Clinically Isolated Syndrome According to McDonald 2010: Intrathecal IgG Synthesis Still Predictive for Conversion to Multiple Sclerosis
Abstract
:1. Introduction
2. Results
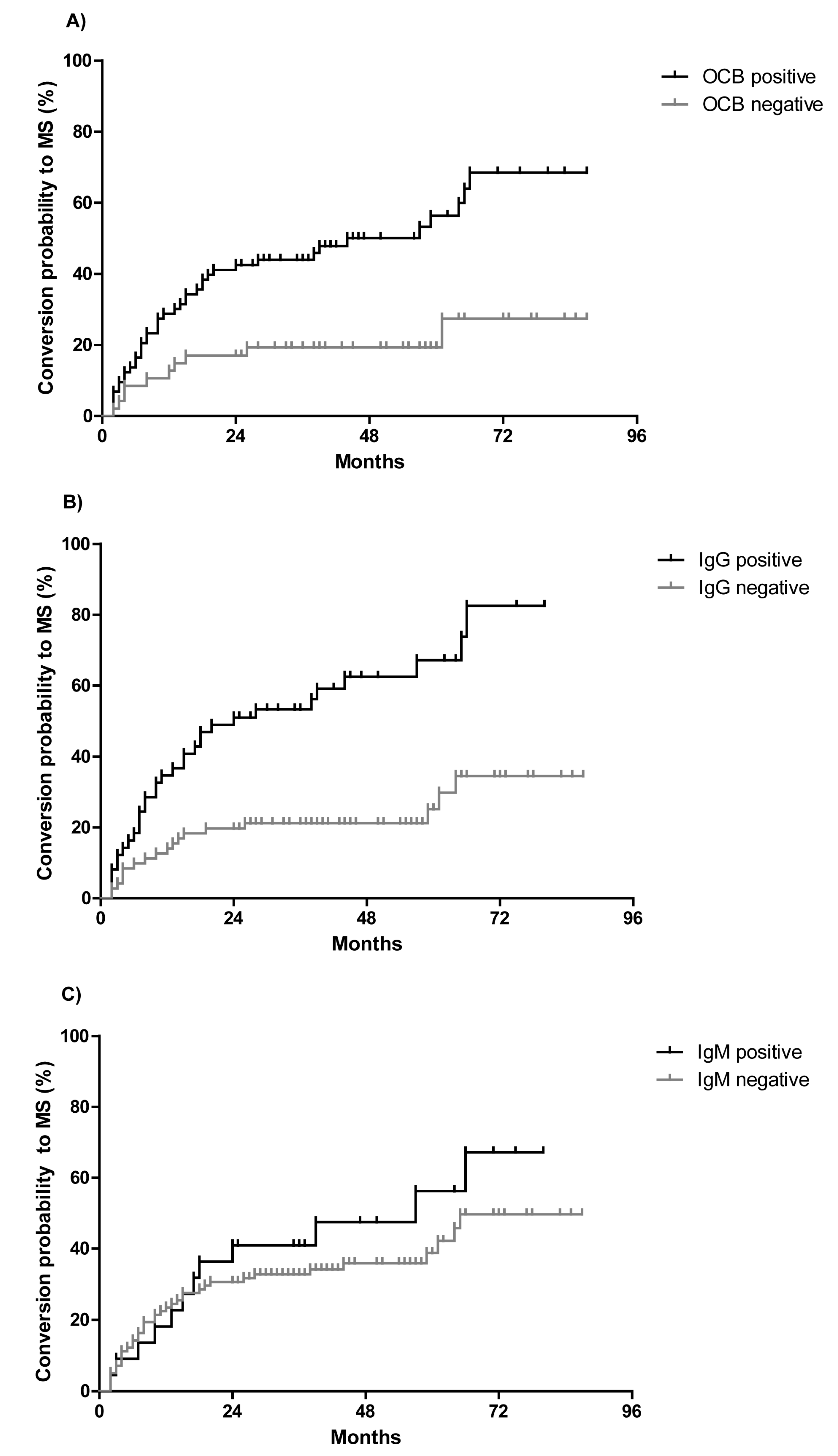
2.1. CSF Parameters Predict Conversion to Multiple Sclerosis (MS) in Clinically Isolated Syndrome (CIS) Patients
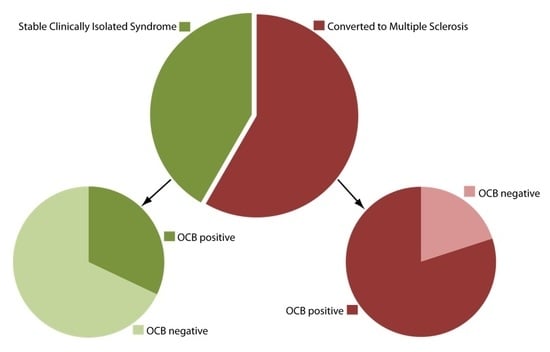
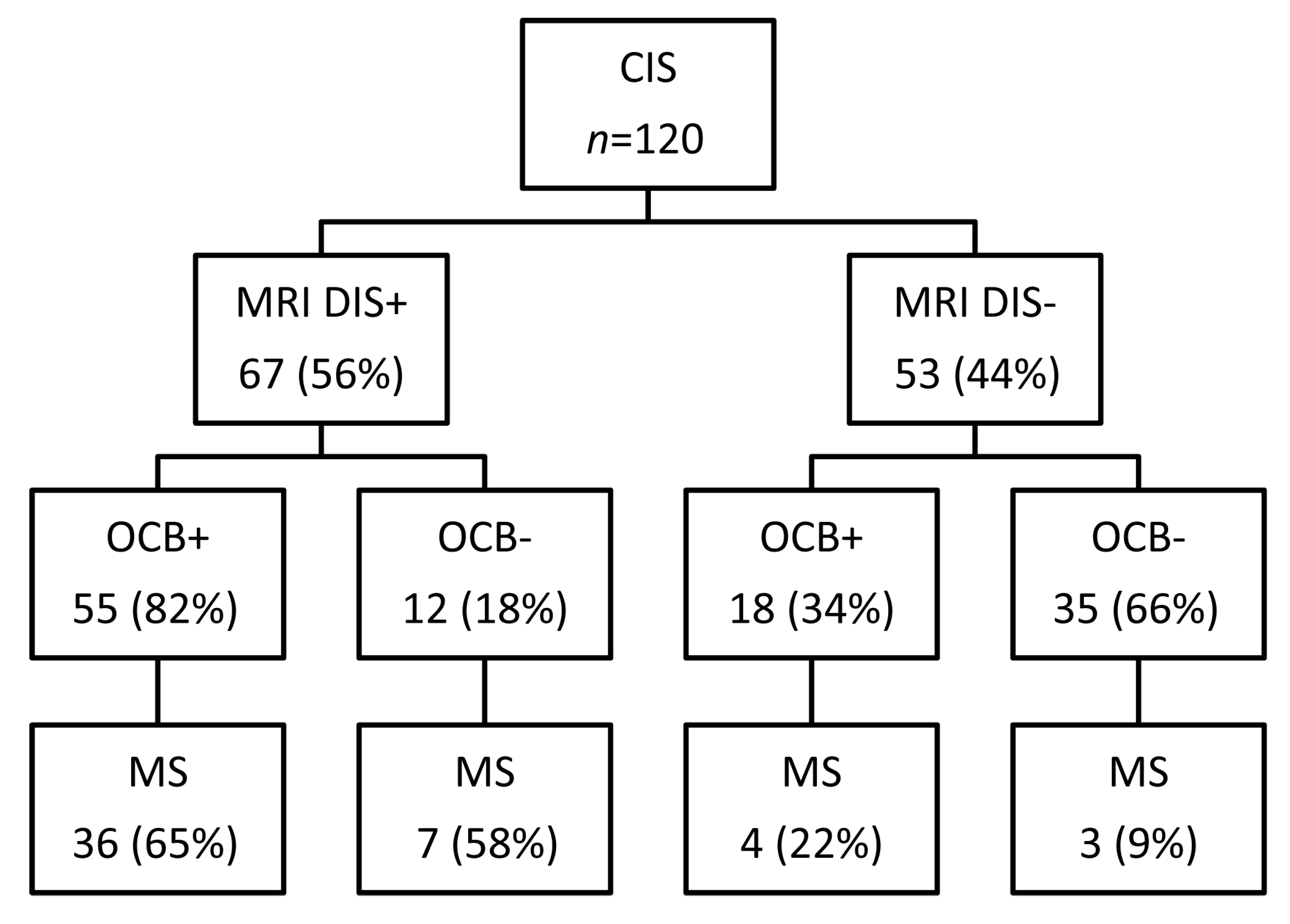
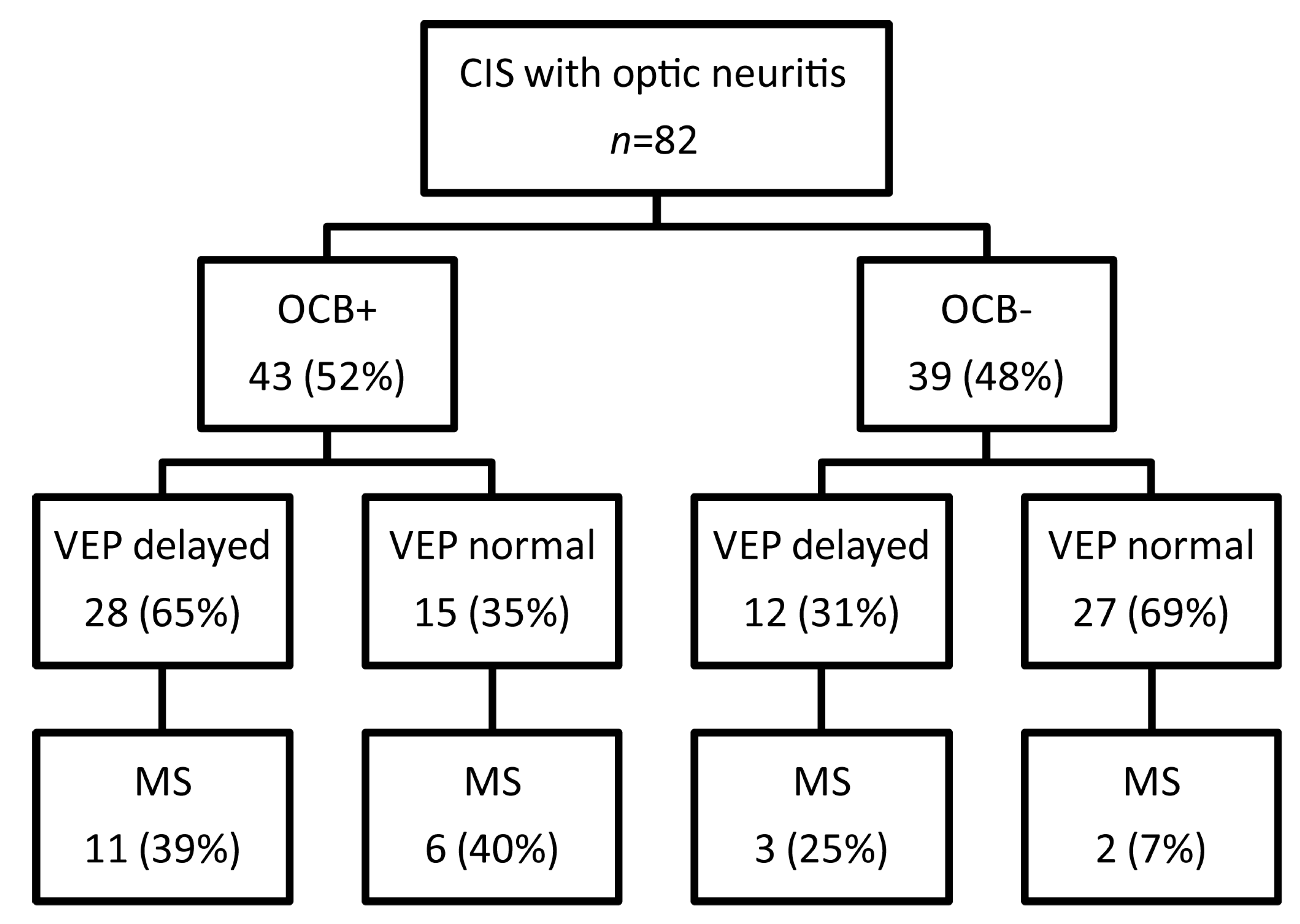
2.2. OCB Predict the Conversion Rate to MS in CIS Patients Who do not Fulfill MRI Dissemination in Space
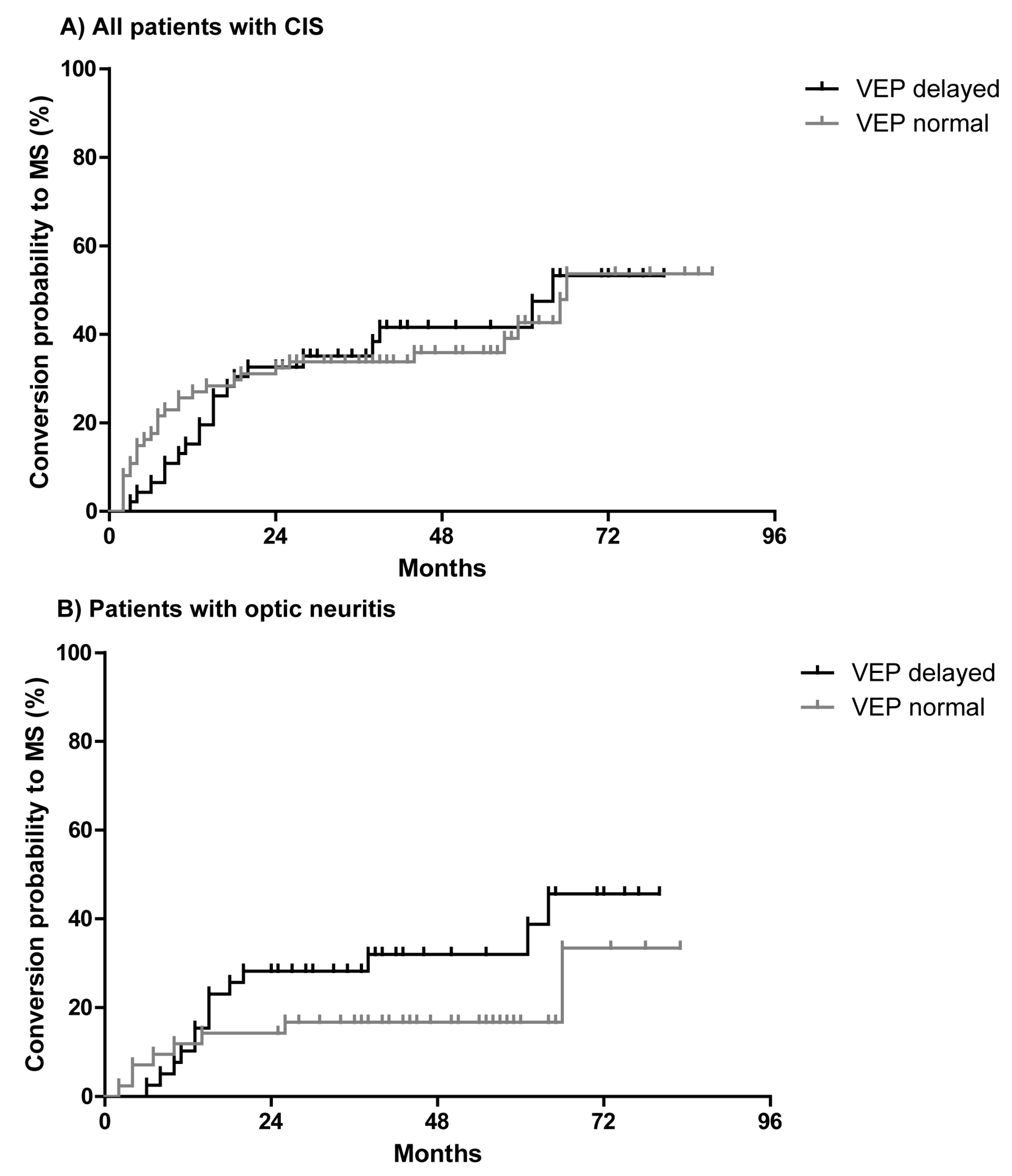
2.3. The Predictive Role of Visual Evoked Potentials for Conversion of CIS to MS
3. Discussion
4. Methods
4.1. Patients
4.2. CSF and Serum Analytical Procedures
4.3. Magnetic Resonance Imaging (MRI)
4.4. Visual Evoked Potentials
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of interest
References
- Miller, D.H.; Chard, D.T.; Ciccarelli, O. Clinically isolated syndromes. Lancet Neurol. 2012, 11, 157–169. [Google Scholar] [CrossRef]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2002, 359, 1221–1231. [Google Scholar] [CrossRef]
- Kuhle, J.; Disanto, G.; Dobson, R.; Adiutori, R.; Bianchi, L.; Topping, J.; Bestwick, J.P.; Meier, U.C.; Marta, M.; Dalla Costa, G.; et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult. Scler. 2015, 21, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Fisniku, L.K.; Brex, P.A.; Altmann, D.R.; Miszkiel, K.A.; Benton, C.E.; Lanyon, R.; Thompson, A.J.; Miller, D.H. Disability and T2 MRI lesions: A 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 2008, 131, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Polman, C.H.; Freedman, M.S.; Edan, G.; Hartung, H.P.; Miller, D.H.; Montalban, X.; Barkhof, F.; Bauer, L.; Jakobs, P.; et al. Treatment with interferon β-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006, 67, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Edan, G.; Kappos, L.; Montalban, X.; Polman, C.H.; Freedman, M.S.; Hartung, H.P.; Miller, D.; Barkhof, F.; Herrmann, J.; Lanius, V.; et al. Long-term impact of interferon β-1b in patients with cis: 8-year follow-up of benefit. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1183–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deangelis, T.M.; Miller, A. Diagnosis of multiple sclerosis. Handb. Clin. Neurol. 2014, 122, 317–342. [Google Scholar] [PubMed]
- Chirapapaisan, N.; Laotaweerungsawat, S.; Chuenkongkaew, W.; Samsen, P.; Ruangvaravate, N.; Thuangtong, A.; Chanvarapha, N. Diagnostic value of visual evoked potentials for clinical diagnosis of multiple sclerosis. Doc. Ophthalmol. Adv. Ophthalmol. 2015, 130, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, P.; Borggrefe-Chappuis, A.; Schindler, C.; Kappos, L. Visual and motor evoked potentials in the course of multiple sclerosis. Brain 2001, 124, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Leocani, L.; Rocca, M.A.; Comi, G. Mri and neurophysiological measures to predict course, disability and treatment response in multiple sclerosis. Curr. Opin. Neurol. 2016, 29, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Schlaeger, R.; D’Souza, M.; Schindler, C.; Grize, L.; Kappos, L.; Fuhr, P. Combined evoked potentials as markers and predictors of disability in early multiple sclerosis. Clin. Neurophysiol. 2012, 123, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Schlaeger, R.; Schindler, C.; Grize, L.; Dellas, S.; Radue, E.W.; Kappos, L.; Fuhr, P. Combined visual and motor evoked potentials predict multiple sclerosis disability after 20 years. Mult. Scler. 2014, 20, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Link, H.; Huang, Y.M. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: An update on methodology and clinical usefulness. J. Neuroimmunol. 2006, 180, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Huss, A.M.; Halbgebauer, S.; Ockl, P.; Trebst, C.; Spreer, A.; Borisow, N.; Harrer, A.; Brecht, I.; Balint, B.; Stich, O.; et al. Importance of cerebrospinal fluid analysis in the era of McDonald 2010 criteria: A German-Austrian retrospective multicenter study in patients with a clinically isolated syndrome. J. Neurol. 2016, 263, 2499–2504. [Google Scholar] [CrossRef] [PubMed]
- Schwenkenbecher, P.; Sarikidi, A.; Wurster, U.; Bronzlik, P.; Suhs, K.W.; Raab, P.; Stangel, M.; Pul, R.; Skripuletz, T. Mcdonald criteria 2010 and 2005 compared: Persistence of high oligoclonal band prevalence despite almost doubled diagnostic sensitivity. Int. J. Mol. Sci. 2016, 17, 1592. [Google Scholar] [CrossRef] [PubMed]
- Tintore, M.; Rovira, A.; Rio, J.; Otero-Romero, S.; Arrambide, G.; Tur, C.; Comabella, M.; Nos, C.; Arevalo, M.J.; Negrotto, L.; et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015, 138, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Tintore, M.; Rovira, A.; Rio, J.; Tur, C.; Pelayo, R.; Nos, C.; Tellez, N.; Perkal, H.; Comabella, M.; Sastre-Garriga, J.; et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology 2008, 70, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Sharief, M.K.; Thompson, E.J. The predictive value of intrathecal immunoglobulin synthesis and magnetic resonance imaging in acute isolated syndromes for subsequent development of multiple sclerosis. Ann. Neurol. 1991, 29, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Ozakbas, S.; Cinar, B.P.; Ozcelik, P.; Baser, H.; Kosehasanogullari, G. Intrathecal igm index correlates with a severe disease course in multiple sclerosis: Clinical and MRI results. Clin. Neurol. Neurosurg. 2017, 160, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Keir, G.; Thompson, E.J.; Giovannoni, G. The clinical significance of an intrathecal monoclonal immunoglobulin band: A follow-up study. Neurology 2003, 60, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Zeman, A.Z.; Kidd, D.; McLean, B.N.; Kelly, M.A.; Francis, D.A.; Miller, D.H.; Kendall, B.E.; Rudge, P.; Thompson, E.J.; McDonald, W.I. A study of oligoclonal band negative multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1996, 60, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Comabella, M.; Fernandez, M.; Martin, R.; Rivera-Vallve, S.; Borras, E.; Chiva, C.; Julia, E.; Rovira, A.; Canto, E.; Alvarez-Cermeno, J.C.; et al. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain 2010, 133, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Corvol, J.C.; Pelletier, D.; Henry, R.G.; Caillier, S.J.; Wang, J.; Pappas, D.; Casazza, S.; Okuda, D.T.; Hauser, S.L.; Oksenberg, J.R.; et al. Abrogation of T cell quiescence characterizes patients at high risk for multiple sclerosis after the initial neurological event. Proc. Natl. Acad. Sci. USA 2008, 105, 11839–11844. [Google Scholar] [CrossRef] [PubMed]
- Lunemann, J.D.; Tintore, M.; Messmer, B.; Strowig, T.; Rovira, A.; Perkal, H.; Caballero, E.; Munz, C.; Montalban, X.; Comabella, M. Elevated epstein-barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann. Neurol. 2010, 67, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Masjuan, J.; Alvarez-Cermeno, J.C.; Garcia-Barragan, N.; Diaz-Sanchez, M.; Espino, M.; Sadaba, M.C.; Gonzalez-Porque, P.; Martinez San Millan, J.; Villar, L.M. Clinically isolated syndromes: A new oligoclonal band test accurately predicts conversion to MS. Neurology 2006, 66, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Tumani, H.; Kiechle, U.; Muche, R.; Richards, G.; Lehmensiek, V.; Ludolph, A.C.; Otto, M. Igg antibodies against measles, rubella, and varicella zoster virus predict conversion to multiple sclerosis in clinically isolated syndrome. PLoS ONE 2009, 4, e7638. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.M.; Spencer, S.; Lazarini, J.; Harp, C.T.; Ward, E.S.; Burgoon, M.; Owens, G.P.; Racke, M.K.; Bennett, J.L.; Frohman, E.M.; et al. Potential of a unique antibody gene signature to predict conversion to clinically definite multiple sclerosis. J. Neuroimmunol. 2009, 213, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Mowry, E.M.; Pesic, M.; Grimes, B.; Deen, S.R.; Bacchetti, P.; Waubant, E. Clinical predictors of early second event in patients with clinically isolated syndrome. J. Neurol. 2009, 256, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.; Kappos, L.; Freedman, M.S.; Edan, G.; Hartung, H.P.; Miller, D.H.; Montalban, X.; Barkhof, F.; Selmaj, K.; Uitdehaag, B.M.; et al. Subgroups of the BENEFIT study: Risk of developing MS and treatment effect of interferon β-1b. J. Neurol. 2008, 255, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tintore, M.; Rovira, A.; Rio, J.; Nos, C.; Grive, E.; Tellez, N.; Pelayo, R.; Comabella, M.; Montalban, X. Is optic neuritis more benign than other first attacks in multiple sclerosis? Ann. Neurol. 2005, 57, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, W.J.; Miller, D.H. Clinically isolated syndromes and the relationship to multiple sclerosis. J. Clin. Neurosci. 2014, 21, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Toosy, A.T.; Mason, D.F.; Miller, D.H. Optic neuritis. Lancet Neurol. 2014, 13, 83–99. [Google Scholar] [CrossRef]
- Alshowaeir, D.; Yiannikas, C.; Garrick, R.; Parratt, J.; Barnett, M.H.; Graham, S.L.; Klistorner, A. Latency of multifocal visual evoked potentials in nonoptic neuritis eyes of multiple sclerosis patients associated with optic radiation lesions. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3758–3764. [Google Scholar] [CrossRef] [PubMed]
- Klistorner, A.; Garrick, R.; Barnett, M.H.; Graham, S.L.; Arvind, H.; Sriram, P.; Yiannikas, C. Axonal loss in non-optic neuritis eyes of patients with multiple sclerosis linked to delayed visual evoked potential. Neurology 2013, 80, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Naismith, R.T.; Tutlam, N.T.; Xu, J.; Shepherd, J.B.; Klawiter, E.C.; Song, S.K.; Cross, A.H. Optical coherence tomography is less sensitive than visual evoked potentials in optic neuritis. Neurology 2009, 73, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Sriram, P.; Wang, C.; Yiannikas, C.; Garrick, R.; Barnett, M.; Parratt, J.; Graham, S.L.; Arvind, H.; Klistorner, A. Relationship between optical coherence tomography and electrophysiology of the visual pathway in non-optic neuritis eyes of multiple sclerosis patients. PLoS ONE 2014, 9, e102546. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, R.; Ahmed, S.; Al-Hashel, J.; Rousseff, R.T.; Alroughani, R. Sensitivity of visual evoked potentials and spectral domain optical coherence tomography in early relapsing remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 12, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Galetta, K.M.; Balcer, L.J. Measures of visual pathway structure and function in ms: Clinical usefulness and role for MS trials. Mult. Scler. Relat. Disord. 2013, 2, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Ellidag, H.Y.; Eren, E.; Erdogan, N.; Ture, S.; Yilmaz, N. Comparison of neurophysiological and MRI findings of patients with multiple sclerosis using oligoclonal band technique. Ann. Neurosci. 2013, 20, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Schwenkenbecher, P.; Chacko, L.P.; Wurster, U.; Pars, K.; Pul, R.; Suhs, K.W.; Stangel, M.; Skripuletz, T. Intrathecal synthesis of anti-Hu antibodies distinguishes patients with paraneoplastic peripheral neuropathy and encephalitis. BMC Neurol. 2016, 16, 136. [Google Scholar] [CrossRef] [PubMed]
- Skripuletz, T.; Schwenkenbecher, P.; Pars, K.; Stoll, M.; Conzen, J.; Bolat, S.; Pul, R.; Vonberg, R.P.; Sedlacek, L.; Wurster, U.; et al. Importance of follow-up cerebrospinal fluid analysis in cryptococcal meningoencephalitis. Dis. Mark. 2014, 2014, 162576. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H. Cerebrospinal fluid—Physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult. Scler. 1998, 4, 99–107. [Google Scholar] [PubMed]
- Reiber, H. External quality assessment in clinical neurochemistry: Survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin. Chem. 1995, 41, 256–263. [Google Scholar] [PubMed]
- Swanton, J.K.; Fernando, K.; Dalton, C.M.; Miszkiel, K.A.; Thompson, A.J.; Plant, G.T.; Miller, D.H. Modification of MRI criteria for multiple sclerosis in patients with clinically isolated syndromes. J. Neurol. Neurosurg. Psychiatry 2006, 77, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.T.; Liu, G.T.; Lavery, A.M.; Liu, G.; Gaetz, W.; Aleman, T.S.; Banwell, B.L. Optical coherence tomography and visual evoked potentials in pediatric MS. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e356. [Google Scholar] [CrossRef] [PubMed]
- Giffroy, X.; Maes, N.; Albert, A.; Maquet, P.; Crielaard, J.M.; Dive, D. Multimodal evoked potentials for functional quantification and prognosis in multiple sclerosis. BMC Neurol. 2016, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Kallmann, B.A.; Fackelmann, S.; Toyka, K.V.; Rieckmann, P.; Reiners, K. Early abnormalities of evoked potentials and future disability in patients with multiple sclerosis. Mult. Scler. 2006, 12, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Joshi, S.; Singh, K.D.; Kumar, A. Visual evoked potentials: Normative values and gender differences. J. Clin. Diagn. Res. 2015, 9, CC12–CC15. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Converted to MS (n = 50) | Stable CIS (n = 70) | p-Value |
|---|---|---|---|
| Age, median (range) | 33 (17–73) | 35 (16–60) | 0.33 |
| Females | 33/50 (66%) | 45/70 (64%) | 0.65 |
| Optic neuritis | 22/82 (27%) | 60/82 (73%) | 0.0001 |
| Paresis/sensory symptoms | 4/7 (57%) | 3/7 (43%) | 0.07 |
| Brainstem symptoms | 9/13 (69%) | 4/13 (31%) | 0.0001 |
| Spinal cord symptoms | 15/18 (83%) | 3/18 (17%) | 0.0001 |
| CSF Findings | Converted to MS (n = 50) | Stable CIS (n = 70) | p-Value |
|---|---|---|---|
| CSF oligoclonal bands | 40/50 (80%) | 33/70 (47%) | <0.0001 |
| Intrathecal synthesis (Reiber graphs) | 32/50 (64%) | 18/70 (26%) | <0.0001 |
| IgG | 32/50 (64%) | 16/70 (23%) | <0.0001 |
| IgM | 12/50 (24%) | 10/70 (14%) | 0.10 |
| IgA | 1/50 (2%) | 3/70 (4%) | 0.68 |
| Pleocytosis (≥5 cells/µl) | 38/50 (76%) | 26/70 (37%) | <0.0001 |
| Lactate (>3.5 mmol/L) | 0 (0%) | 0 (0%) | 1 |
| Protein (>500 mg/L) | 13/50 (26%) | 14/70 (20%) | 0.31 |
| Blood-CSF-barrier dysfunction | 12/50 (24%) | 17/70 (24%) | 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwenkenbecher, P.; Sarikidi, A.; Bönig, L.; Wurster, U.; Bronzlik, P.; Sühs, K.-W.; Pul, R.; Stangel, M.; Skripuletz, T. Clinically Isolated Syndrome According to McDonald 2010: Intrathecal IgG Synthesis Still Predictive for Conversion to Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 2061. https://doi.org/10.3390/ijms18102061
Schwenkenbecher P, Sarikidi A, Bönig L, Wurster U, Bronzlik P, Sühs K-W, Pul R, Stangel M, Skripuletz T. Clinically Isolated Syndrome According to McDonald 2010: Intrathecal IgG Synthesis Still Predictive for Conversion to Multiple Sclerosis. International Journal of Molecular Sciences. 2017; 18(10):2061. https://doi.org/10.3390/ijms18102061
Chicago/Turabian StyleSchwenkenbecher, Philipp, Anastasia Sarikidi, Lena Bönig, Ulrich Wurster, Paul Bronzlik, Kurt-Wolfram Sühs, Refik Pul, Martin Stangel, and Thomas Skripuletz. 2017. "Clinically Isolated Syndrome According to McDonald 2010: Intrathecal IgG Synthesis Still Predictive for Conversion to Multiple Sclerosis" International Journal of Molecular Sciences 18, no. 10: 2061. https://doi.org/10.3390/ijms18102061