The Diagnostic, Prognostic, and Therapeutic Utility of Molecular Testing in a Patient with Waldenstrom’s Macroglobulinemia
Abstract
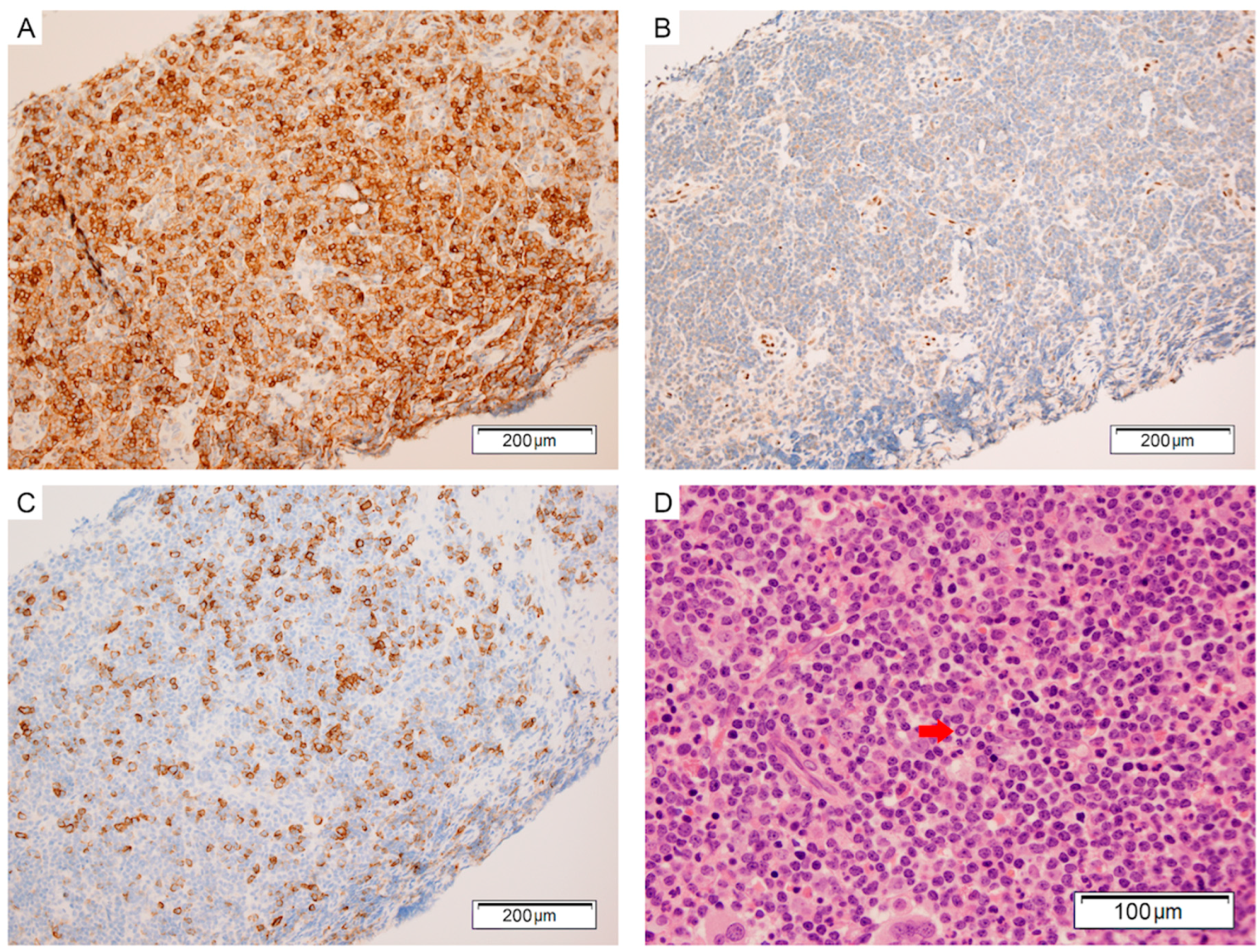
:1. Case Presentation
2. Discussion
2.1. Diagnostic Relevance of MYD88 L265P
2.2. Prognostic Implications of MYD88 in WM
2.3. Current and Future Treatment Approaches for Patients with WM
3. Conclusions
Author Contributions
Conflicts of Interest
References
- Ngo, V.N.; Young, R.M.; Schmitz, R.; Jhavar, S.; Xiao, W.; Lim, K.H.; Kohlhammer, H.; Xu, W.; Yang, Y.; Zhao, H.; et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011, 470, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhou, Y.; Liu, X.; Xu, L.; Cao, Y.; Manning, R.J.; Patterson, C.J.; Buhrlage, S.J.; Gray, N.; Tai, Y.T.; et al. A mutation in MYD88 (l265p) supports the survival of lymphoplasmacytic cells by activation of bruton tyrosine kinase in waldenstrom macroglobulinemia. Blood 2013, 122, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Sheehy, P.; Manning, R.J.; Patterson, C.J.; Tripsas, C.; et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N. Engl. J. Med. 2012, 367, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hunter, Z.R.; Yang, G.; Zhou, Y.; Cao, Y.; Liu, X.; Morra, E.; Trojani, A.; Greco, A.; Arcaini, L.; et al. MYD88 L265P in Waldenstrom macroglobulinemia, immunoglobulin M monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction. Blood 2013, 121, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Xu, L.; Hunter, Z. MYD88 mutations and response to ibrutinib in Waldenstrom’s macroglobulinemia. N. Engl. J. Med. 2015, 373, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Poulain, S.; Roumier, C.; Galiegue-Zouitina, S.; Daudignon, A.; Herbaux, C.; Aiijou, R.; Lainelle, A.; Broucqsault, N.; Bertrand, E.; Manier, S.; et al. Genome wide snp array identified multiple mechanisms of genetic changes in Waldenstrom macroglobulinemia. Am. J. Hematol. 2013, 88, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Kuzu, I.; Dogan, A.; Dirnhofer, S.; Chan, J.K.; Sander, B.; Ott, G.; Xerri, L.; Quintanilla-Martinez, L.; Campo, E. The many faces of small B cell lymphomas with plasmacytic differentiation and the contribution of MYD88 testing. Virchows Arch. 2016, 468, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, A.; Curiel-Olmo, S.; Mollejo, M.; Cereceda, L.; Martinez, N.; Montes-Moreno, S.; Almaraz, C.; Revert, J.B.; Piris, M.A. MYD88 (L265P) somatic mutation in marginal zone B-cell lymphoma. Am. J. Surg. Pathol. 2015, 39, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Varettoni, M.; Arcaini, L.; Zibellini, S.; Boveri, E.; Rattotti, S.; Riboni, R.; Corso, A.; Orlandi, E.; Bonfichi, M.; Gotti, M.; et al. Prevalence and clinical significance of the MYD88 (L265P) somatic mutation in Waldenstrom’s macroglobulinemia and related lymphoid neoplasms. Blood 2013, 121, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Trillos, A.; Pinyol, M.; Navarro, A.; Aymerich, M.; Jares, P.; Juan, M.; Rozman, M.; Colomer, D.; Delgado, J.; Gine, E.; et al. Mutations in TLR/MYD88 pathway identify a subset of young chronic lymphocytic leukemia patients with favorable outcome. Blood 2014, 123, 3790–3796. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Takata, K.; Chuang, S.S.; Miyata-Takata, T.; Sato, Y.; Satou, A.; Hashimoto, Y.; Tamura, M.; Nagakita, K.; Ohnishi, N.; et al. Frequent MYD88 L265P and CD79B mutations in primary breast diffuse large B-cell lymphoma. Am. J. Surg. Pathol. 2016, 40, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Cao, Y.; Xu, L.; Yang, G.; Liu, X.; Hunter, Z.R. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood 2014, 123, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Romero, D.L.; Chaudhary, D. IRAK4 kinase as a novel therapeutic agent in the ABC subtype of diffuse large B cell lymphoma. Blood 2012, 120, 62. [Google Scholar]
- Treon, S.P.; Ioakimidis, L.; Soumerai, J.D.; Patterson, C.J.; Sheehy, P.; Nelson, M.; Willen, M.; Matous, J.; Mattern, J., 2nd; Diener, J.G.; et al. Primary therapy of Waldenstrom macroglobulinemia with bortezomib, dexamethasone, and rituximab: Wmctg clinical trial 05–180. J. Clin. Oncol. 2009, 27, 3830–3835. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Tripsas, C.K.; Meid, K.; Kanan, S.; Sheehy, P.; Chuma, S.; Xu, L.; Cao, Y.; Yang, G.; Liu, X.; et al. Carfilzomib, rituximab, and dexamethasone (CaRD) treatment offers a neuropathy-sparing approach for treating Waldenstrom’s macroglobulinemia. Blood 2014, 124, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Garcia-Sanz, R.; Gavriatopoulou, M.; Morel, P.; Kyrtsonis, M.C.; Michalis, E.; Kartasis, Z.; Leleu, X.; Palladini, G.; Tedeschi, A.; et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): Long-term results of a phase 2 study of the european myeloma network (EMN). Blood 2013, 122, 3276–3282. [Google Scholar] [CrossRef] [PubMed]
- Burhlage, S. Kinome targets and inhibitors. In Proceedings of the 8th International Workshop on Waldenstrom’s Macroglobulinaemia, London, UK, 13–17 August 2014. [Google Scholar]
- Treon, S.P.; Tripsas, C.K.; Meid, K.; Warren, D.; Varma, G.; Green, R.; Argyropoulos, K.V.; Yang, G.; Cao, Y.; Xu, L.; et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N. Engl. J. Med. 2015, 372, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Paludo, J.; Abeykoon, J.P.; Kumar, S.; Shreders, A.; Ailawadhi, S.; Gertz, M.A.; Kourelis, T.; King, R.L.; Reeder, C.B.; Leung, N.; et al. Dexamethasone, rituximab and cyclophosphamide for relapsed and/or refractory and treatment-naive patients with Waldenstrom macroglobulinemia. Br. J. Haematol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rummel, M.J.; Niederle, N.; Maschmeyer, G.; Banat, G.A.; von Grunhagen, U.; Losem, C.; Kofahl-Krause, D.; Heil, G.; Welslau, M.; Balser, C.; et al. Bendamustine plus rituximab versus chop plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013, 381, 1203–1210. [Google Scholar] [CrossRef]
- Tam, C.S.; Trotman, J.; Opat, S.; Marlton, P.; Cull, G.; Simpson, D.; Ku, M.; Ritchie, D.; Verner, E.; Ratnasingam, S.; et al. High major response rate, including very good partial responses (VGPR), in patients (pts) with Waldenstrom macroglobulinemia (WM) treated with the highly specific BTK inhibitor bgb-3111: Expansion phase results from an ongoing phase I study. Blood 2016, 128, 1216. [Google Scholar]
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Puvvada, S.; Kipps, T.J.; Anderson, M.A.; Salem, A.H.; et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-hodgkin lymphoma. J. Clin. Oncol. 2017, 35, 826–833. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, C.K.; Leslie, C.; Grove, C.S.; Van Vliet, C.; Cheah, C.Y. The Diagnostic, Prognostic, and Therapeutic Utility of Molecular Testing in a Patient with Waldenstrom’s Macroglobulinemia. Int. J. Mol. Sci. 2017, 18, 2038. https://doi.org/10.3390/ijms18102038
Chin CK, Leslie C, Grove CS, Van Vliet C, Cheah CY. The Diagnostic, Prognostic, and Therapeutic Utility of Molecular Testing in a Patient with Waldenstrom’s Macroglobulinemia. International Journal of Molecular Sciences. 2017; 18(10):2038. https://doi.org/10.3390/ijms18102038
Chicago/Turabian StyleChin, Collin K., Connull Leslie, Carolyn S. Grove, Chris Van Vliet, and Chan Yoon Cheah. 2017. "The Diagnostic, Prognostic, and Therapeutic Utility of Molecular Testing in a Patient with Waldenstrom’s Macroglobulinemia" International Journal of Molecular Sciences 18, no. 10: 2038. https://doi.org/10.3390/ijms18102038




