Evaluating Changes in Cell-Wall Components Associated with Clubroot Resistance Using Fourier Transform Infrared Spectroscopy and RT-PCR
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biochemical Changes in the Cell Wall
2.2. Transcription of Cell-Wall and Lignin-Related Genes
3. Materials and Methods
3.1. Plant Materials
3.2. Pathogen Inoculum and Plant Inoculation
3.3. Root Sampling
3.4. FTIR Spectroscopic Analysis
3.5. Transcription of Cell-Wall and Lignin-Related Genes in Canola Root Samples
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FTIR | Fourier transform infrared spectroscopy |
| qRT-PC | Quantitative PCR |
| CR | Clubroot resistance |
| PCA | Principal component analysis |
| R | Resistant |
| S | Susceptible |
| Pb | Plasmodiophorabrassicae |
| Dpi | Days post-inoculation |
References
- Dixon, G.R. The occurrence and economic impact of Plasmodiophorabrassicae and clubroot disease. J. Plant Growth Regul. 2009, 28, 194–202. [Google Scholar] [CrossRef]
- Howard, R.J.; Strelkov, S.E.; Harding, M.W. Clubroot of cruciferous crops—New perspectives on an old disease. Can. J. Plant Pathol. Rev. Can. Phytopathol. 2010, 32, 43–57. [Google Scholar] [CrossRef]
- Donald, C.; Porter, I. Integrated control of clubroot. J. Plant Growth Regul. 2009, 28, 289–303. [Google Scholar] [CrossRef]
- Hirai, M. Genetic analysis of clubroot resistance in Brassica crops. Breed. Sci. 2006, 56, 223–229. [Google Scholar] [CrossRef]
- Voorrips, R.E.; Jongerius, M.C.; Kanne, H.J. Mapping of two genes for resistance to clubroot (Plasmodiophorabrassicae) in a population of doubled haploid lines of Brassica oleracea by means of RFLP and AFLP markers. Theor. Appl. Genet. 1997, 94, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Diederichsen, E.; Frauen, M.; Schondelmaier, J.; Jung, C. Genetic mapping of clubroot resistance genes in oilseed rape. Theor. Appl. Genet. 2008, 116, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Harada, T.; Kubo, N.; Tsukada, M.; Suwabe, K.; Matsumoto, S. A novel locus for clubroot resistance in Brassica rapa and its linkage markers. Theor. Appl. Genet. 2004, 108, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Suwabe, K.; Tsukazaki, H.; Iketani, H.; Hatakeyama, K.; Fujimura, M.; Nunome, T.; Fukuoka, H.; Matsumoto, S.; Hirai, M. Identification of two loci for resistance to clubroot (Plasmodiophorabrassicae Woronin) in Brassica rapa L. Theor. Appl. Genet. 2003, 107, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Saito, A.; Hayashida, N.; Taguchi, G.; Matsumoto, E. Mapping of isolate-specific QTLs for clubroot resistance in Chinese cabbage (Brassica rapa L. ssppekinensis). Theor. Appl. Genet. 2008, 117, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Matsumoto, E.; Aruga, D.; Kitagawa, S.; Matsumura, H.; Hayashida, N. Molecular characterization of the CRa gene conferring clubroot resistance in Brassica rapa. Plant Mol. Biol. 2012, 80, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.G.; Song, T.; Falk, K.C.; Zhang, X.G.; Liu, X.J.; Chang, A.; Lahlali, R.; McGregor, L.; Gossen, B.D.; Peng, G.; et al. Fine mapping of Rcr1 and analyses of its effect on transcriptome patterns during infection by Plasmodiophorabrassicae. BMC Genom. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, E.; Frauen, M.; Linders, E.G.A.; Hatakeyama, K.; Hirai, M. Status and perspectives of clubroot resistance breeding in crucifer crops. J. Plant Growth Regul. 2009, 28, 265–281. [Google Scholar] [CrossRef]
- Bradshaw, J.E.; Gemmell, D.J.; Wilson, R.N. Transfer of resistance to clubroot (Plasmodiophorabrassicae) to swedes (Brassica napus L. varnapobrassicaPeterm) from B-rapa. Ann. Appl. Biol. 1997, 130, 337–348. [Google Scholar] [CrossRef]
- Piao, Z.Y.; Ramchiary, N.; Lim, Y.P. Genetics of clubroot resistance in brassica species. J. Plant Growth Regul. 2009, 28, 252–264. [Google Scholar] [CrossRef]
- Rahman, H.; Peng, G.; Yu, F.; Falk, K.C.; Kulkarni, M.; Selvaraj, G. Genetics and breeding for clubroot resistance in Canadian spring canola (Brassica napus L.). Can. J. Plant Pathol. 2014, 36, 122–134. [Google Scholar] [CrossRef]
- Peng, G.; Falk, K.C.; Gugel, R.K.; Franke, C.; Yu, F.Q.; James, B.; Strelkov, S.E.; Hwang, S.F.; McGregor, L. Sources of resistance to Plasmodiophorabrassicae (clubroot) pathotypes virulent on canola. Can. J. Plant Pathol. 2014, 36, 89–99. [Google Scholar] [CrossRef]
- Matsumoto, E.; Ueno, H.; Aruga, D.; Sakamoto, K.; Hayashida, N. Accumulation of Three clubroot resistance genes through marker-assisted selection in Chinese cabbage (Brassica rapa ssp. pekinensis). J. Jpn. Soc. Hortic. Sci. 2012, 81, 184–190. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.F.; Manolii, V.P.; Cao, T.; Feindel, D. Emergence of new virulence phenotypes of Plasmodiophorabrassicae on canola (Brassica napus) in Alberta, Canada. Eur. J. Plant Pathol. 2016, 145, 517–529. [Google Scholar] [CrossRef]
- Fuchs, H.; Sacristan, M.D. Identification of a gene in Arabidopsis thaliana controlling resistance to clubroot (Plasmodiophorabrassicae) and characterization of the resistance response. Mol. Plant Microbe Interact. 1996, 9, 91–97. [Google Scholar] [CrossRef]
- Jubault, M.; Hamon, C.; Gravot, A.; Lariagon, C.; Delourme, R.; Bouchereau, A.; Manzanares-Dauleux, M.J. Differential regulation of root arginine catabolism and polyamine metabolism in clubroot-susceptible and partially resistant Arabidopsis genotypes. Plant Physiol. 2008, 146, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lahlali, R.; Liu, X.; Karunakaran, C. Infrared spectroscopy combined with imaging a new developing analytical tool in health and plant science. Appl. Spectrosc. Rev. 2016, 51, 466–483. [Google Scholar] [CrossRef]
- Conrad, A.O.; Bonello, P. Application of infrared and raman spectroscopy for the identification of disease resistant trees. Front. Plant Sci. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.R.; de Haseth, J.A. Fourier Transform Infrared Spectrometry, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1986. [Google Scholar]
- McCann, M.C.; Chen, L.; Roberts, K.; Kemsley, E.K.; Sene, C.; Carpita, N.C.; Stacey, N.J.; Wilson, R.H. Infrared microspectroscopy: Sampling heterogeneity in plant cell wall composition and architecture. Physiol. Plant. 1997, 100, 729–738. [Google Scholar] [CrossRef]
- Mccann, M.C.; Hammouri, M.; Wilson, R.; Belton, P.; Roberts, K. Fourier-transform infrared microspectroscopy is a new way to look at plant-cell walls. Plant Physiol. 1992, 100, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Gorzsas, A.; Stenlund, H.; Persson, P.; Trygg, J.; Sundberg, B. Cell-specific chemotyping and multivariate imaging by combined FT-IR microspectroscopy and orthogonal projections to latent structures (OPLS) analysis reveals the chemical landscape of secondary xylem. Plant J. 2011, 66, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Mucciolo, A.; Humbel, B.M.; Nawrath, C. Transmission fourier transform infrared microspectroscopy allows simultaneous assessment of cutin and cell-wall polysaccharides of Arabidopsis petals. Plant J. 2013, 74, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Benning, L.G.; Phoenix, V.R.; Ferris, F.G. Characterization of metal-cyanobacteria sorption reactions: A combined macroscopic and infrared spectroscopic investigation. Environ. Sci. Technol. 2004, 38, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B. Biological Applications of Infrared Spectroscopy; John Wiley & Sons: Chichester, UK, 1997. [Google Scholar]
- Dumas, P.; Miller, L. The use of synchrotron infrared microspectroscopy in biological and biomedical investigations. Vib. Spectrosc. 2003, 32, 3–21. [Google Scholar] [CrossRef]
- Hawkins, S.A.; Park, B.; Poole, G.H.; Gottwald, T.R.; Windham, W.R.; Albano, J.; Lawrence, K.C. Comparison of FTIR spectra between huanglongbing (citrus greening) and other citrus maladies. J. Agric. Food Chem. 2010, 58, 6007–6010. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Solla, A.; Woodward, S.; Gil, L. Fourier transform-infrared spectroscopy as a new method for evaluating host resistance in the Dutch elm disease complex. Tree Physiol. 2005, 25, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Dorado, J.; Almendros, G.; Field, J.A.; Sierra-Alvarez, R. Infrared spectroscopy analysis of hemp (Cannabis sativa) after selective delignification by Bjerkanderasp at different nitrogen levels. Enzyme Microb. Technol. 2001, 28, 550–559. [Google Scholar] [CrossRef]
- Lahlali, R.; Karunakaran, C.; Wang, L.P.; Willick, I.; Schmidt, M.; Liu, X.; Borondics, F.; Forseille, L.; Fobert, P.R.; Tanino, K.; et al. Synchrotron based phase contrast X-ray imaging combined with FTIR spectroscopy reveals structural and biomolecular differences in spikelets play a significant role in resistance to Fusarium in wheat. BMC Plant Biol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by DeconvolvedFtir spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Shadle, G.L.; Wesley, S.V.; Korth, K.L.; Chen, F.; Lamb, C.; Dixon, R.A. Phenylpropanoid compounds and disease resistance in transgenic tobacco with altered expression of L-phenylalanine ammonia-lyase. Phytochemistry 2003, 64, 153–161. [Google Scholar] [CrossRef]
- Barber, M.S.; Mitchell, H.J. Regulation of phenylpropanoid metabolism in relation to lignin biosynthesis in plants. Int. Rev. Cytol. 1997, 172, 243–293. [Google Scholar]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Fischer, G.; Braun, S.; Thissen, R.; Dott, W. FT-IR spectroscopy as a tool for rapid identification and intra-species characterization of airborne filamentous fungi. J. Microbiol. Methods 2006, 64, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Wolkers, W.F.; Oliver, A.E.; Tablin, F.; Crowe, J.H. A fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr. Res. 2004, 339, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D. Use of infrared spectroscopy for in-field measurement and phenotyping of plant Properties: Instrumentation, data analysis, and examples. Appl. Spectrosc. Rev. 2014, 49, 564–584. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D.F. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Solla, A.; Woodward, S.; Gil, L. Detection of differential changes in lignin composition of elm xylem tissues inoculated with Ophiostoma novo-ulmi using fourier transform-infrared spectroscopy. For. Pathol. 2007, 37, 187–191. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Lahlali, R.; Jiang, Y.F.; Kumar, S.; Karunakaran, C.; Liu, X.; Borondics, F.; Hallin, E.; Bueckert, R. ATR-FTIR spectroscopy reveals involvement of lipids and proteins of intact pea pollen grains to heat stress tolerance. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Solla, A.; Coimbra, M.A.; Gil, L. Metabolic fingerprinting allows discrimination between Ulmuspumila and U-minor, and between U-minor clones of different susceptibility to Dutch elm disease. For. Pathol. 2008, 38, 244–256. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Guerrada, R.; Costa, A.M.R.; Serrano, M.S.; Sánchez, M.E.; Coelho, A.C. Temporal metabolic profiling of the Quercussuber–Phytophthoracinnamomi system by middle-infrared spectroscopy. For. Pathol. 2016, 46, 122–133. [Google Scholar] [CrossRef]
- Conrad, A.O.; Rodriguez-Saona, L.E.; McPherson, B.A.; Wood, D.L.; Bonello, P. Identification of Quercusagrifolia (coast live oak) resistant to the invasive pathogen Phytophthoraramorum in native stands using Fourier-transform infrared (FT-IR) spectroscopy. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Selvendran, R.R. Analysis of cell-wall material from plant-Tissues—Extraction and purification. Phytochemistry 1975, 14, 1011–1017. [Google Scholar] [CrossRef]
- York, W.S.; Darvill, A.G.; Mcneil, M.; Stevenson, T.T.; Albersheim, P. Isolation and characterization of plant-cell walls and cell-wall components. Methods Enzymol. 1986, 118, 3–40. [Google Scholar]
- Kircher, M.; Kelso, J. High-throughput DNA sequencing—Concepts and limitations. Bioessays 2010, 32, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.J.; Hall, S.A.; Stratford, R.; Hall, J.L.; Barber, M.S. Differential induction of cinnamyl alcohol dehydrogenase during defensive lignification in wheat (Triticumaestivum L.): Characterisation of the major inducible form. Planta 1999, 208, 31–37. [Google Scholar] [CrossRef]
- MauchMani, B.; Slusarenko, A.J. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of arabidopsis to Peronosporaparasitica. Plant Cell 1996, 8, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhu, D.F.; Zhou, B.; Peng, W.S.; Lin, J.Z.; Huang, X.Q.; He, R.Q.; Zhuo, Y.H.; Peng, D.; Tang, D.Y.; et al. Over-expression of the AtGA2ox8 gene decreases the biomass accumulation and lignification in rapeseed (Brassica napus L.). J. Zhejiang Univ. Sci. B 2010, 11, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Devos, S.; Vissenberg, K.; Verbelen, J.P.; Prinsen, E. Infection of Chinese cabbage by Plasmodiophorabrassicae leads to a stimulation of plant growth: Impacts on cell wall metabolism and hormone balance. New Phytol. 2005, 166, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, J.P.; Vissenberg, K.; Kerstens, S.; Le, J. Cell expansion in the epidermis: Microtubules, cellulose orientation and wall loosening enzymes. J. Plant Physiol. 2001, 158, 537–543. [Google Scholar] [CrossRef]
- Lahlali, R.; McGregor, L.; Song, T.; Gossen, B.D.; Narisawa, K.; Peng, G. Heteroconiumchaetospira induces resistance to clubroot via upregulation of host genes involved in jasmonic acid, ethylene, and auxin biosynthesis. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Lahlali, R.; Peng, G. Suppression of clubroot by Clonostachysrosea via antibiosis and induced host resistance. Plant Pathol. 2014, 63, 447–455. [Google Scholar] [CrossRef]
- Frova, C. Glutathione transferases in the genomics era: New insights and perspectives. Biomol. Eng. 2006, 23, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Ghanashyam, C.; Bhattacharjee, A. Comprehensive expression analysis suggests overlapping and specific roles of rice glutathione S-transferase genes during development and stress responses. BMC Genom. 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Olsztyńska-Janus, S.; Gąsior-Głogowska, M.; Szymborska-Małek, K.; Czarnik-Matusewicz, B.; Komorowska, M. Specific application of vibrational spectroscopy in biomedical engineering. In Biomedical Engineering, Trends, Research and Technologies; Komorowska, M., Olsztynska-Janus, S., Eds.; In-Tech: Shanghai, China, 2011; pp. 91–120. [Google Scholar]
- Peng, G.; Lahlali, R.; Hwang, S.F.; Pageau, D.; Hynes, R.K.; Mcdonald, M.R.; Gossen, B.D.; Strelkov, S.E. Special Issue: Crop rotation, cultivar resistance, and fungicides/biofungicides for managing clubroot (Plasmodiophorabrassicae) on canola. Can. J. Plant Pathol. 2014, 36, 99–112. [Google Scholar] [CrossRef]
- Lahlali, R.; Peng, G.; McGregor, L.; Gossen, B.D.; Hwang, S.F.; McDonald, M. Mechanisms of the biofungicide Serenade (Bacillus subtilis QST713) in suppressing clubroot. Biocontrol Sci. Technol. 2011, 21, 1351–1362. [Google Scholar] [CrossRef]
- Nafisi, M.; Fimognari, L.; Sakuragi, Y. Interplays between the cell wall and phytohormones in interaction between plants and necrotrophic pathogens. Phytochemistry 2015, 112, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Voigt, C.A. Callose-mediated resistance to pathogenic intruders in plant defense-related papillae. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Dixon, R.A. Altering the cell wall and its impact on plant disease: From forage to bioenergy. Annu. Rev. Phytopathol. 2014, 52, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.; Pastor, V.; Robert, J.; Flors, V.; Mauch-Mani, B.; Ton, J. Callose deposition: A multifaceted plant defense response. Mol. Plant Microbe Interact. 2011, 24, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.J.; Kjellbom, P.; Lamb, C.J. Elicitor-induced and wound-induced oxidative cross-linking of a proline-rich plant-cell wall protein—A novel, rapid defense response. Cell 1992, 70, 21–30. [Google Scholar] [CrossRef]
- O'Brien, J.A.; Daudi, A.; Finch, P.; Butt, V.S.; Whitelegge, J.P.; Souda, P.; Ausubel, F.M.; Bolwell, G.P. A peroxidase-dependent apoplastic oxidative burst in cultured arabidopsis cells functions in MAMP-elicited defense. Plant Physiol. 2012, 158, 2013–2027. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, S.E.; Manolii, V.P.; Cao, T.; Xue, S.; Hwang, S.F. Pathotype classification of Plasmodiophorabrassicae and its occurrence in Brassica napus in Alberta, Canada. J. Phytopathol. 2007, 155, 706–712. [Google Scholar] [CrossRef]
- Lahlali, R.; Peng, G.; Gossen, B.D.; McGregor, L.; Yu, F.Q.; Hynes, R.K.; Hwang, S.F.; McDonald, M.R.; Boyetchko, S.M. Evidence that the biofungicide serenade (Bacillus subtilis) suppresses clubroot on canola via antibiosis and induced host resistance. Phytopathology 2013, 103, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; McGregor, L.; Lahlali, R.; Gossen, B.D.; Hwang, S.F.; Adhikari, K.K.; Strelkov, S.E.; McDonald, M.R. Potential biological control of clubroot on canola and crucifer vegetable crops. Plant Pathol. 2011, 60, 566–574. [Google Scholar] [CrossRef]
- Mcdonald, M.; Sharma, K.; Nieuwelaar, A.V.; Gossen, B.D. A comparison of clubroot resistance in Brassica vegetable crops. Phytopathology 2012, 102, 78. [Google Scholar]
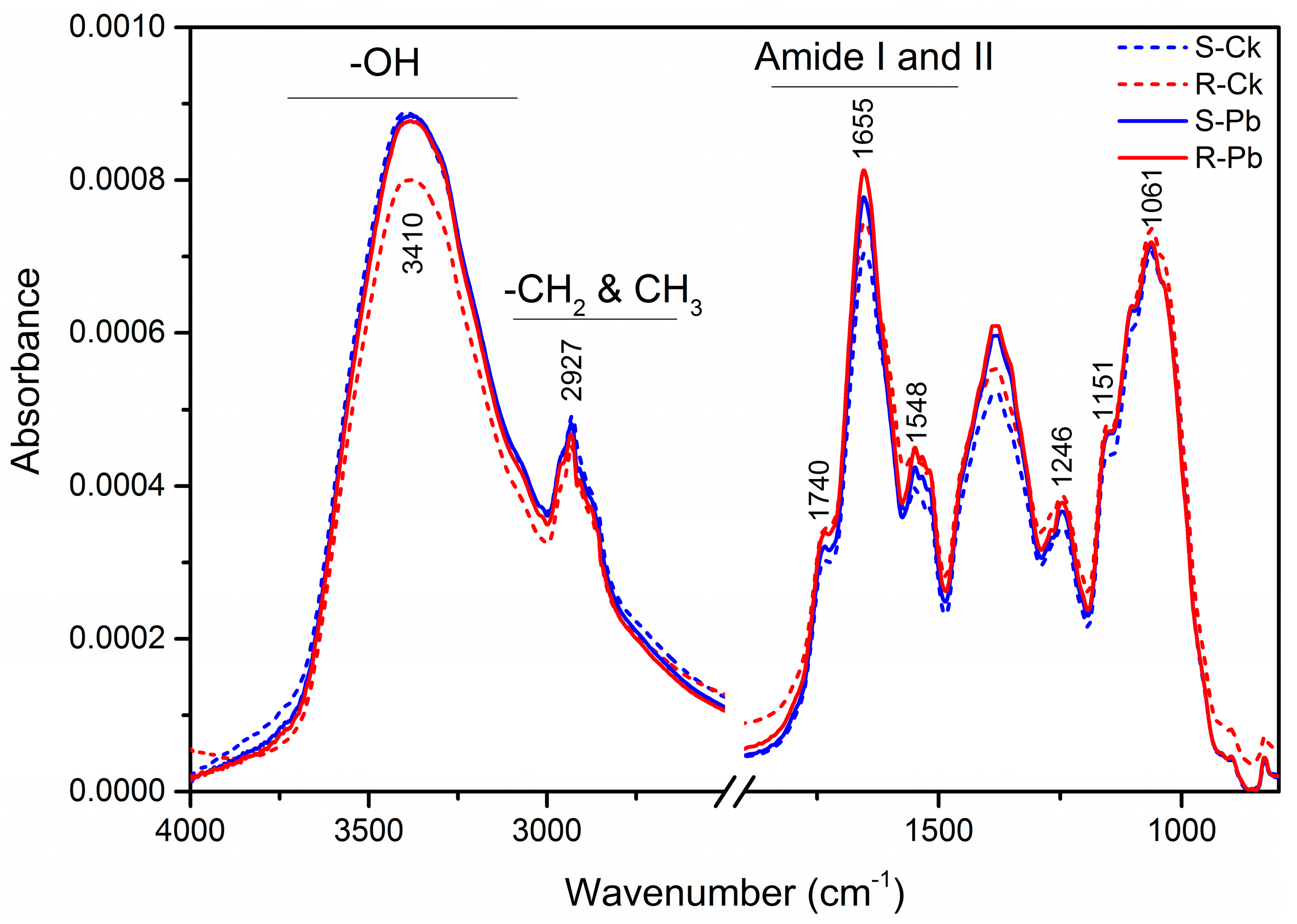
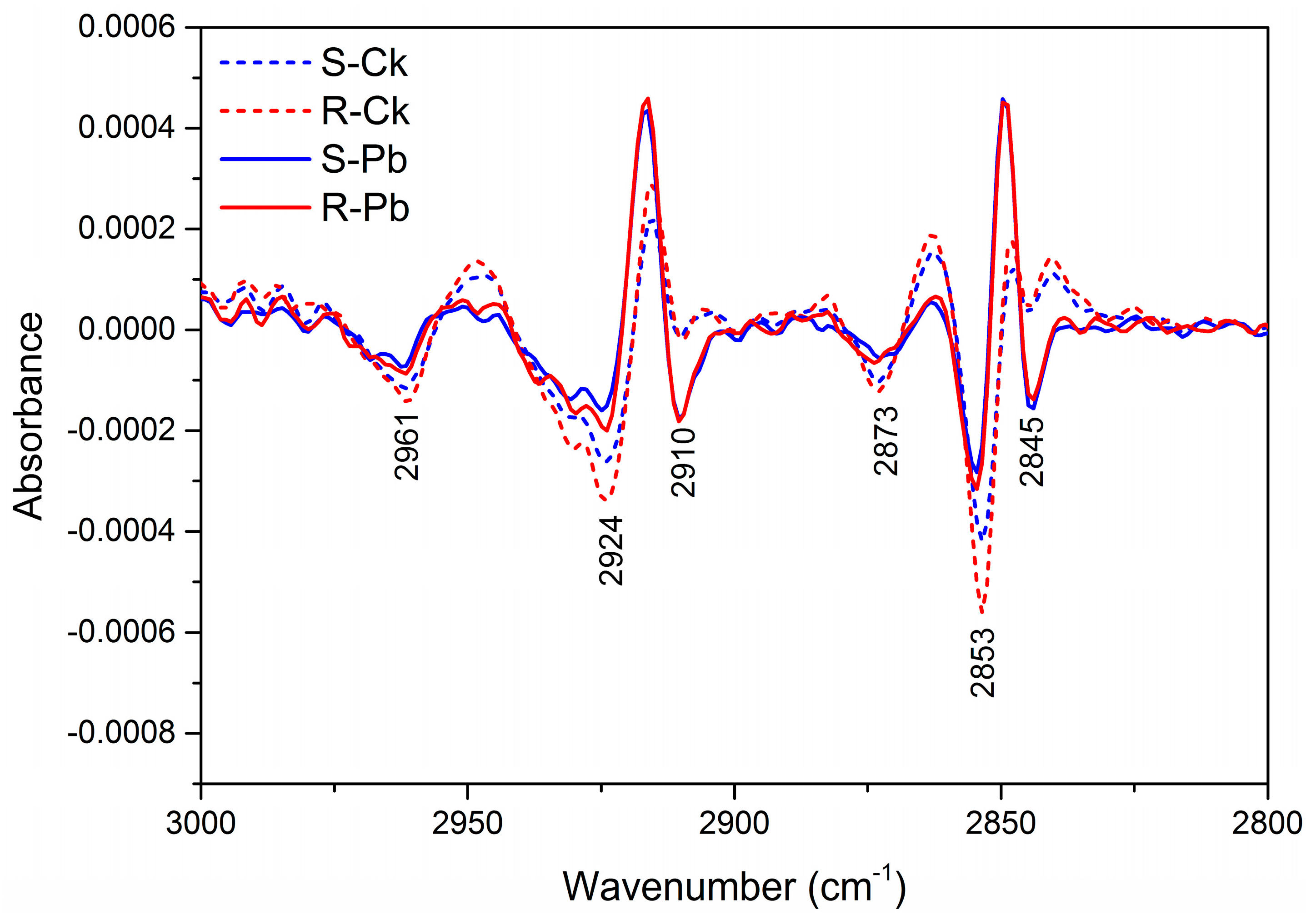
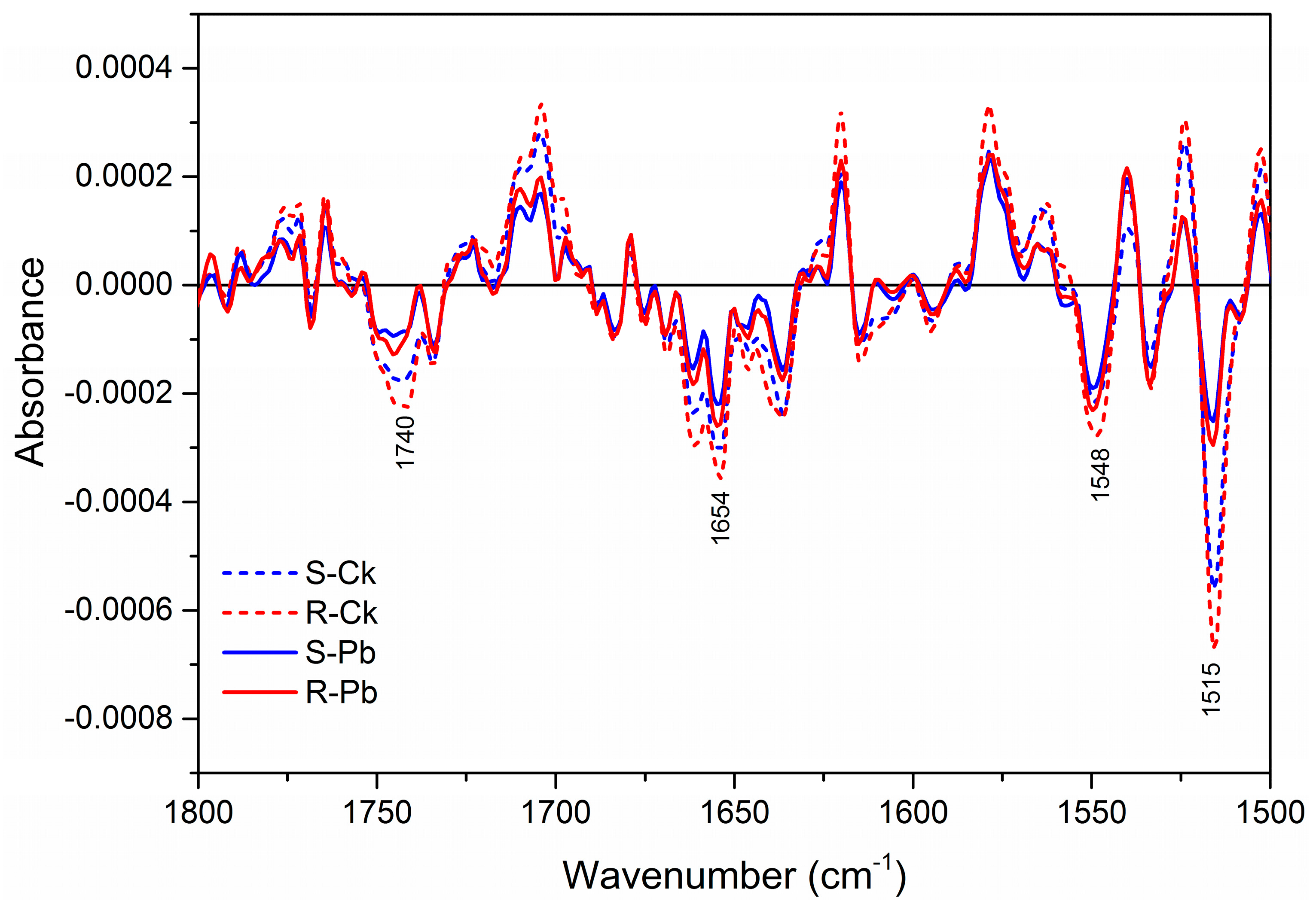
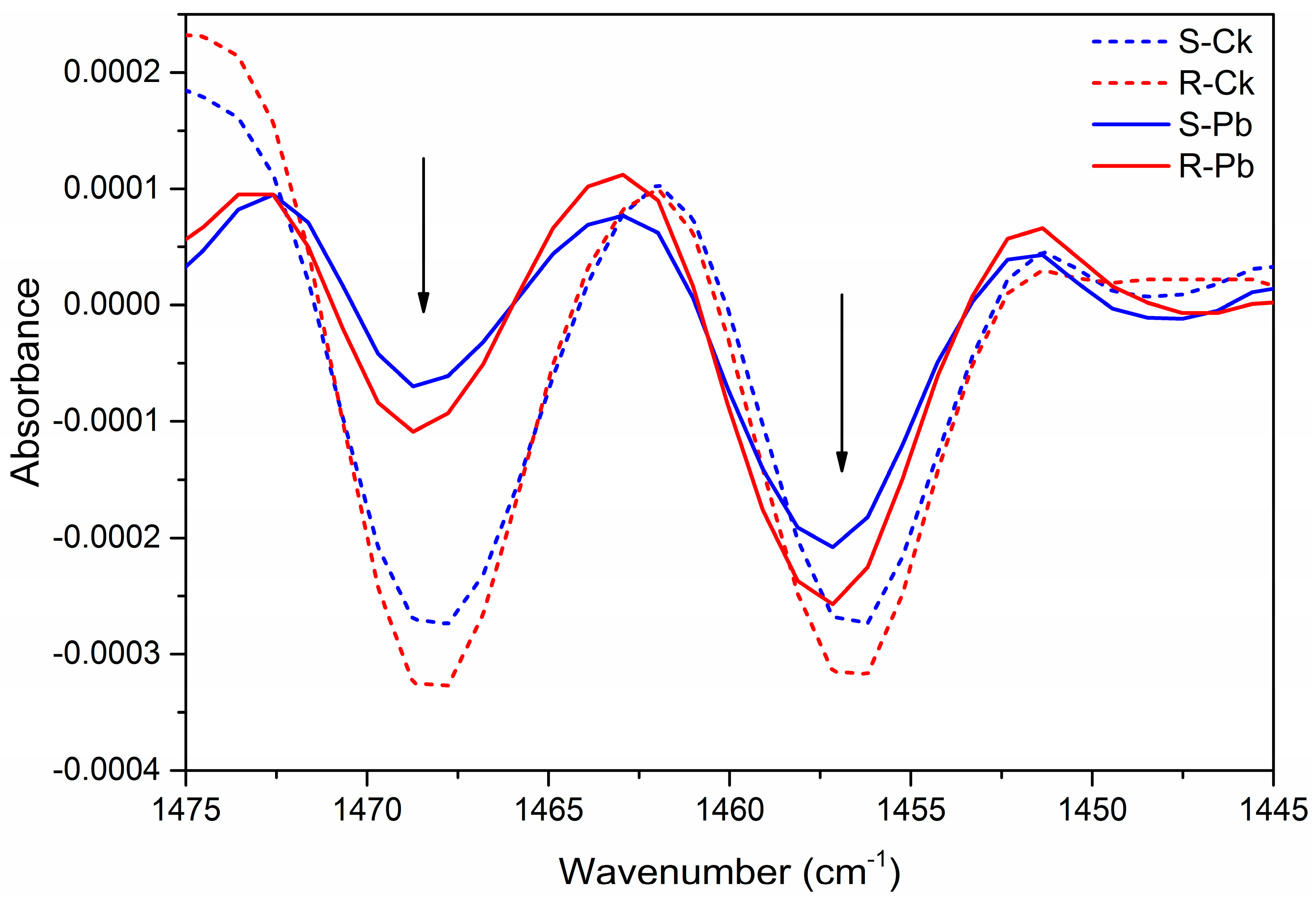

| Wavenumber (cm−1) | Absorption Peak Location and Assignment | Components |
|---|---|---|
| 3410 | –OH groups, NH stretching | Proteins (Amide A) |
| 2927 | C–H stretch (asym.) of CH2 | Lipid acyl chains |
| 1740 | C=O stretching: carbonyl ester compounds | Pectin |
| 1650 | –C=O– and –C=N– stretching | Proteins (Amide I) |
| 1548 | N–H bending and C–N stretching | Proteins (Amide II) |
| 1518 | CH2 and CH3 methylene chain stretching | Lignin |
| 1246 | C–O, –CH2- stretching and bending, P–O stretching | Hemicellulose |
| 1151 | C–O–C asymmetric stretching, PO2 stretching | Cellulose |
| 1061 | C–O–C symmetric stretching | Cellulose |
| Amide A | Acyl Lipids | Carbonyl Ester | Amide I | Amide II | Phenolic Ring | |
|---|---|---|---|---|---|---|
| S-CK | 564.0 ± 7.4a | 34.7± 1.5ab | 10.8 ± 0.5ab | 61.8 ± 1.9a | 14.7 ± 0.7b | 4.1 ± 0.2a |
| S-Pb | 620.3 ± 8.7c | 36.1 ± 1.1b | 10.7 ± 0.5ab | 68.6 ± 1.7c | 15.2 ± 0.7b | 5.3 ± 0.3b |
| R-CK | 597.0 ± 8.8b | 35.6 ± 1.1ab | 9.9 ± 0.5a | 63.8 ± 1.6ab | 15.0 ± 0.6b | 4.1 ± 0.2a |
| R-Pb | 550.9 ± 9.5a | 33.7 ± 1.1a | 11.0 ± 0.4b | 65.7 ± 1.9bc | 13.6 ± 0.5a | 6.1 ± 0.2c |
| Lignin | Hemicellulose | Cellulose | Pectin | |
|---|---|---|---|---|
| S-CK | 3.3 ± 0.2a | 8.3 ± 0.2a | 62.8 ± 2.8b | 0.8 ± 0.0a |
| S-PK | 3.7 ± 0.1b | 8.7 ± 0.2b | 60.7 ± 1.5b | 0.8 ± 0.1a |
| R-CK | 3.2 ± 0.1a | 8.2 ± 0.2a | 64.0 ± 2.4b | 0.8 ± 0.1a |
| R-Pb | 4.0 ± 0.1c | 8.7 ± 0.2b | 55.3 ± 2.5a | 0.9 ± 0.1b |
| Non-Inoculated (Ck) | Inoculated (Pb) | |||
|---|---|---|---|---|
| S | R | S | R | |
| β-sheet (PAL) 1 | 46.4c 2 | 44.2b | 43.5a | 48.3d |
| α-helices | 24.2c | 22.0b | 26.0d | 21.5a |
| Others | 29.5a | 33.8d | 30.4c | 30.2b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahlali, R.; Song, T.; Chu, M.; Yu, F.; Kumar, S.; Karunakaran, C.; Peng, G. Evaluating Changes in Cell-Wall Components Associated with Clubroot Resistance Using Fourier Transform Infrared Spectroscopy and RT-PCR. Int. J. Mol. Sci. 2017, 18, 2058. https://doi.org/10.3390/ijms18102058
Lahlali R, Song T, Chu M, Yu F, Kumar S, Karunakaran C, Peng G. Evaluating Changes in Cell-Wall Components Associated with Clubroot Resistance Using Fourier Transform Infrared Spectroscopy and RT-PCR. International Journal of Molecular Sciences. 2017; 18(10):2058. https://doi.org/10.3390/ijms18102058
Chicago/Turabian StyleLahlali, Rachid, Tao Song, Mingguang Chu, Fengqun Yu, Saroj Kumar, Chithra Karunakaran, and Gary Peng. 2017. "Evaluating Changes in Cell-Wall Components Associated with Clubroot Resistance Using Fourier Transform Infrared Spectroscopy and RT-PCR" International Journal of Molecular Sciences 18, no. 10: 2058. https://doi.org/10.3390/ijms18102058







