High Endogenous Accumulation of ω-3 Polyunsaturated Fatty Acids Protect against Ischemia-Reperfusion Renal Injury through AMPK-Mediated Autophagy in Fat-1 Mice
Abstract
:1. Introduction
2. Results
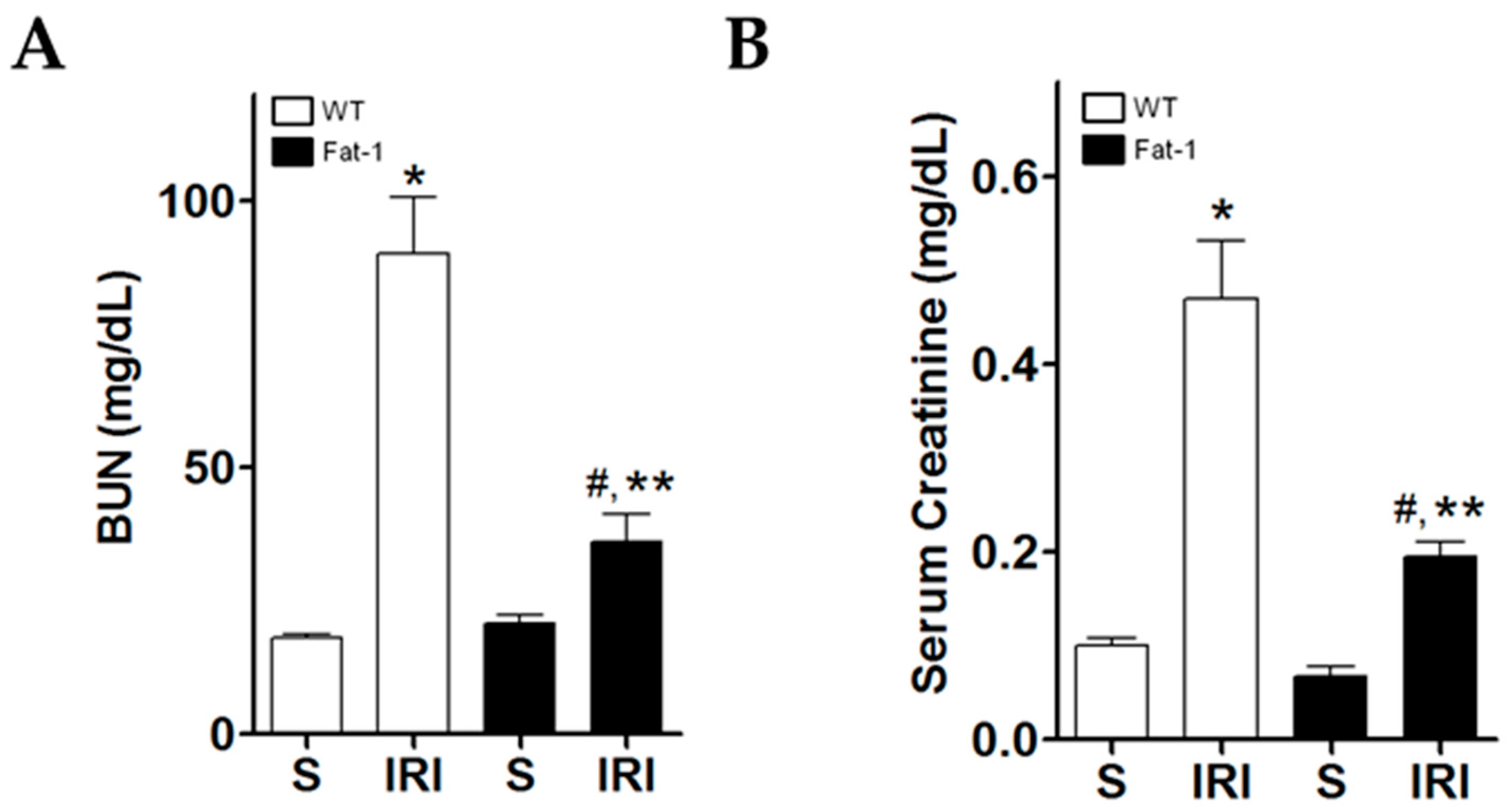
2.1. Effects of Endogenous ω3-Polyunsaturated Fatty Acids on Renal Function
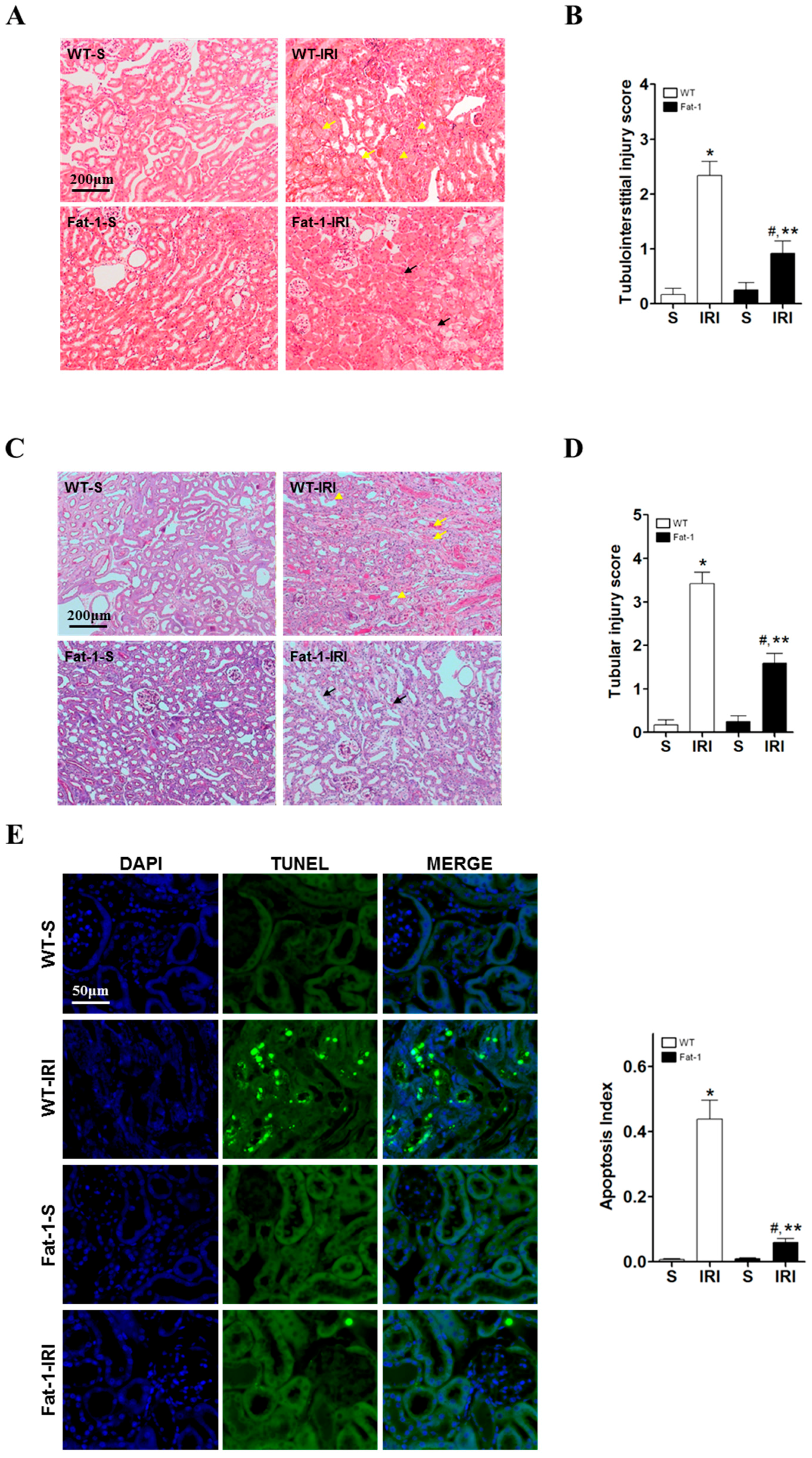
2.2. Effects of Endogenous ω3-Polyunsaturated Fatty Acids on Renal Tissue Injury
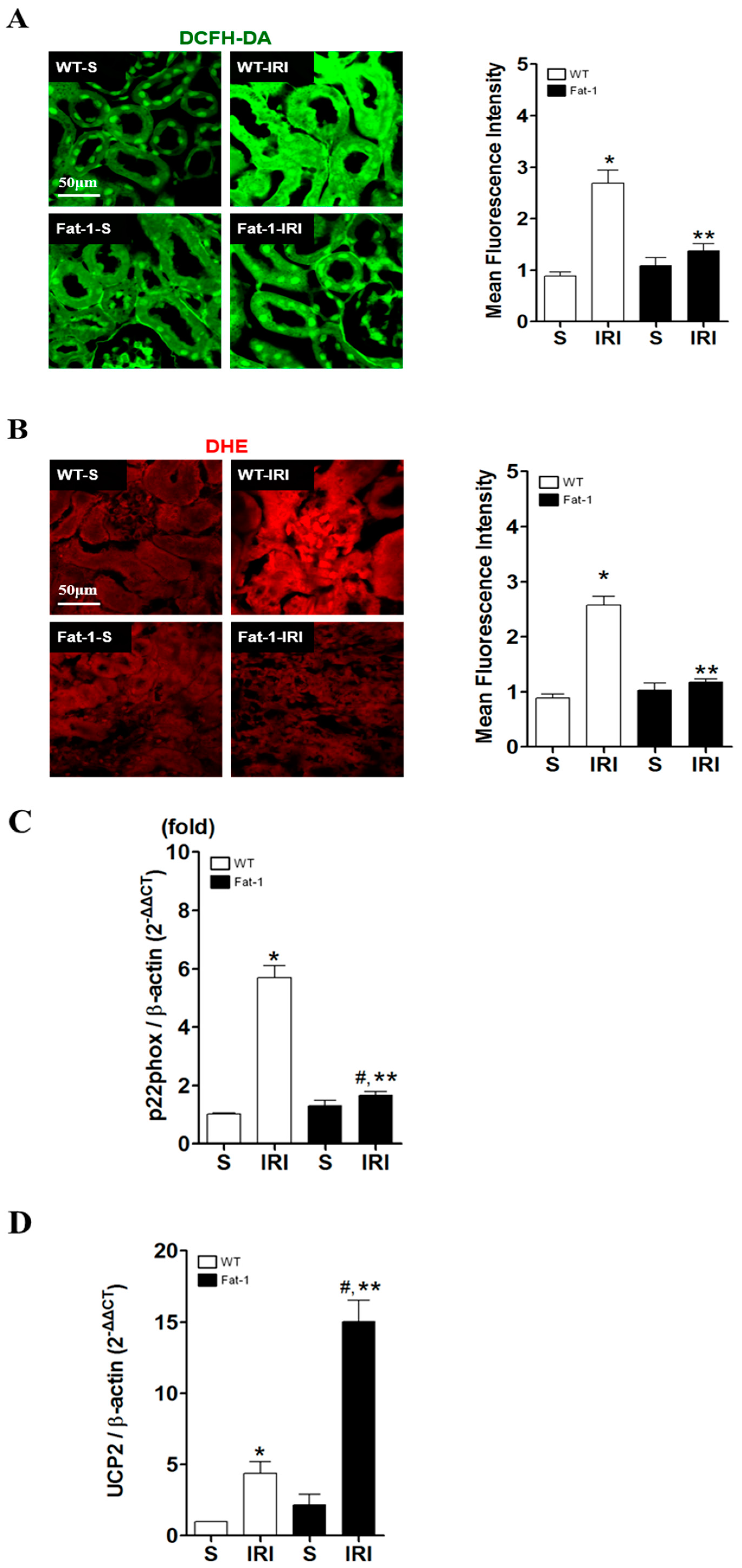
2.3. Effects of Endogenous ω3-Polyunsaturated Fatty Acids on Oxidative Stress
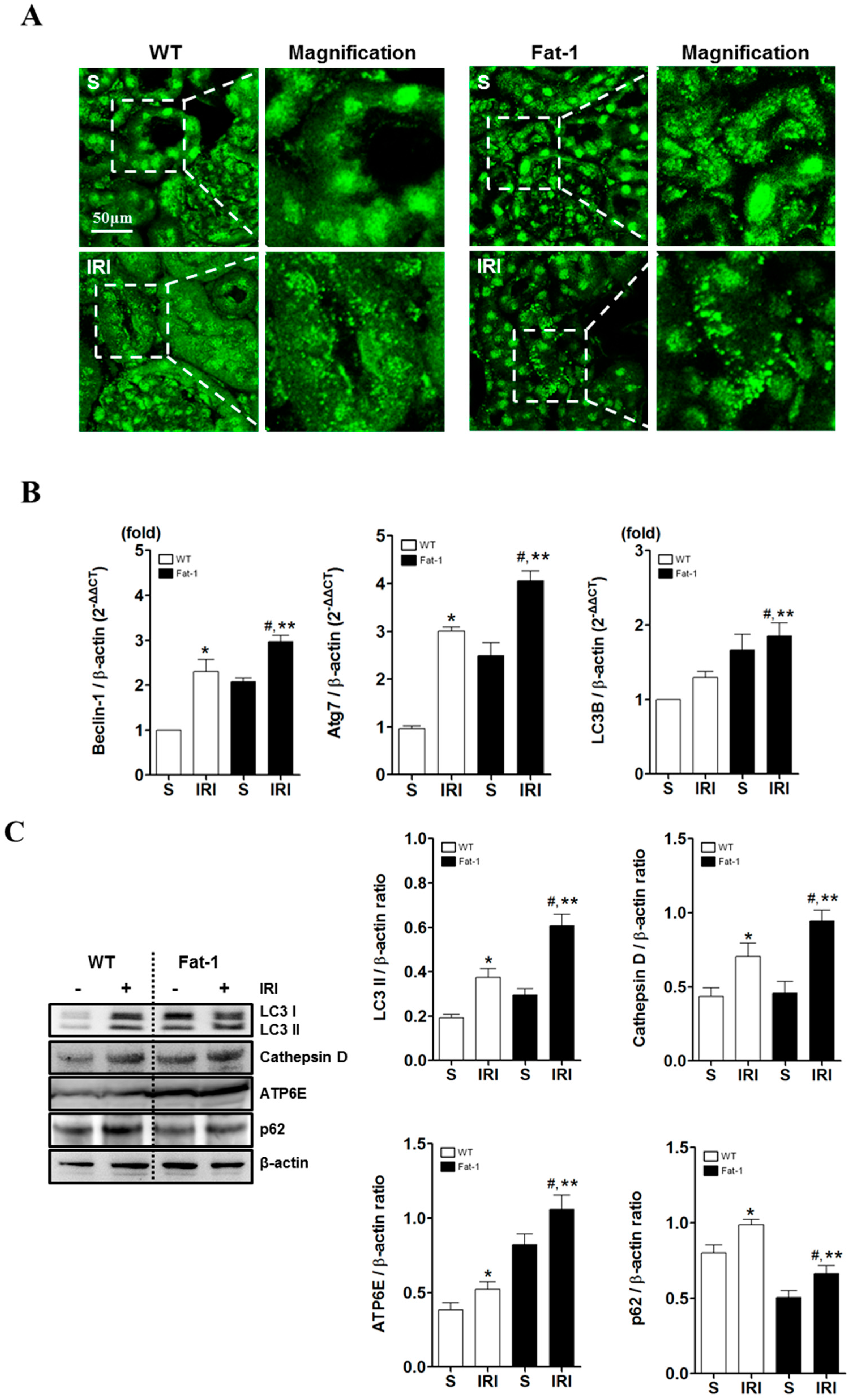
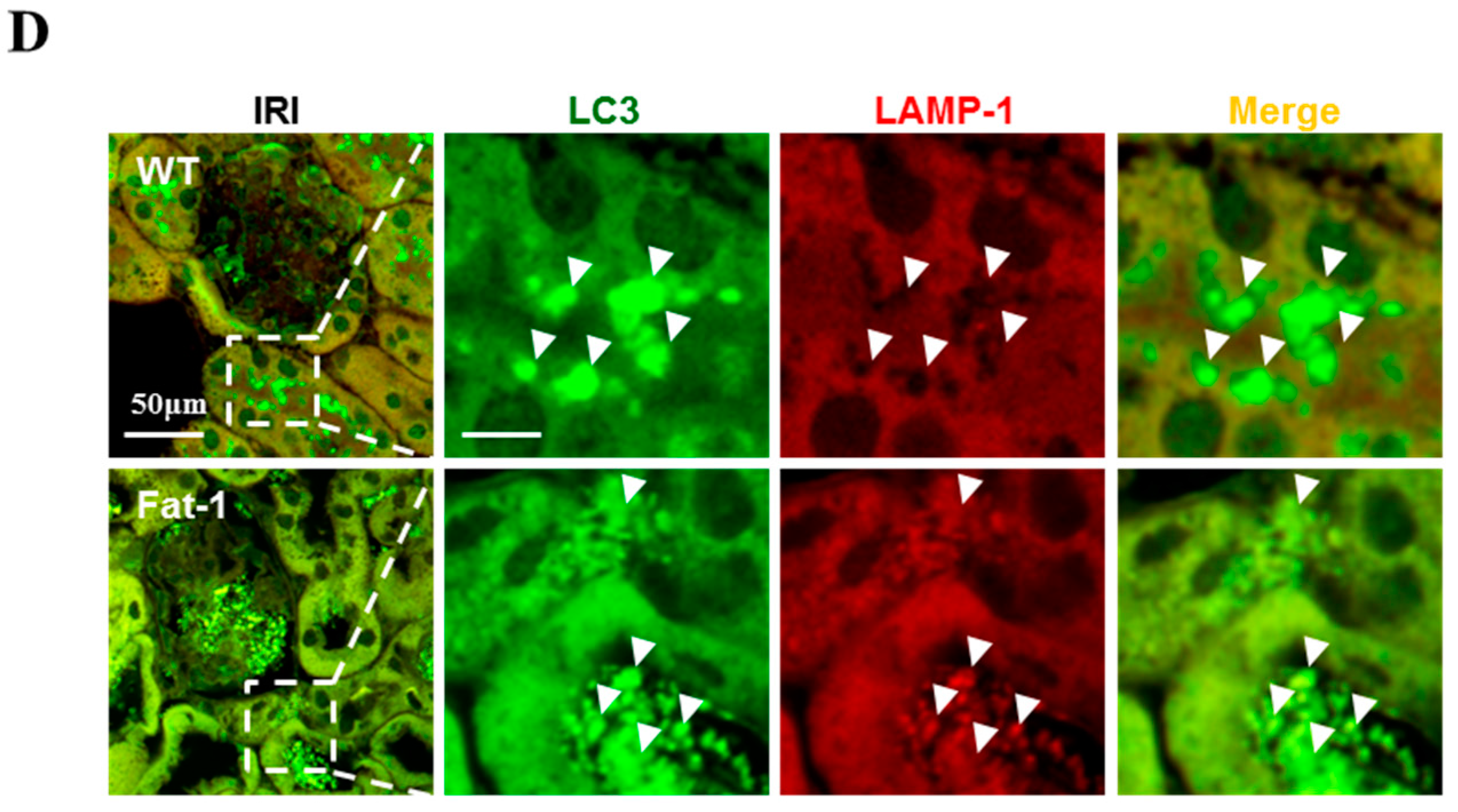
2.4. Effects of Endogenous ω3-Polyunsaturated Fatty Acids on Autophagy Activation
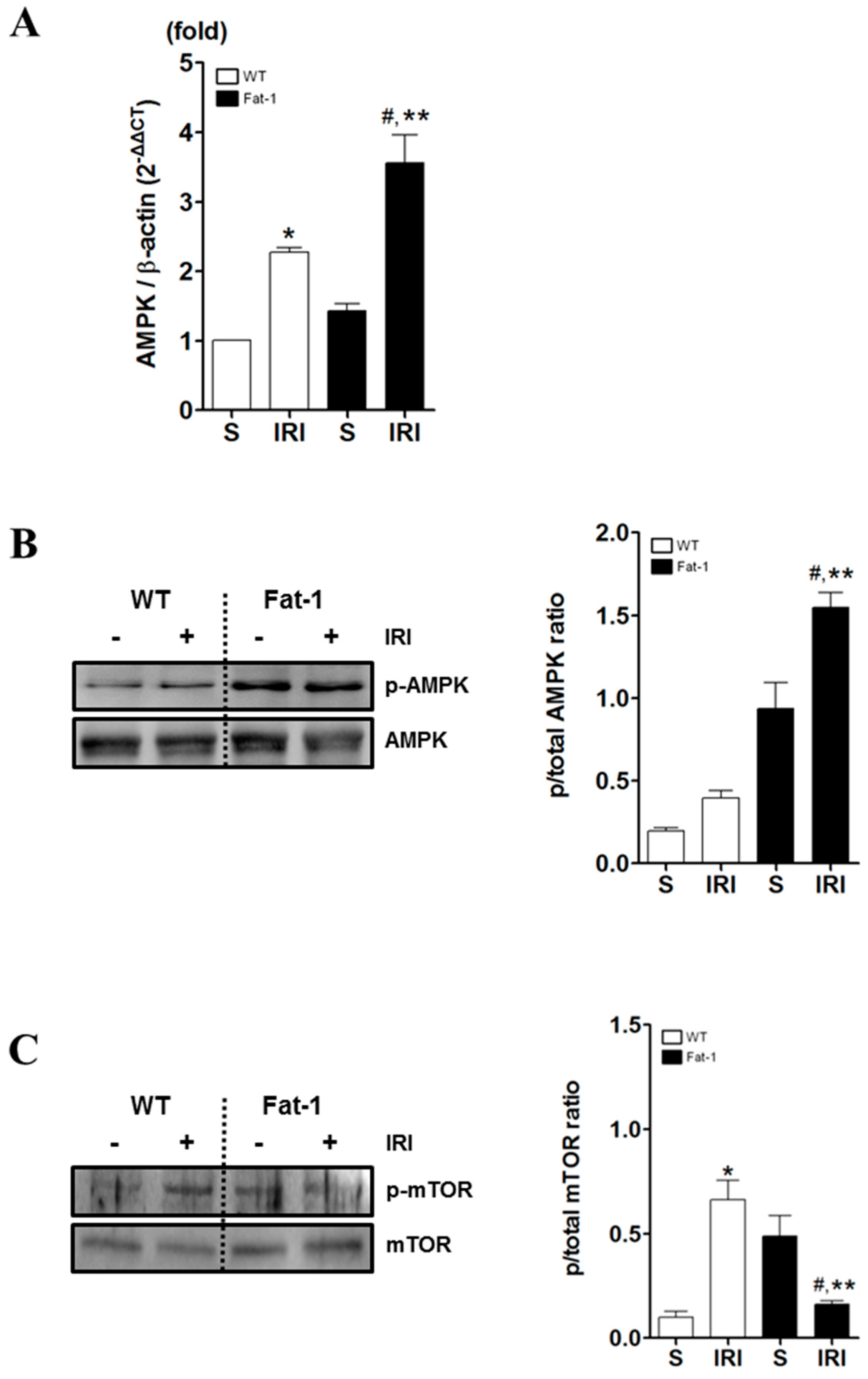
2.5. Effects of Endogenous ω3-Polyunsaturated Fatty Acids on Adenosine Monophosphate-Activated Protein Kinase Signaling
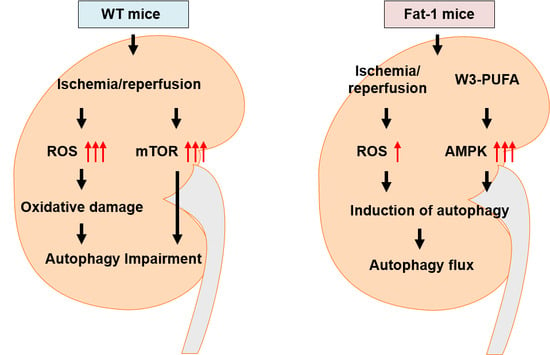
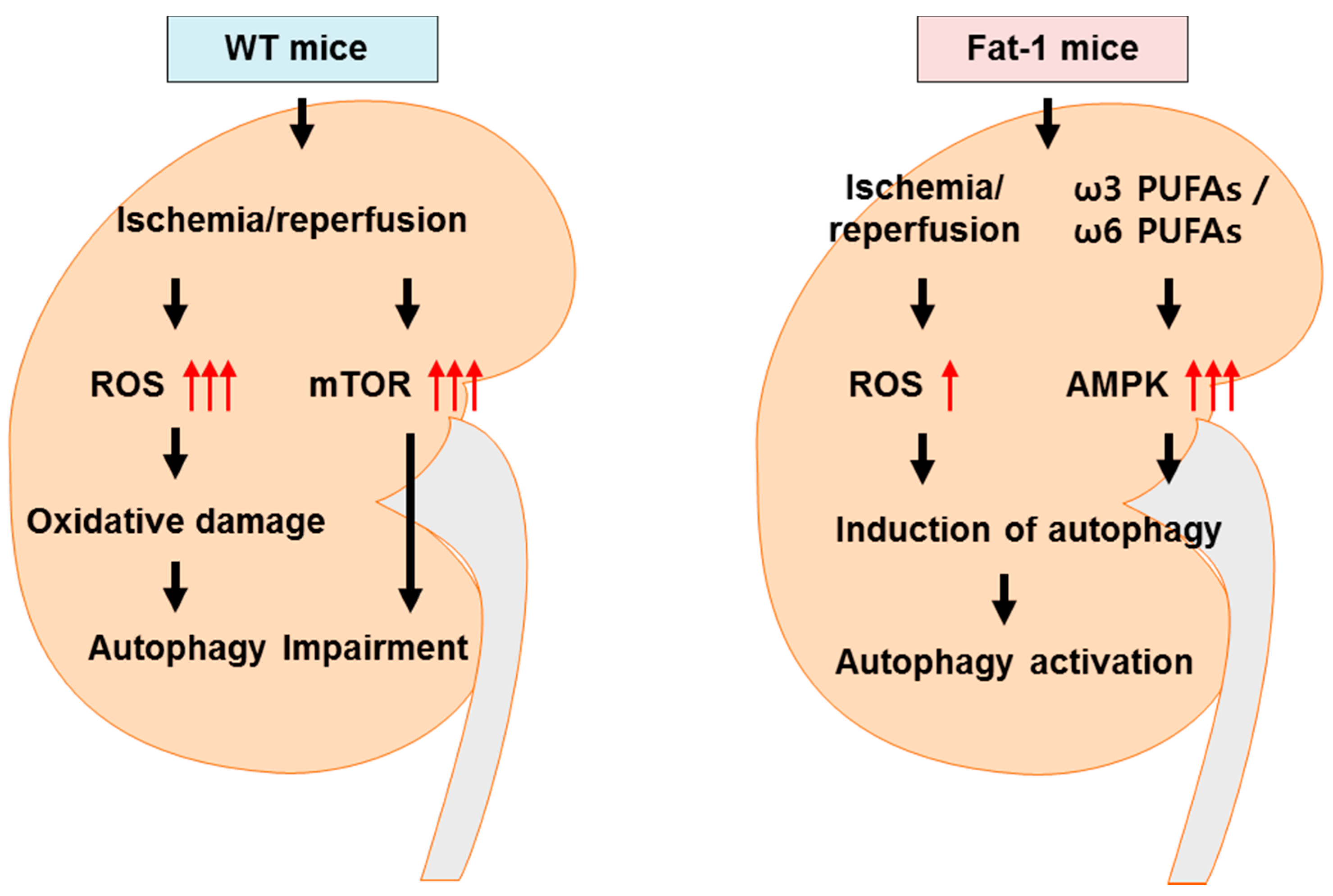
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Blood and Tissue Preparation
4.3. Tissue Injury Score
4.4. Confocal Microscopy
4.5. Western Blot Analysis
4.6. Measurement of Reactive Oxygen Species Production
4.7. Real-Time Polymerase Chain Reaction
4.8. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Staining
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IRI | Ischemia-reperfusion Injury |
| ω3-PUFAs | ω3-Polyunsaturated fatty acids |
| AKI | Acute kidney injury |
| WT | Wild-type |
| mTOR | Mammalian target of rapamycin |
| AMPK | AMP-activated protein kinase |
| ROS | Reactive oxygen species |
| UCP2 | Uncoupling protein 2 |
| LC3 | Microtubule-associated protein 1A/1B-light chain |
| SQSTM1 | Sequestosome 1 |
References
- Cooper, J.E.; Wiseman, A.C. Acute kidney injury in kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2013, 22, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.E.; Jeong, J.Y.; Lim, B.J.; Chung, S.; Chang, Y.K.; Lee, S.J.; Na, K.R.; Kim, S.Y.; Shin, Y.T.; Lee, K.W. Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am. J. Physiol. Ren. Physiol. 2009, 297, F362–F370. [Google Scholar] [CrossRef] [PubMed]
- Versteilen, A.M.; Di Maggio, F.; Leemreis, J.R.; Groeneveld, A.B.; Musters, R.J.; Sipkema, P. Molecular mechanisms of acute renal failure following ischemia/reperfusion. Int. J. Artif. Organs 2004, 27, 1019–1029. [Google Scholar] [PubMed]
- Vercauteren, S.R.; Ysebaert, D.K.; Van Rompay, A.R.; de Greef, K.E.; de Broe, M.E. Acute ischemia/reperfusion injury after isogeneic kidney transplantation is mitigated in a rat model of chronic renal failure. Am. J. Transpl. 2003, 3, 570–580. [Google Scholar] [CrossRef]
- Zweier, J.L.; Talukder, M.A. The role of oxidants and free radicals in reperfusion injury. Cardiovasc. Res. 2006, 70, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Hsu, S.P.; Yang, C.C.; Chien, C.T.; Wang, N.P. Hypoxic preconditioning reinforces HIF-alpha-dependent HSP70 signaling to reduce ischemic renal failure-induced renal tubular apoptosis and autophagy. Life Sci. 2010, 86, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Choi, M.E. Autophagy in kidney health and disease. Antioxid. Redox Signal. 2014, 20, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Liu, K.; Luo, J.; Dong, Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am. J. Pathol. 2010, 176, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Feng, D.; Chen, G.; Chen, M.; Zheng, Q.; Song, P.; Ma, Q.; Zhu, C.; Wang, R.; Qi, W.; et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 2012, 14, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Arakawa, S.; Fujitani, K.; Yamaguchi, H.; Mizuta, T.; Kanaseki, T.; Komatsu, M.; Otsu, K.; Tsujimoto, Y.; Shimizu, S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 2009, 461, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, X. AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation. Autophagy 2011, 7, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Bhayana, S.; Baisantry, A.; Kraemer, T.D.; Wrede, C.; Hegermann, J.; Brasen, J.H.; Bockmeyer, C.; Ulrich Becker, J.; Ochs, M.; Gwinner, W.; et al. Autophagy in kidney transplants of sirolimus treated recipients. J. Nephropathol. 2017, 6, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Satriano, J.; Sharma, K. Autophagy and metabolic changes in obesity-related chronic kidney disease. Nephrol. Dial. Transpl. 2013, 28 (Suppl. S4), iv29–iv36. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.S.; Huang, L.; Belousova, T.; Lu, L.; Yang, Y.; Reddel, R.; Chang, A.; Ju, H.; DiMattia, G.; Tong, Q.; et al. Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J. Am. Soc. Nephrol. 2015, 26, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, P.; Zou, M.H. AMP-activated protein kinase, stress responses and cardiovascular diseases. Clin. Sci. 2012, 122, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Lempiainen, J.; Finckenberg, P.; Levijoki, J.; Mervaala, E. AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br. J. Pharmacol. 2012, 166, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdalla, F.C.; Abeliovich, H.; Abraham, R.T.; Acevedo-Arozena, A.; Adeli, K.; Agholme, L.; Agnello, M.; Agostinis, P.; Aguirre-Ghiso, J.A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012, 8, 445–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moscat, J.; Diaz-Meco, M.T. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 2009, 137, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Psakhye, I.; Jentsch, S. Autophagic clearance of polyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell 2014, 158, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Yasuda, S.; Geleijnse, J.M.; Shimokawa, H. Fish oil and omega-3 fatty acids in cardiovascular disease: Do they really work? Eur. Heart J. 2012, 33, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Leder, L.; Kolehmainen, M.; Narverud, I.; Dahlman, I.; Myhrstad, M.C.; de Mello, V.D.; Paananen, J.; Carlberg, C.; Schwab, U.; Herzig, K.H.; et al. Effects of a healthy Nordic diet on gene expression changes in peripheral blood mononuclear cells in response to an oral glucose tolerance test in subjects with metabolic syndrome: A SYSDIET sub-study. Genes Nutr. 2016, 11, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathod, R.; Kale, A.; Joshi, S. Novel insights into the effect of vitamin B(1)(2) and omega-3 fatty acids on brain function. J. Biomed. Sci. 2016, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Appleton, K.M.; Sallis, H.M.; Perry, R.; Ness, A.R.; Churchill, R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst. Rev. 2015, 5, CD004692. [Google Scholar] [CrossRef]
- Luo, C.; Ren, H.; Wan, J.B.; Yao, X.; Zhang, X.; He, C.; So, K.F.; Kang, J.X.; Pei, Z.; Su, H. Enriched endogenous omega-3 fatty acids in mice protect against global ischemia injury. J. Lipid Res. 2014, 55, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [PubMed]
- Hassan, I.R.; Gronert, K. Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J. Immunol. 2009, 182, 3223–3232. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Yao, J.; Liu, X.; Fan, H.; Zhang, F.; Li, Z.; Tian, X.; Zhou, Y. Fish-oil emulsion (omega-3 polyunsaturated fatty acids) attenuates acute lung injury induced by intestinal ischemia-reperfusion through Adenosine 5′-monophosphate-activated protein kinase-sirtuin1 pathway. J. Surg. Res. 2014, 187, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Shao, S.H.; Xu, Z.X.; Hennessy, B.; Ding, Z.; Larrea, M.; Kondo, S.; Dumont, D.J.; Gutterman, J.U.; Walker, C.L.; et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat. Cell Biol. 2007, 9, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.O.; Wang, X.; Hitron, J.A.; Zhang, Z.; Cheng, S.; Budhraja, A.; Ding, S.; Lee, J.C.; Shi, X. Cadmium induces autophagy through ROS-dependent activation of the LKB1-AMPK signaling in skin epidermal cells. Toxicol. Appl. Pharmacol. 2011, 255, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Walker, C.L. The role of LKB1 and AMPK in cellular responses to stress and damage. FEBS Lett. 2011, 585, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jing, K.; Jeong, S.; Kim, N.; Song, K.S.; Heo, J.Y.; Park, J.H.; Seo, K.S.; Han, J.; Park, J.I.; et al. The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53. BioMed Res. Int. 2013, 2013, 568671. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Jeong, S.; Jing, K.; Shin, S.; Kim, S.; Heo, J.Y.; Kweon, G.R.; Park, S.K.; Wu, T.; Park, J.I.; et al. Docosahexaenoic Acid induces cell death in human non-small cell lung cancer cells by repressing mtor via ampk activation and pi3k/akt inhibition. BioMed Res. Int. 2015, 2015, 239764. [Google Scholar] [CrossRef] [PubMed]
- Menesi, D.; Kitajka, K.; Molnar, E.; Kis, Z.; Belleger, J.; Narce, M.; Kang, J.X.; Puskas, L.G.; Das, U.N. Gene and protein expression profiling of the fat-1 mouse brain. Prostaglandins Leukot Essent Fatty Acids 2009, 80, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [PubMed]
- Yang, C.S.; Yuk, J.M.; Kim, J.J.; Hwang, J.H.; Lee, C.H.; Kim, J.M.; Oh, G.T.; Choi, H.S.; Jo, E.K. Small heterodimer partner-targeting therapy inhibits systemic inflammatory responses through mitochondrial uncoupling protein 2. PLoS ONE 2013, 8, e63435. [Google Scholar] [CrossRef] [PubMed]
- Bak, D.H.; Zhang, E.; Yi, M.H.; Kim, D.K.; Lim, K.; Kim, J.J.; Kim, D.W. High omega3-polyunsaturated fatty acids in fat-1 mice prevent streptozotocin-induced Purkinje cell degeneration through BDNF-mediated autophagy. Sci. Rep. 2015, 5, 15465. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.C.; Woods, A.; Jones, N.A.; Davison, M.D.; Carling, D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem. J. 2000, 345 Pt 3, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, W.; Fuhro, R.; Andry, C.C.; Rennke, H.; Abernathy, V.E.; Koh, J.S.; Valeri, R.; Levine, J.S. Rapamycin impairs recovery from acute renal failure: Role of cell-cycle arrest and apoptosis of tubular cells. Am. J. Physiol. Ren. Physiol. 2001, 281, F693–F706. [Google Scholar]
- Yuan, Q.; Hong, S.; Han, S.; Zeng, L.; Liu, F.; Ding, G.; Kang, Y.; Mao, J.; Cai, M.; Zhu, Y.; et al. Preconditioning with physiological levels of ethanol protect kidney against ischemia/reperfusion injury by modulating oxidative stress. PLoS ONE 2011, 6, e25811. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, E. Induction of oxidative stress in kidney. Int. J. Nephrol. 2012, 2012, 465897. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Basnakian, A.G.; Shah, S.V. Apoptotic pathways in ischemic acute renal failure. Kidney Int. 2004, 66, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Liu, J.; Yan, X.Y.; Zhang, Y.; Zhang, J.J.; Zhang, L.C.; Sun, L.K. Cytoprotective Effect of the UCP2-SIRT3 signaling pathway by decreasing mitochondrial oxidative stress on cerebral ischemia-reperfusion injury. Int. J. Mol. Sci. 2017, 18, 1599. [Google Scholar]
- Gang, G.T.; Hwang, J.H.; Kim, Y.H.; Noh, J.R.; Kim, K.S.; Jeong, J.Y.; Choi, D.E.; Lee, K.W.; Jung, J.Y.; Shong, M.; et al. Protection of NAD(P)H:quinone oxidoreductase 1 against renal ischemia/reperfusion injury in mice. Free Radic. Biol. Med. 2014, 67, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Han, I.Y.; Kim, Y.; Kim, Y.H.; Choi, I.W.; Seo, S.K.; Jung, S.Y.; Park, S.; Kang, M.S. The NADPH oxidase inhibitor DPI can abolish hypoxia-induced apoptosis of human kidney proximal tubular epithelial cells through Bcl2 up-regulation via ERK activation without ROS reduction. Life Sci. 2015, 126, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Komatsu, M. Selective degradation of p62 by autophagy. Semin. Immunopathol. 2010, 32, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Gong, J.L.; Xing, T.Y.; Zheng, S.P.; Ding, W. Autophagy protein p62/SQSTM1 is involved in HAMLET-induced cell death by modulating apotosis in U87MG cells. Cell Death Dis. 2013, 4, e550. [Google Scholar] [CrossRef] [PubMed]
- Williams-Bey, Y.; Boularan, C.; Vural, A.; Huang, N.N.; Hwang, I.Y.; Shan-Shi, C.; Kehrl, J.H. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-kappaB activation and enhancing autophagy. PLoS ONE 2014, 9, e97957. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Kim, J.J.; Lee, H.M.; Jin, H.S.; Lee, S.H.; Park, J.H.; Kim, S.J.; Kim, J.M.; Han, Y.M.; Lee, M.S.; et al. The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy 2014, 10, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.S.; Noh, H.S.; Ha, J.H.; Ahn, J.S.; Hahm, J.R.; Cho, H.Y.; Kim, D.R. Cathepsin D inhibits oxidative stress-induced cell death via activation of autophagy in cancer cells. Cancer Lett. 2012, 323, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Lee, H.M.; Shin, D.M.; Kim, W.; Yuk, J.M.; Jin, H.S.; Lee, S.H.; Cha, G.H.; Kim, J.M.; Lee, Z.W.; et al. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 2012, 11, 457–468. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwon, D.H.; Hwang, T.W.; Ro, J.-Y.; Kang, Y.-J.; Jeong, J.Y.; Kim, D.-K.; Lim, K.; Kim, D.W.; Choi, D.E.; Kim, J.-J. High Endogenous Accumulation of ω-3 Polyunsaturated Fatty Acids Protect against Ischemia-Reperfusion Renal Injury through AMPK-Mediated Autophagy in Fat-1 Mice. Int. J. Mol. Sci. 2017, 18, 2081. https://doi.org/10.3390/ijms18102081
Gwon DH, Hwang TW, Ro J-Y, Kang Y-J, Jeong JY, Kim D-K, Lim K, Kim DW, Choi DE, Kim J-J. High Endogenous Accumulation of ω-3 Polyunsaturated Fatty Acids Protect against Ischemia-Reperfusion Renal Injury through AMPK-Mediated Autophagy in Fat-1 Mice. International Journal of Molecular Sciences. 2017; 18(10):2081. https://doi.org/10.3390/ijms18102081
Chicago/Turabian StyleGwon, Do Hyeong, Tae Woong Hwang, Ju-Ye Ro, Yoon-Joong Kang, Jin Young Jeong, Do-Kyung Kim, Kyu Lim, Dong Woon Kim, Dae Eun Choi, and Jwa-Jin Kim. 2017. "High Endogenous Accumulation of ω-3 Polyunsaturated Fatty Acids Protect against Ischemia-Reperfusion Renal Injury through AMPK-Mediated Autophagy in Fat-1 Mice" International Journal of Molecular Sciences 18, no. 10: 2081. https://doi.org/10.3390/ijms18102081