Cross-Linked Amylose Bio-Plastic: A Transgenic-Based Compostable Plastic Alternative
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Lipid Content
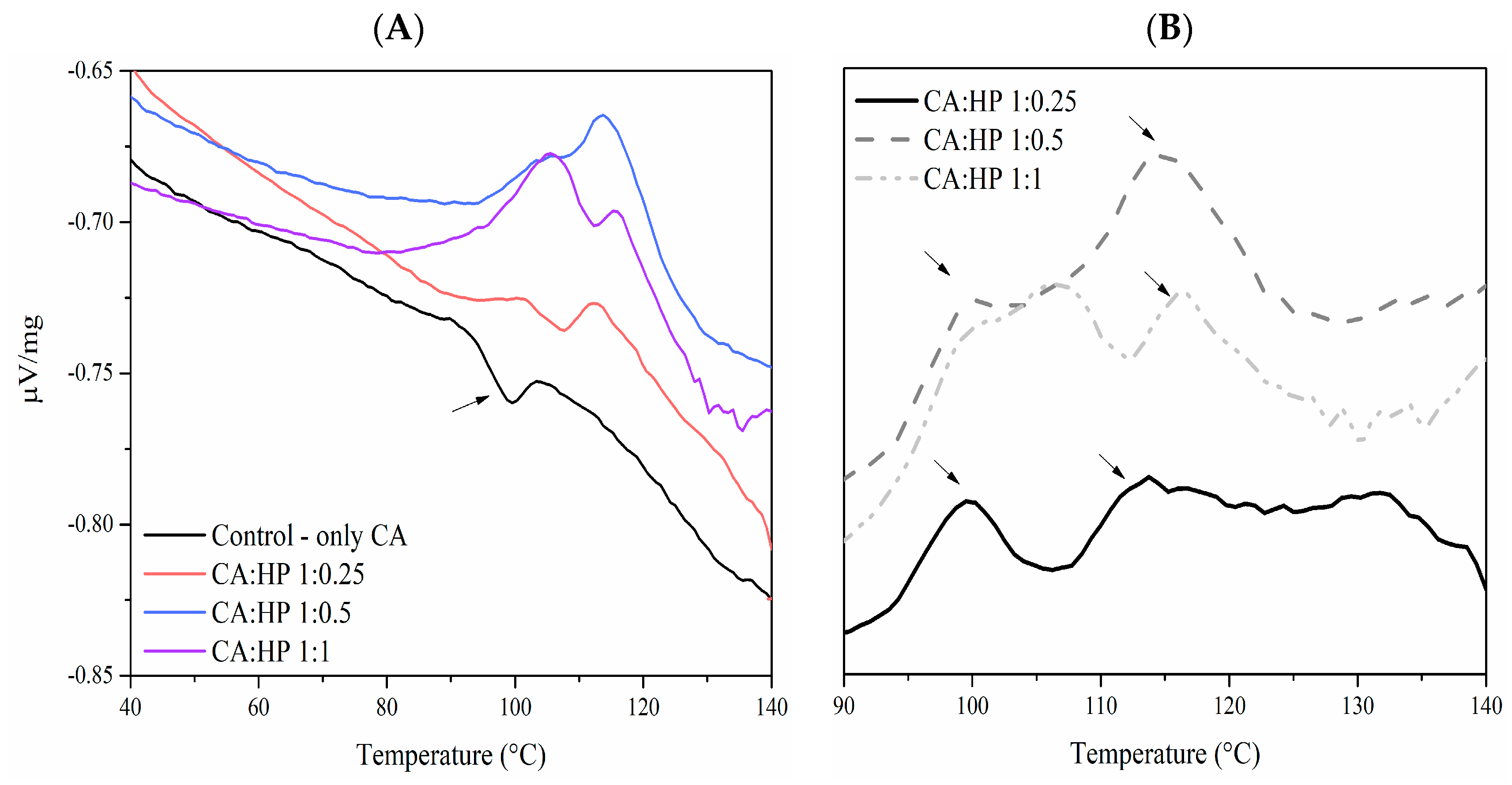
2.2. Monitoring of Cross-Linking Reaction
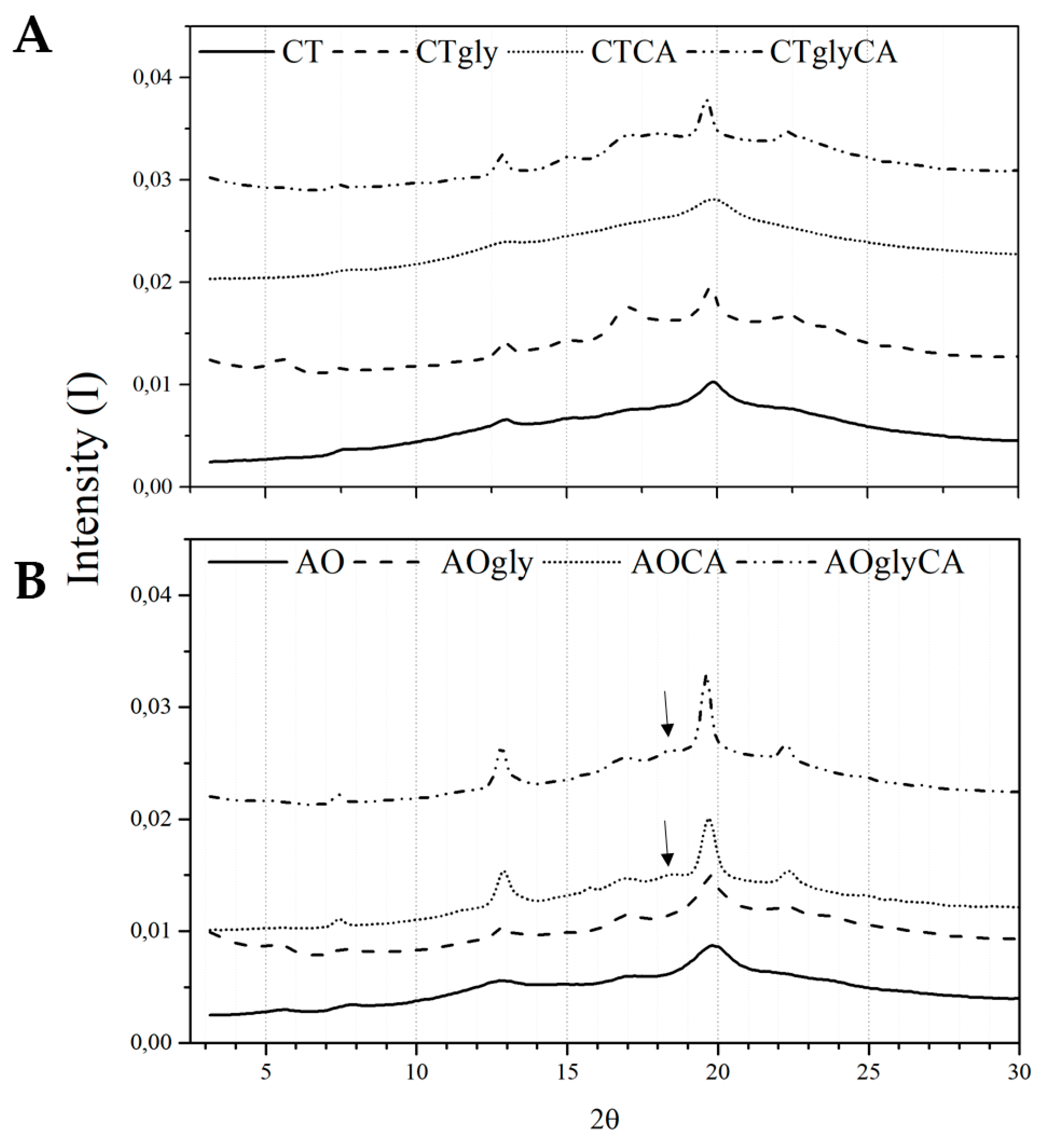
2.3. Crystalline Properties of the Extrudates
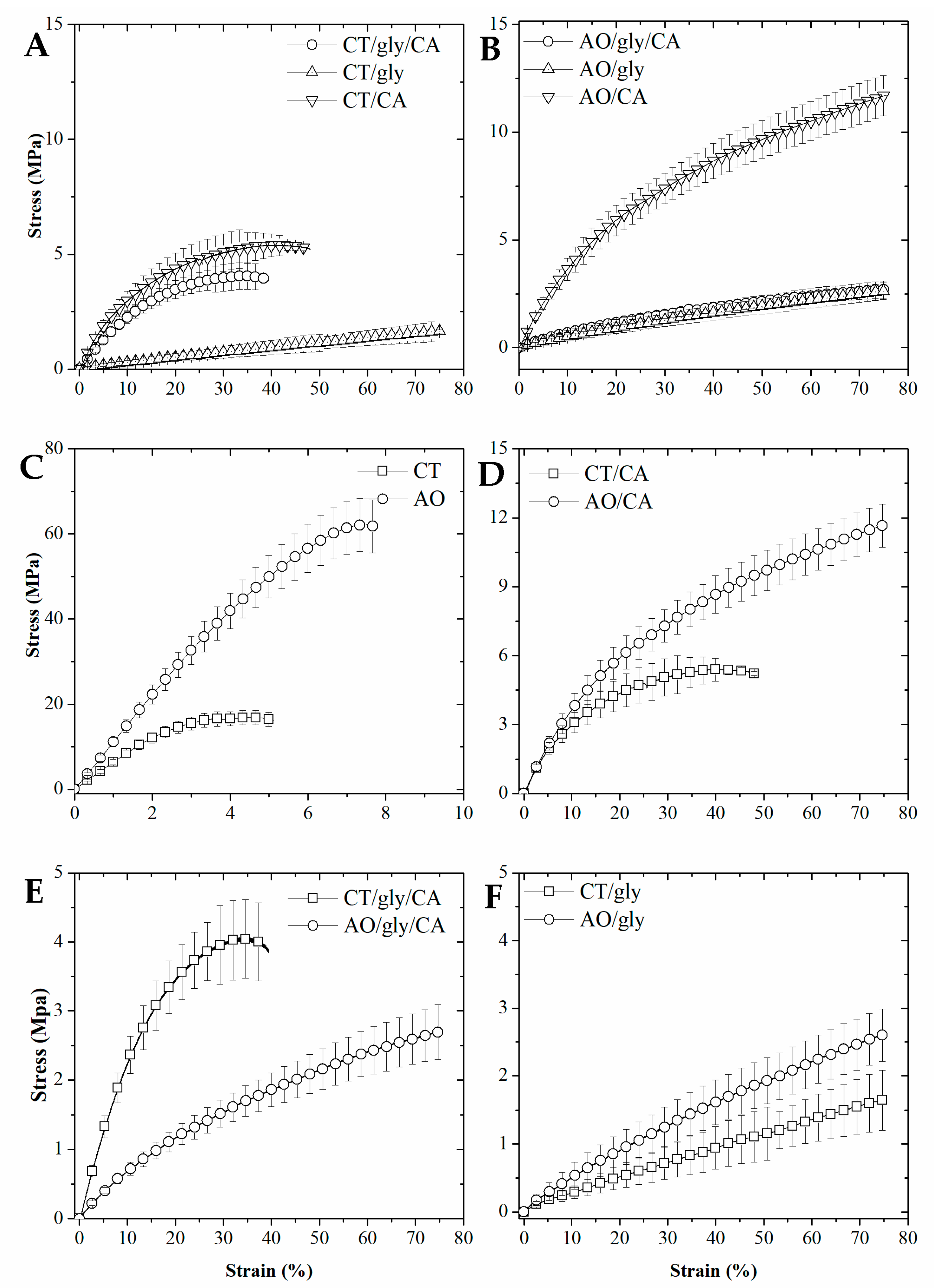
2.4. Mechanical Properties and Glass Transition
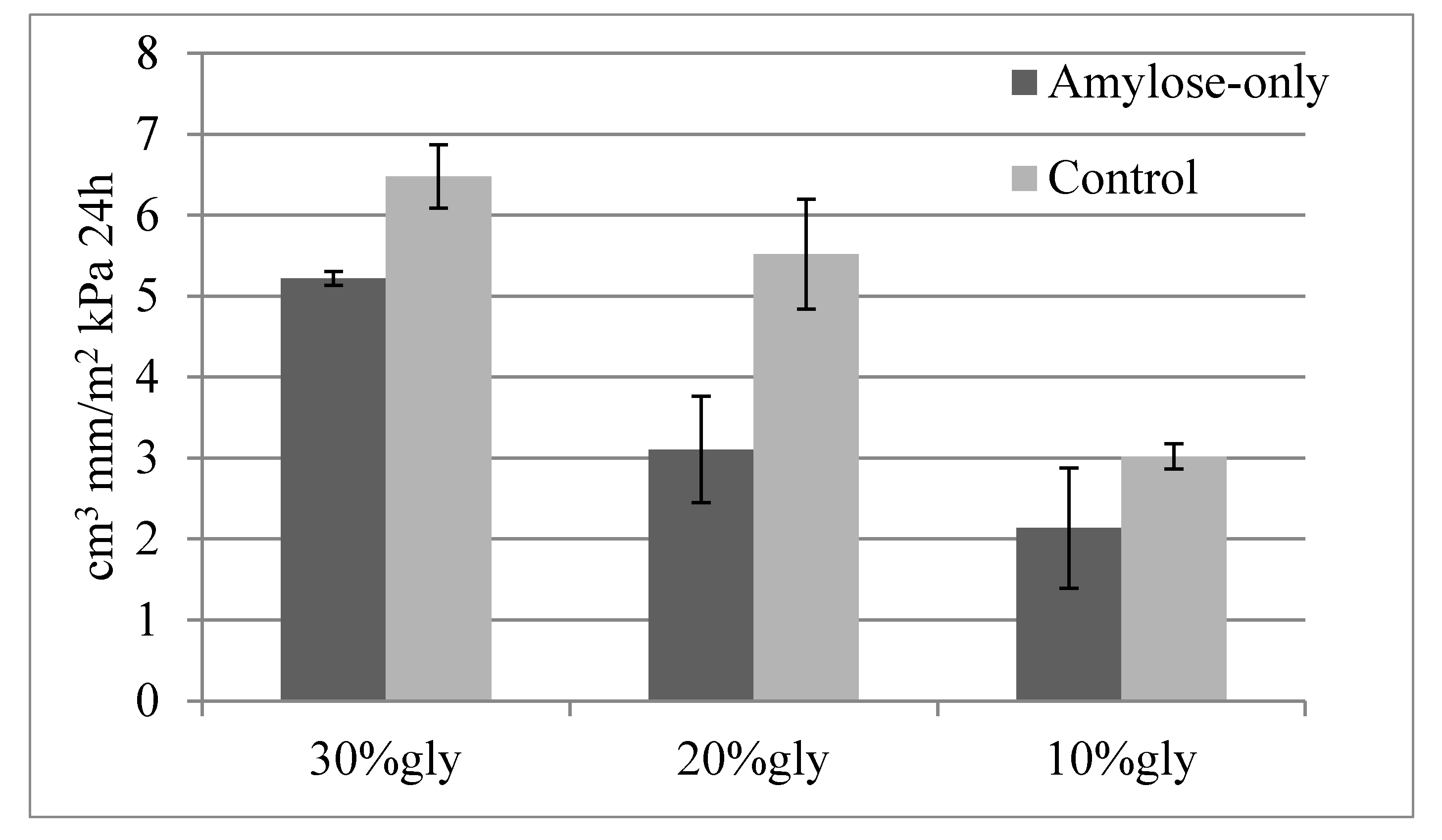
2.5. Permeability Tests
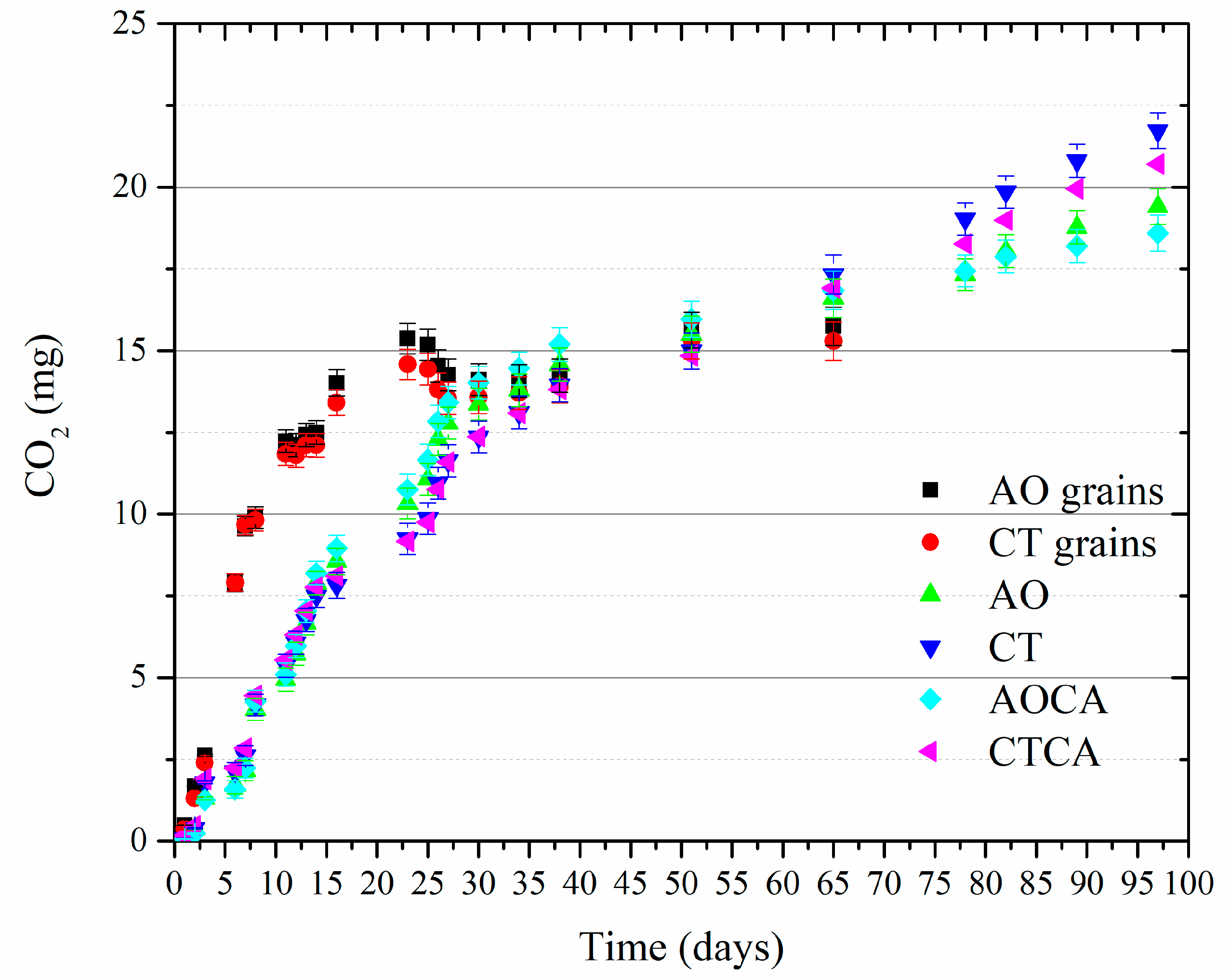
2.6. Biodegradation of Grains and Extruded Prototypes
3. Materials and Method
3.1. Materials
3.2. Methods
3.2.1. Determination of Lipids
3.2.2. Cross-Linking Evaluation by Differential Scanning Calorimetry
3.2.3. Melt Processing
3.2.4. Fourier Transform Infrared Spectroscopy
3.2.5. Wide-Angle X-ray Scattering
3.2.6. Mechanical Properties
3.2.7. Dynamic Mechanical Analysis
3.2.8. Permeability to Gases
3.2.9. Biodegradation Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Commission. On a European Strategy on Plastic Waste in the Environment; European Commission: Brussels, Belgium, 2003. [Google Scholar]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. Engl. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.B.; Quinn, B. 5-Microplastics, standardisation and spatial distribution. In Microplastic Pollutants; Elsevier: Kidlington, UK, 2017; pp. 101–130. [Google Scholar]
- Crawford, C.B.; Quinn, B. 7-The biological impacts and effects of contaminated microplastics. In Microplastic Pollutants; Elsevier: Kidlington, UK, 2017; pp. 159–178. [Google Scholar]
- Sagnelli, D.; Hebelstrup, K.H.; Leroy, E.; Rolland-Sabaté, A.; Guilois, S.; Kirkensgaard, J.J.K.; Mortensen, K.; Lourdin, D.; Blennow, A. Plant-crafted starches for bioplastics production. Carbohydr. Polym. 2016, 152, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Jantanasakulwong, K.; Leksawasdi, N.; Seesuriyachan, P.; Wongsuriyasak, S.; Techapun, C.; Ougizawa, T. Reactive blending of thermoplastic starch and polyethylene-graft-maleic anhydride with chitosan as compatibilizer. Carbohydr. Polym. 2016, 153, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R.; Montero, B. Processing and characterization of polyols plasticized-starch reinforced with microcrystalline cellulose. Carbohydr. Polym. 2016, 149, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Sagnelli, D.; Kirkensgaard, J.J.K.; Giosafatto, C.V.L.; Ogrodowicz, N.; Kruczał, K.; Mikkelsen, M.S.; Maigret, J.-E.; Lourdin, D.; Mortensen, K.; Blennow, A. All-natural bio-plastics using starch-betaglucan composites. Carbohydr. Polym. 2017, 172, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Biliaderis, C.G. Non-Equilibrium Melting of Amylose-V Complexes. Carbohydr. Polym. 1986, 6, 269–288. [Google Scholar] [CrossRef]
- Wokadala, O.C.; Ray, S.S.; Emmambux, M.N. Occurrence of amylose–lipid complexes in teff and maize starch biphasic pastes. Carbohydr. Polym. 2012, 90, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Biliaderis, C.G.; Page, C.M.; Slade, L.; Sirett, R.R. Thermal Behavior of Amylose-Lipid Complexes. Carbohydr. Polym. 1985, 5, 367–389. [Google Scholar] [CrossRef]
- Genkina, N.K.; Kiseleva, V.I.; Martirosyan, V.V. Different types of V amylose-lipid inclusion complexes in maize extrudates revealed by DSC analysis. Starch Stärke 2015, 67, 752–755. [Google Scholar] [CrossRef]
- Le Bail, P.; Bizot, H.; Ollivon, M.; Keller, G.; Bourgaux, C.; Buléon, A. Monitoring the crystallization of amylose–lipid complexes during maize starch melting by synchrotron X-ray diffraction. Biopolymers 1999, 50. [Google Scholar] [CrossRef]
- Lourdin, D.; Della Valle, G.; Colonna, P. Influence of amylose content on starch films and foams. Carbohydr. Polym. 1995, 27, 261–270. [Google Scholar] [CrossRef]
- Morrison, W.R.; Milligan, T.P.; Azudin, M.N. A relationship between the amylose and lipid contents of starches from diploid cereals. J. Cereal Sci. 1984, 2, 257–271. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Gilbert, E.P.; Gidley, M.J. A novel approach for calculating starch crystallinity and its correlation with double helix content: A combined XRD and NMR study. Biopolymers 2008, 89, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Mariniello, L.; Giosafatto, C.V. L.; Moschetti, G.; Aponte, M.; Masi, P.; Sorrentino, A.; Porta, R. Fennel Waste-Based Films Suitable for Protecting Cultivations. Biomacromolecules 2007, 8, 3008–3014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carciofi, M.; Blennow, A.; Jensen, S.L.; Shaik, S.S.; Henriksen, A.; Buleon, A.; Holm, P.B.; Hebelstrup, K.H. Concerted suppression of all starch branching enzyme genes in barley produces amylose-only starch granules. BMC Plant Biol. 2012, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Coventry, A.M. Solvent Extraction of Fatty Acids from Amylose Inclusion Complexes. Starch Stärke 1989, 41, 24–27. [Google Scholar] [CrossRef]
- Rouser, G.; Fleischer, S.; Yamamoto, A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing and Materials. Standard Test Method for Determining Aerobic Biodegradation in Soil of Plastic Materials or Residual Plastic Materials after Composting; ASTM D5988-03; American Society for Testing and Materials: Philadelphia, PA, USA, 2003. [Google Scholar]
- European Committee for Standardization. Determination of pH in Soil, Sewage sludge and Biowast; CEN/BT/Task-Force-151. STD5151; European Committee for Standardization: Brussels, Belgium, 2005. [Google Scholar]

| Sample | FFA (%) | PPH (%) |
|---|---|---|
| Amylose-only | 0.04 | 0.9 |
| Control | 0.001 | 0.5 |
| Permebility | CO2 (cm3·mm/m2·kPa 24 h) | O2 (cm3·mm/m2·kPa 24 h) | WVP (cm3·mm/m2·kPa 24 h) |
|---|---|---|---|
| AO/gly | 4.0 ± 0.2 | 0.6 ± 0.03 | 0.1 ± 0.01 |
| AO/gly/CA | 0.4 ± 0.1 | 1 | 0.1 ± 0.03 |
| CT/gly | NA $ | NA $ | 0.1 |
| CT/gly/CA | 0.5 | 0.5 | 0.02 |
| Mater-Bi (S-301) | 5.0 ± 0.03 | 0.7 ± 0.005 | 0.04 ± 0.002 |
| Mater-Bi ZIO1U/C | 5.0 ± 0.02 | 0.5 ± 0.003 | Na |
| Mater-Bi (Z) | Na | Na | 33 a |
| LD-PE | Na | Na | 0.5 a |
| Cross-Linking | Glycerol | Water/CA | Starch | ||||
|---|---|---|---|---|---|---|---|
| 135 °C | 125 °C | 115 °C | 115 °C | 105 °C | 80 °C | 40 °C | 40 °C |
 | |||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagnelli, D.; Hooshmand, K.; Kemmer, G.C.; Kirkensgaard, J.J.K.; Mortensen, K.; Giosafatto, C.V.L.; Holse, M.; Hebelstrup, K.H.; Bao, J.; Stelte, W.; et al. Cross-Linked Amylose Bio-Plastic: A Transgenic-Based Compostable Plastic Alternative. Int. J. Mol. Sci. 2017, 18, 2075. https://doi.org/10.3390/ijms18102075
Sagnelli D, Hooshmand K, Kemmer GC, Kirkensgaard JJK, Mortensen K, Giosafatto CVL, Holse M, Hebelstrup KH, Bao J, Stelte W, et al. Cross-Linked Amylose Bio-Plastic: A Transgenic-Based Compostable Plastic Alternative. International Journal of Molecular Sciences. 2017; 18(10):2075. https://doi.org/10.3390/ijms18102075
Chicago/Turabian StyleSagnelli, Domenico, Kourosh Hooshmand, Gerdi Christine Kemmer, Jacob J. K. Kirkensgaard, Kell Mortensen, Concetta Valeria L. Giosafatto, Mette Holse, Kim H. Hebelstrup, Jinsong Bao, Wolfgang Stelte, and et al. 2017. "Cross-Linked Amylose Bio-Plastic: A Transgenic-Based Compostable Plastic Alternative" International Journal of Molecular Sciences 18, no. 10: 2075. https://doi.org/10.3390/ijms18102075







