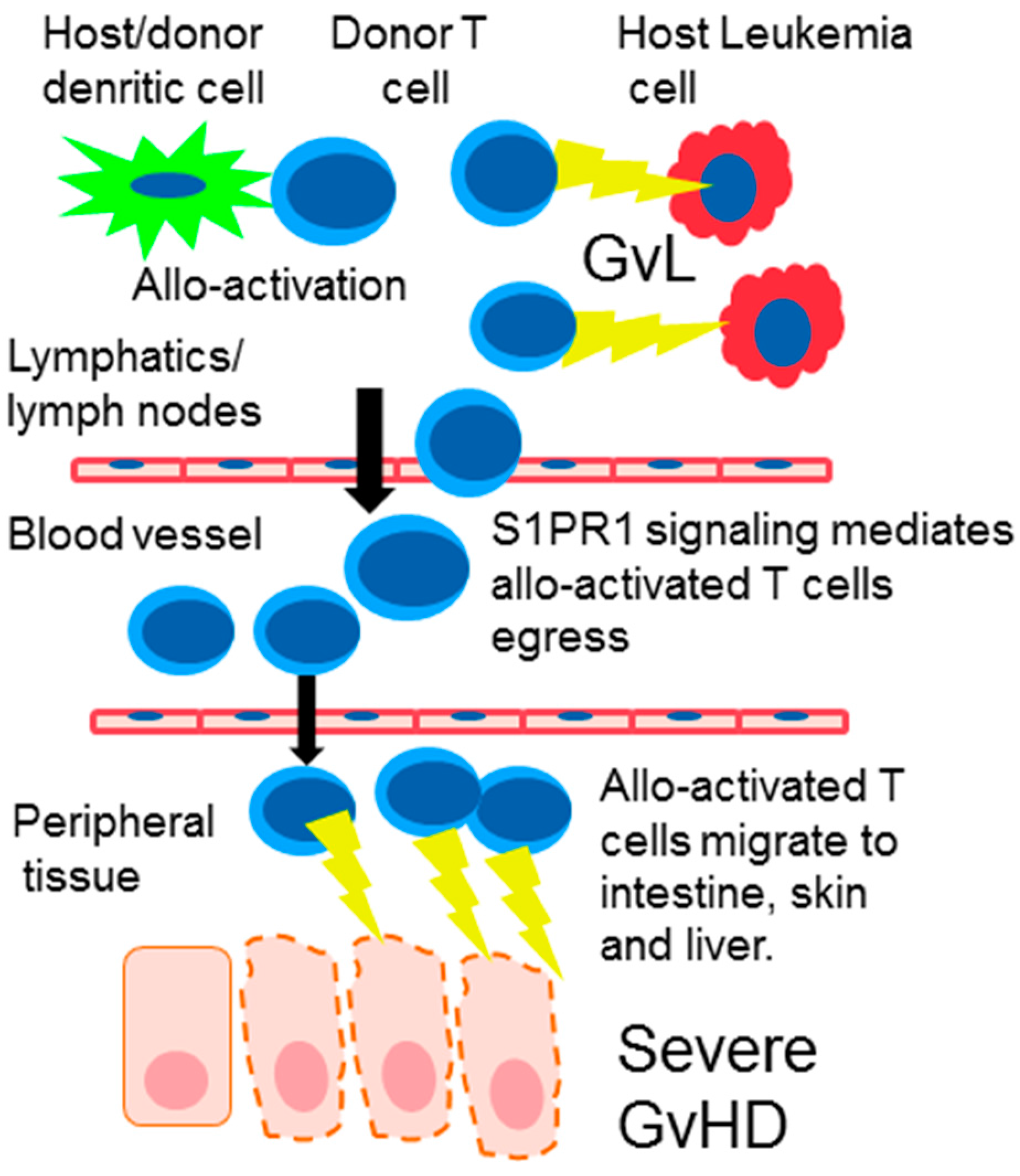
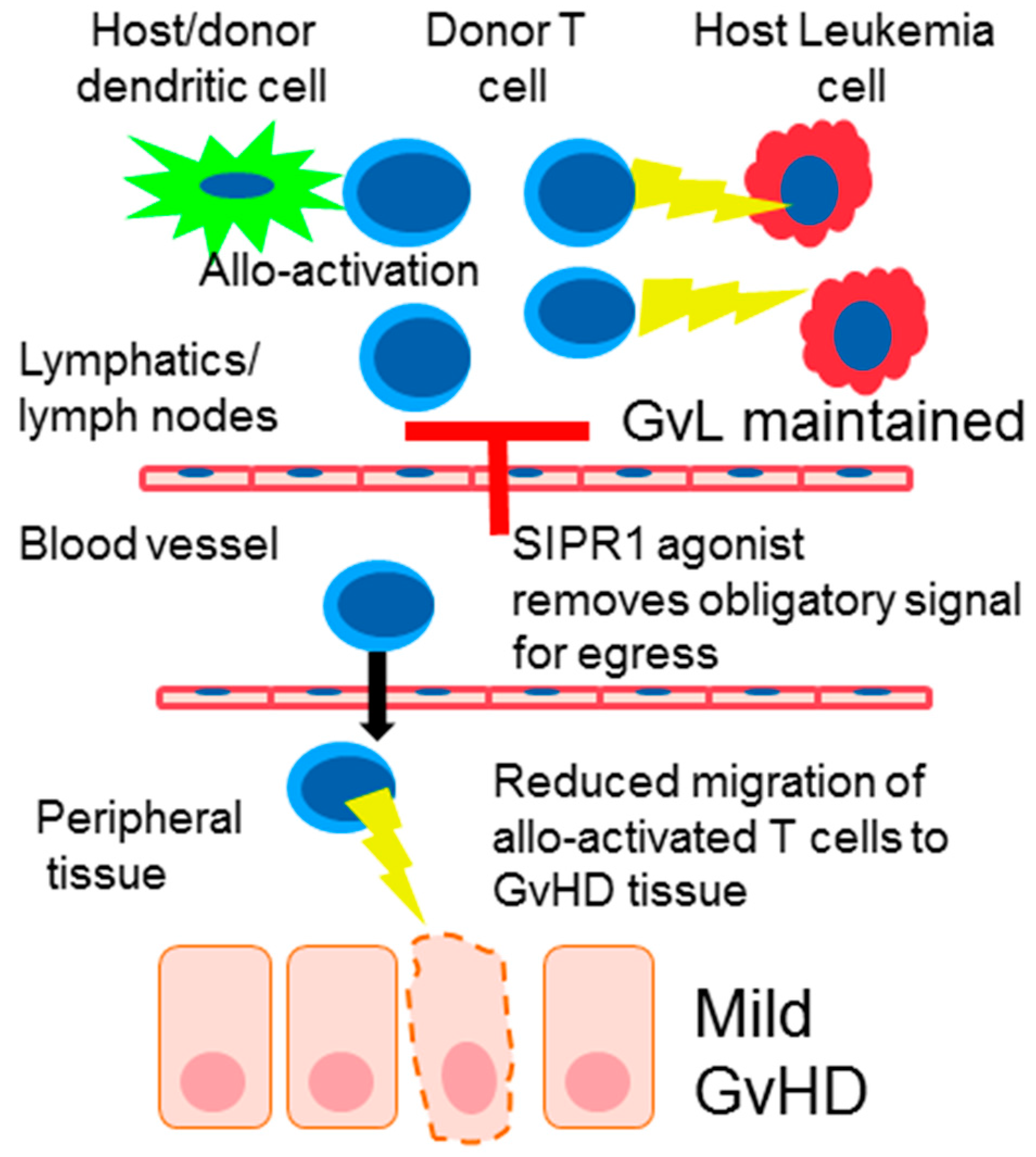
4. S1PR Modulation in Graft-Versus-Host Disease (GvHD)
5. Macrophages, Dendritic Cells and T Cell Activation
6. Donor T Cell Apoptosis and Egress from Lymphoid Tissue
7. The Effect of S1PR Signaling on T Regulatory Cells (Tregs) in GvHD
8. SIPR Signaling in Endothelial Cells
9. The Effect of SIPR Signaling on the Graft vs. Leukemia Effect (GvL)
10. S1P and Engraftment
11. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ng, Y.Y.; Baert, M.R.; de Haas, E.F.; Pike-Overzet, K.; Staal, F.J. Isolation of human and mouse hematopoietic stem cells. Methods Mol. Biol. 2009, 506, 13–21. [Google Scholar] [PubMed]
- Ghimire, S.; Weber, D.; Mavin, E.; Wang, X.N.; Dickinson, A.M.; Holler, E. Pathophysiology of gvhd and other hsct-related major complications. Front. Immunol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; Krotkiewski, H.; Hogan, E. Signaling and regulatory functions of bioactive sphingolipids as therapeutic targets in multiple sclerosis. Neurochem. Res. 2012, 37, 1154–1169. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.F. Sphingolipids in inflammation: Pathological implications and potential therapeutic targets. Br. J. Pharmacol. 2009, 158, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Radue, E.-W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Chiba, K. Fty720 story. Its discovery and the following accelerated development of sphingosine 1-phosphate receptor agonists as immunomodulators based on reverse pharmacology. Perspect. Med. Chem. 2008, 1, 11–23. [Google Scholar]
- Pan, S.; Gray, N.S.; Gao, W.; Mi, Y.; Fan, Y.; Wang, X.; Tuntland, T.; Che, J.; Lefebvre, S.; Chen, Y.; et al. Discovery of baf312 (siponimod), a potent and selective s1p receptor modulator. ACS Med. Chem. Lett. 2013, 4, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.; Sun, S.-Y.; Hajdu, R.; Bergstrom, J.; Card, D.; Doherty, G.; Hale, J.; Keohane, C.; Meyers, C.; Milligan, J.; et al. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J. Pharmacol. Exp. Ther. 2004, 309, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Hla, T.; Brinkmann, V. Sphingosine 1-phosphate (s1p): Physiology and the effects of s1p receptor modulation. Neurology 2011, 76, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate in coagulation and inflammation. Semin. Immunopathol. 2012, 34, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Oo, M.L.; Thangada, S.; Wu, M.T.; Liu, C.H.; Macdonald, T.L.; Lynch, K.R.; Lin, C.Y.; Hla, T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 2007, 282, 9082–9089. [Google Scholar] [CrossRef] [PubMed]
- Oo, M.L.; Chang, S.H.; Thangada, S.; Wu, M.T.; Rezaul, K.; Blaho, V.; Hwang, S.I.; Han, D.K.; Hla, T. Engagement of s1p-degradative mechanisms leads to vascular leak in mice. J. Clin. Investig. 2011, 121, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Mullershausen, F.; Zecri, F.; Cetin, C.; Billich, A.; Guerini, D.; Seuwen, K. Persistent signaling induced by fty720-phosphate is mediated by internalized s1p1 receptors. Nat. Chem. Biol. 2009, 5, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ito, T.; Matsuda, C.; Miao, G.; Tanemura, M.; Nishida, T.; Nozawa, M.; Matsuda, H.; Sawa, Y. Inhibition of donor-derived t cells trafficking into target organs by fty720 during acute graft-versus-host disease in small bowel transplantation. Clin. Exp. Immunol. 2006, 146, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on s1p receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Sachs, T.; Asavaroengchai, W.; Bronson, R.; Sykes, M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with fty720. J. Clin. Investig. 2003, 111, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J. Classification systems for chronic graft-versus-host disease. Blood 2017, 129, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Socié Gerard, B.B.R. Acute graft-versus-host disease: From the bench to the bedside. Blood 2009, 114, 4327–4336. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.A.D.; DiPersio, J.F. Mouse models of graft-versus-host disease: Advances and limitations. Dis. Model. Mech. 2011, 4, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Duffner, U.A.; Maeda, Y.; Cooke, K.R.; Reddy, P.; Ordemann, R.; Liu, C.; Ferrara, J.L.M.; Teshima, T. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J. Immunol. 2004, 172, 7393–7398. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Panther, E.; Corinti, S.; Morelli, A.; Ferrari, D.; Herouy, Y.; Dichmann, S.; Mockenhaupt, M.; Gebicke-Haerter, P.; Di Virgilio, F.; et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of th2 immune responses. FASEB 2002, 16, 625–627. [Google Scholar] [CrossRef]
- Muller, H.; Hofer, S.; Kaneider, N.; Neuwirt, H.; Mosheimer, B.; Mayer, G.; Konwalinka, G.; Heufler, C.; Tiefenthaler, M. The immunomodulator fty720 interferes with effector functions of human monocyte-derived dendritic cells. Eur. J. Immunol. 2005, 35, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A.; Ehrhardt, M.J.; Lees, C.J.; Tolar, J.; Weigel, B.J.; Panoskaltsis-Mortari, A.; Serody, J.S.; Brinkmann, V.; Blazar, B.R. Insights into the mechanism of fty720 and compatibility with regulatory t cells for the inhibition of graft-versus-host disease (gvhd). Blood 2007, 110, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Alegre, M.-L.; Frauwirth, K.A.; Thompson, C.B. T-cell regulation by cd28 and ctla-4. Nat. Rev. Immunol. 2001, 1, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Sartory, N.; Zahn, N.; Geisslinger, G.; Radeke, H.H.; Stein, J.M. Fty720 ameliorates th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J. Immunol. 2007, 178, 2458–2468. [Google Scholar] [CrossRef] [PubMed]
- Oaks, J.J.; Santhanam, R.; Walker, C.J.; Roof, S.; Harb, J.G.; Ferenchak, G.; Eisfeld, A.-K.; Van Brocklyn, J.R.; Briesewitz, R.; Saddoughi, S.A.; et al. Antagonistic activities of the immunomodulator and pp2a-activating drug fty720 (fingolimod, gilenya) in jak2-driven hematologic malignancies. Blood 2013, 122, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Patmanathan, S.N.; Yap, L.F.; Murray, P.G.; Paterson, I.C. The antineoplastic properties of fty720: Evidence for the repurposing of fingolimod. J. Cell. Mol. Med. 2015, 19, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Baroja, M.L.; Vijayakrishnan, L.; Bettelli, E.; Darlington, P.J.; Chau, T.A.; Ling, V.; Collins, M.; Carreno, B.M.; Madrenas, J.; Kuchroo, V.K. Inhibition of ctla-4 function by the regulatory subunit of serine/threonine phosphatase 2a. J. Immunol. 2002, 168, 5070–5078. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A.; Noelle, R.J.; Blazar, B.R. Cd4(+)cd25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 2001, 193, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.D.; Haxhinasto, S.A.; Anderson, S.M.; Stefanopoulos, D.E.; Fogal, S.E.; Adusumalli, P.; Desai, S.N.; Patnaude, L.A.; Lukas, S.M.; Ryan, K.R.; et al. Circulating monocytes are reduced by sphingosine-1-phosphate receptor modulators independently of s1p3. J. Immunol. 2013, 190, 3533–3540. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Ma, S.; Lin, D.; Mei, Y.; Gong, H.; Lei, L.; Chen, Y.; Zhao, Y.; Hu, B.; Wu, Y.; et al. The s1p1 receptor-selective agonist cym-5442 reduces the severity of acute gvhd by inhibiting macrophage recruitment. Cell. Mol. Immunol. 2015, 12, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, S.; Nakayama, T.; Murata, M.; Nishida, T.; Terakura, S.; Saito, S.; Kato, T.; Mizuno, H.; Imahashi, N.; Seto, A.; et al. Dexamethasone palmitate ameliorates macrophages-rich graft-versus-host disease by inhibiting macrophage functions. PLoS ONE 2014, 9, e96252. [Google Scholar] [CrossRef] [PubMed]
- Blazar, B.R.; Aukerman, S.L.; Vallera, D.A. Effect of recombinant human macrophage colony-stimulating factor in irradiated murine recipients of t-cell-depleted allogeneic or non-depleted syngeneic bone marrow transplants. Blood 1992, 79, 1636–1642. [Google Scholar] [PubMed]
- Alexander, K.A.; Flynn, R.; Lineburg, K.E.; Kuns, R.D.; Teal, B.E.; Olver, S.D.; Lor, M.; Raffelt, N.C.; Koyama, M.; Leveque, L.; et al. Csf-1-dependant donor-derived macrophages mediate chronic graft-versus-host disease. J. Clin. Investig. 2014, 124, 4266–4280. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.E.; Srinivasan, S.; Lynch, K.R.; Proia, R.L.; Ferdek, P.; Hedrick, C.C. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ. Res. 2008, 102, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Andreas, B.; Schulz, S.; Jeanette, B.; Georg, F.B.; Courtney, B.W.; Edward, I.H.; Enosh, M.B.; Yu-An, C.; Christopher, H.C.; Robert, S.N. In vivo analyses of early events in acute graft-versus-host diseasereveal sequential infiltration of T-cell subsets. Blood 2005, 106, 1113–1122. [Google Scholar]
- Ushiki, T.; Kizaka-Kondoh, S.; Ashihara, E.; Tanaka, S.; Masuko, M.; Hirai, H.; Kimura, S.; Aizawa, Y.; Maekawa, T.; Hiraoka, M. Noninvasive tracking of donor cell homing by near-infrared fluorescence imaging shortly after bone marrow transplantation. PLoS ONE 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, D.; Asakura, S.; Matsuoka, K.; Sakoda, Y.; Koyama, M.; Aoyama, K.; Tanimoto, M.; Teshima, T. Fty720 enhances the activation-induced apoptosis of donor t cells and modulates graft-versus-host disease. Eur. J. Immunol. 2007, 37, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ohtsuki, M.; Shimano, K.; Mochizuki, S.; Oshita, K.; Murata, M.; Sugahara, K.; Sato, N.; Hoshino, Y.; Chiba, K. Immunosuppressive activity of fty720, sphingosine 1-phosphate receptor agonist: Ii. Effect of fty720 and fty720-phosphate on host-versus-graft and graft-versus-host reaction in mice. Transplant. Proc. 2005, 37, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.; Fanq, V.; Chen, C.; Serasinghe, M.; Verma, A.; Muller, J.; Chaluvadi, V.S.; Dustin, M.L.; Hla, T.; Elemento, O.; et al. Lymphatic endothelial s1p promotes mitochondrialfunction and survival in naive T cells. Nature 2017, 546, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Fujino, M.; Li, X.K.; Guo, L.; Kitazawa, Y.; Funeshima, N.; Fukuda, S.; Kimura, H.; Miyashita, T.; Okuyama, T.; Amano, T.; et al. T-cell apoptosis triggered by fty720 via mitochondrial pathway. Transplant. Proc. 2001, 33, 3084–3085. [Google Scholar] [CrossRef]
- Li, X.K.; Shinomiya, T.; Enosawa, S.; Kakefuda, T.; Amemiya, H.; Suzuki, S. Induction of lymphocyte apoptosis by a novel immunosuppressant fty720: Relation with fas, bcl-2 and bax expression. Transplant. Proc. 1997, 29, 1267–1268. [Google Scholar] [CrossRef]
- Nagahara, Y.; Enosawa, S.; Ikekita, M.; Suzuki, S.; Shinomiya, T. Evidence that fty720 induces T cell apoptosis in vivo. Immunopharmacology 2000, 48, 75–85. [Google Scholar] [CrossRef]
- Hoffmann, P.; Ermann, J.; Edinger, M.; Fathman, C.G.; Strober, S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 2002, 196, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A.; Lees, C.J.; Blazar, B.R. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood 2002, 99, 3493–3499. [Google Scholar] [CrossRef] [PubMed]
- Edinger, M.; Hoffmann, P.; Ermann, J.; Drago, K.; Fathman, C.G.; Strober, S.; Negrin, R.S. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003, 9, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Brunstein, C.G.; Miller, J.S.; Cao, Q.; McKenna, D.H.; Hippen, K.L.; Curtsinger, J.; Defor, T.; Levine, B.L.; June, C.H.; Rubinstein, P.; et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood 2011, 117, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, K.; Burns, S.; Shrestha, S.; Chi, H. The s1p1-mtor axis directs the reciprocal differentiation of th1 and treg cells. Nat. Immunol. 2010, 11, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Huu, D.L.; Matsushita, T.; Jin, G.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Fujimoto, M. Fty720 ameliorates murine sclerodermatous chronic graft-versus-host disease by promoting expansion of splenic regulatory cells and inhibiting immune cell infiltration into skin. Arthritis Rheum. 2013, 65, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, B.C.; Sahner, S.; Gregor, M.; Tsakiris, D.A.; Jeanneret, C.; Pober, J.S.; Gratwohl, A. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet 2002, 359, 2078–2083. [Google Scholar] [CrossRef]
- Shulman, H.M.; Sullivan, K.M.; Weiden, P.L.; McDonald, G.B.; Striker, G.E.; Sale, G.E.; Hackman, R.; Tsoi, M.S.; Storb, R.; Thomas, E.D. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 seattle patients. Am. J. Med. 1980, 69, 204–217. [Google Scholar] [CrossRef]
- Dumler, J.S.; Beschorner, W.E.; Farmer, E.R.; Di Gennaro, K.A.; Saral, R.; Santos, G.W. Endothelial-cell injury in cutaneous acute graft-versus-host disease. Am. J. Pathol. 1989, 135, 1097–1103. [Google Scholar] [PubMed]
- Luft, T.; Dietrich, S.; Falk, C.; Conzelmann, M.; Hess, M.; Benner, A.; Neumann, F.; Isermann, B.; Hegenbart, U.; Ho, A.D.; et al. Steroid-refractory gvhd: T-cell attack within a vulnerable endothelial system. Blood 2011, 118, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Andrulis, M.; Dietrich, S.; Longerich, T.; Koschny, R.; Burian, M.; Schmitt-Graf, A.; Schirmacher, P.; Ho, A.D.; Dreger, P.; Luft, T. Loss of endothelial thrombomodulin predicts response to steroid therapy and survival in acute intestinal graft-versus-host disease. Haematologica 2012, 97, 1674–1677. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Estrada-Hernandez, T.; Paik, J.-H.; Wu, M.-T.; Venkataraman, K.; Brinkmann, V.; Claffey, K.; Hla, T. Phosphorylation and action of the immunomodulator fty720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 2003, 278, 47281–47290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Chen, Y.X.; Sun, J.; Guo, L.; Zeng, Z.C. Fty720, a sphingosine-1-phosphate (s1p) receptor modulator, protects sinusoid endothelial cells from radiation injury in vitro. Hepatol. Int. 2015, 9, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, J.; Erwin, P.A.; Dantas, A.P.; Chen, H.; Michel, T. Vegf induces s1p1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 10664–10669. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Hla, T. S1p control of endothelial integrity. Curr. Top. Microbiol. Immunol. 2014, 378, 85–105. [Google Scholar] [PubMed]
- Singer, II; Tian, M.; Wickham, L.A.; Lin, J.; Matheravidathu, S.S.; Forrest, M.J.; Mandala, S.; Quackenbush, E.J. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J. Immunol. 2005, 175, 7151–7161. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, F.; Fozza, C. Disease relapse after haematopoietic stem cell transplantation: Risk factors and treatment. Best Pract. Res. Clin. Haematol. 2007, 20, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Savani, B.N.; Mielke, S.; Reddy, N.; Goodman, S.; Jagasia, M.; Rezvani, K. Management of relapse after allo-sct for aml and the role of second transplantation. Bone Marrow Transpl. 2009, 44, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Butturini, A.; Gale, R.P. T cell depletion in bone marrow transplantation for leukemia: Current results and future directions. Bone Marrow Transpl. 1988, 3, 265–279. [Google Scholar]
- Vaessen, L.M.; van Besouw, N.M.; Mol, W.M.; IJzermans, J.N.; Weimar, W. Fty720 treatment of kidney transplant patients: A differential effect on B cells, naïve T cells, memory T cells and NK cells. Transpl. Immunol. 2006, 15, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Beckham, T.H.; Cheng, C.J.; Marrison, S.T.; Norris, J.S.; Liu, X. Interdiction of sphingolipid metabolism to improve standard cancer therapies. Adv. Cancer Res. 2013, 117, 1–36. [Google Scholar] [PubMed]
- McCracken, A.N.; McMonigle, R.J.; Tessier, J.; Fransson, R.; Perryman, M.S.; Chen, B.; Keebaugh, A.; Selwan, E.; Barr, S.A.; Kim, S.M.; et al. Phosphorylation of a constrained azacyclic fty720 analog enhances anti-leukemic activity without inducing s1p receptor activation. Leukemia 2017, 31, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Golan, K.; Vagima, Y.; Ludin, A.; Itkin, T.; Cohen-Gur, S.; Kalinkovich, A.; Kollet, O.; Kim, C.; Schajnovitz, A.; Ovadya, Y.; et al. S1p promotes murine progenitor cell egress and mobilization via s1p1-mediated ros signaling and sdf-1 release. Blood 2012, 119, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Whetton, A.D.; Lu, Y.; Pierce, A.; Carney, L.; Spooncer, E. Lysophospholipids synergistically promote primitive hematopoietic cell chemotaxis via a mechanism involving vav 1. Blood 2003, 102, 2798–2802. [Google Scholar] [CrossRef] [PubMed]
- Ryser, M.F.; Ugarte, F.; Lehmann, R.; Bornhauser, M.; Brenner, S. S1p(1) overexpression stimulates s1p-dependent chemotaxis of human CD34+ hematopoietic progenitor cells but strongly inhibits sdf-1/cxcr4-dependent migration and in vivo homing. Mol. Immunol. 2008, 46, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Jackson, J.; Baulch-Brown, C. Enumeration of bone marrow ‘homing’ haemopoietic stem cells from g-csf-mobilised normal donors and influence on engraftment following allogeneic transplantation. Bone Marrow Transpl. 2001, 28, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Boehmler, A.M.; Seitz, G.; Kuci, S.; Wiesner, T.; Brinkmann, V.; Kanz, L.; Mohle, R. The sphingosine 1-phosphate receptor agonist fty720 supports cxcr4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood 2004, 103, 4478–4486. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.D.; Hoffman, R.; Zanjani, E.D. Stem Cell Transplantation: Biology, Processes, Therapy; Wiley: New York, NY, USA, 2006. [Google Scholar]
- Walter, D.H.; Rochwalsky, U.; Reinhold, J.; Seeger, F.; Aicher, A.; Urbich, C.; Spyridopoulos, I.; Chun, J.; Brinkmann, V.; Keul, P.; et al. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the cxcr4-dependent signaling pathway via the s1p3 receptor. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, K.W.; Hanqoc, G.; Mantel, C.R.; Broxmeyer, H.E. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 2004, 305, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Onai, N.; Zhang, Y.; Yoneyama, H.; Kitamura, T.; Ishikawa, S.; Matsushima, K. Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with bone marrow-hematopoietic progenitor cells expressing sdf-1-intrakine. Blood 2000, 96, 2074–2080. [Google Scholar] [PubMed]
- Kim, C.H.; Wu, W.; Wysoczynski, M.; Abdel-Latif, A.; Sunkara, M.; Morris, A.; Kucia, M.; Ratajczak, J.; Ratajczak, M.Z. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: A novel role for bioactive lipids and soluble c5b-c9 as homing factors. Leukemia 2012, 26, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Kim, C.H. Bioactive sphingolipids and complement cascade as new emerging regulators of stem cell mobilization and homing. J. Stem Cell Res. Ther. 2013, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A.; Kelly, R.M.; Bade, N.D.; Smith, M.J.; Stefanski, H.E.; Blazar, B.R. Fty720 markedly increases alloengraftment but does not eliminate host anti-donor T cells that cause graft rejection upon its withdrawal. Biol. Blood Marrow Transpl. 2012, 18, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Lee, H.; Wysoczynski, M.; Wan, W.; Marlicz, W.; Laughlin, M.J.; Kucia, M.; Janowska-Wieczorek, A.; Ratajczak, J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement. Leukemia 2010, 24, 976–985. [Google Scholar] [PubMed]
- Passweg, J.R.; Baldomero, H.; Bader, P.; Bonini, C.; Cesaro, S.; Dreger, P.; Duarte, R.F.; Dufour, C.; Kuball, J.; Farge-Bancel, D.; Gennery, A.; et al. Hematopoietic stem cell transplantation in Europe 2014: More than 40,000 transplants annually. Bone Marrow Transplant. 2016, 51, 786–792. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, P.; O’Sullivan, C.; Gergely, P. Sphingosine 1-Phosphate Signaling and Its Pharmacological Modulation in Allogeneic Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2017, 18, 2027. https://doi.org/10.3390/ijms18102027
Smith P, O’Sullivan C, Gergely P. Sphingosine 1-Phosphate Signaling and Its Pharmacological Modulation in Allogeneic Hematopoietic Stem Cell Transplantation. International Journal of Molecular Sciences. 2017; 18(10):2027. https://doi.org/10.3390/ijms18102027
Chicago/Turabian StyleSmith, Philip, Catherine O’Sullivan, and Peter Gergely. 2017. "Sphingosine 1-Phosphate Signaling and Its Pharmacological Modulation in Allogeneic Hematopoietic Stem Cell Transplantation" International Journal of Molecular Sciences 18, no. 10: 2027. https://doi.org/10.3390/ijms18102027




