Unexpected High Intragenomic Variation in Two of Three Major Pest Thrips Species Does Not Affect Ribosomal Internal Transcribed Spacer 2 (ITS2) Utility for Thrips Identification
Abstract
:1. Introduction
2. Results
2.1. DNA Sequence Analysis
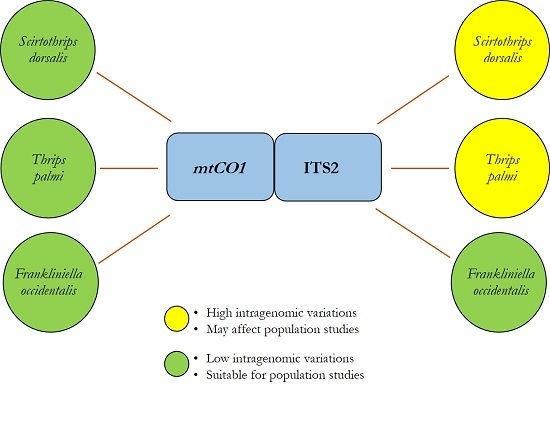
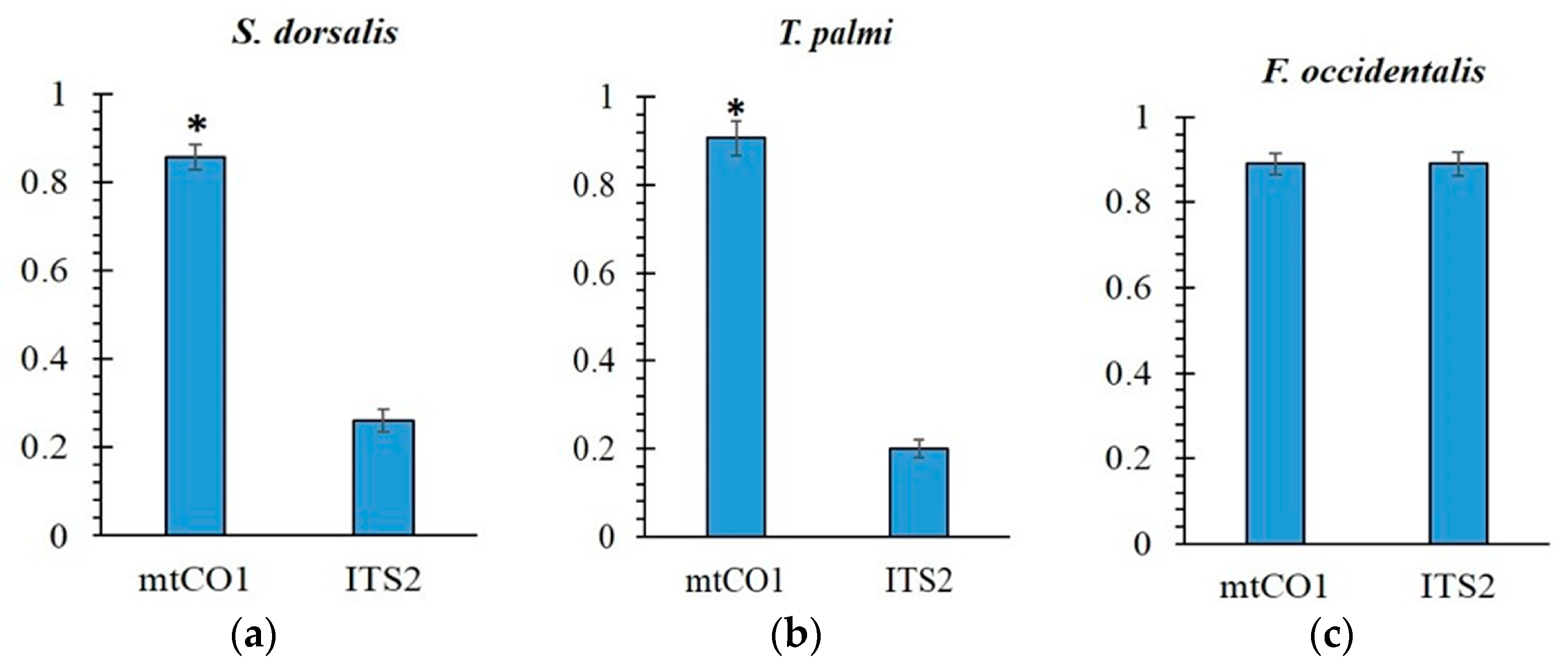
2.2. Intragenomic and Intergenomic Variation
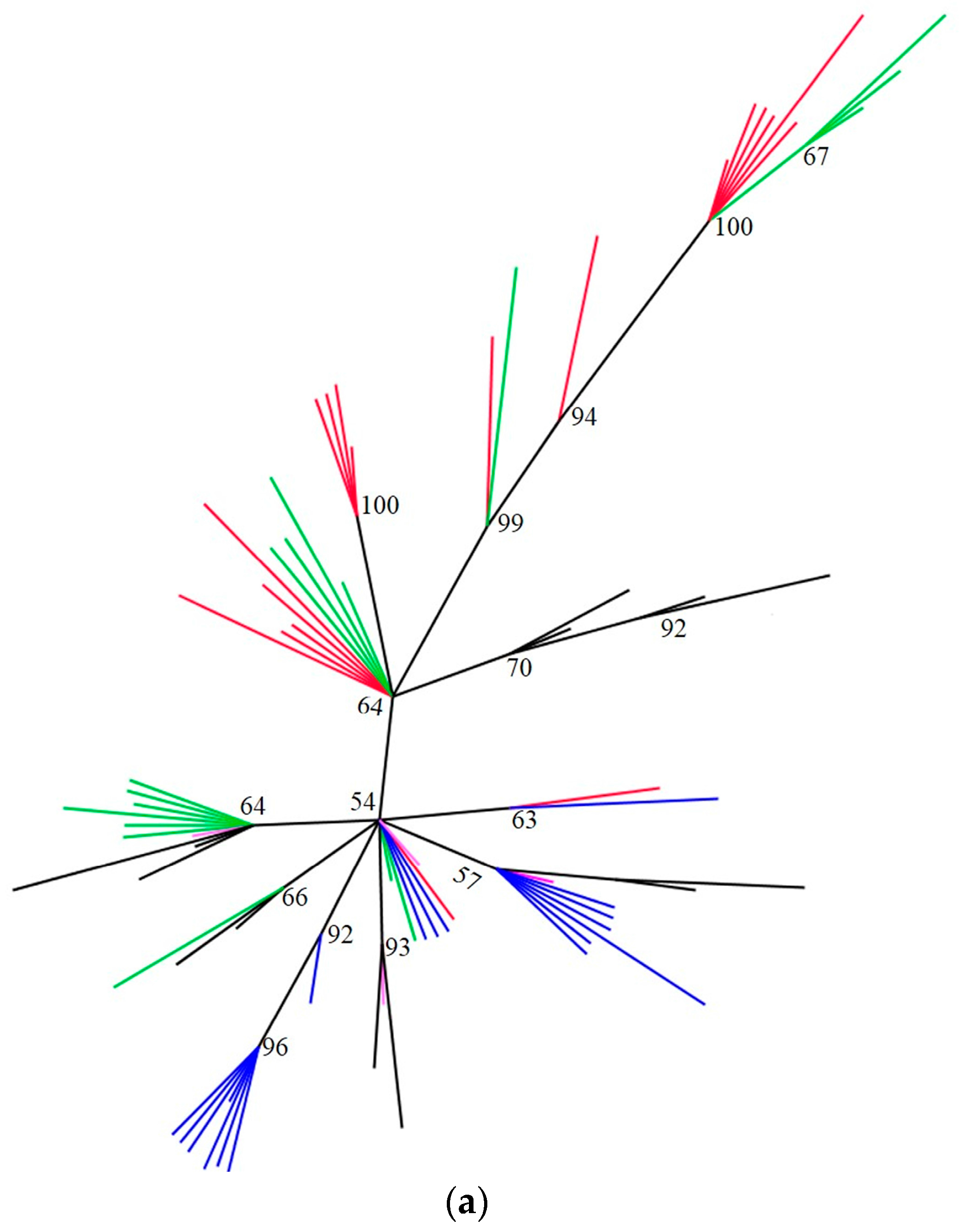
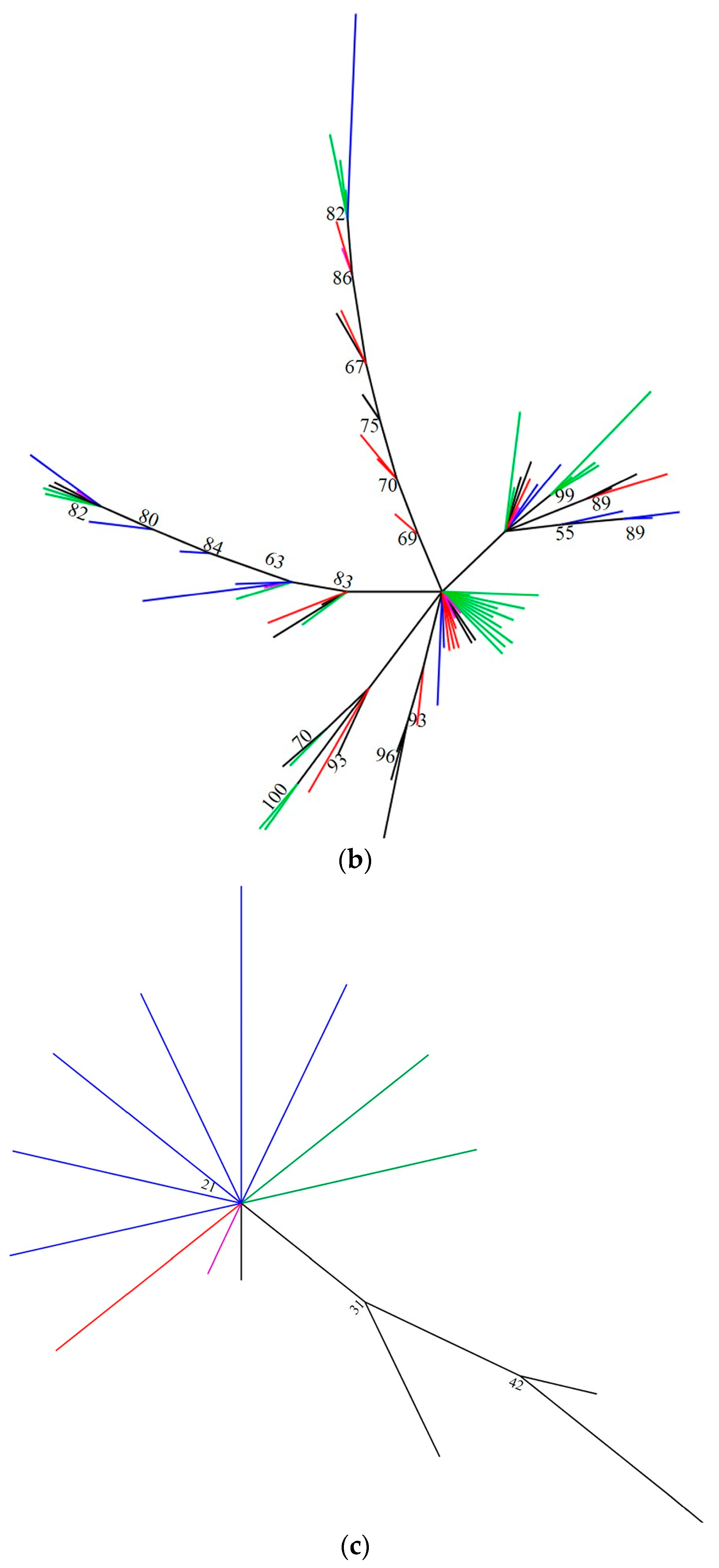
2.3. Phylogenetic Analyses
3. Discussion
4. Materials and Methods
4.1. Thrips Sampling
4.2. Morphological Identification of Thrips
4.3. DNA Processing
4.4. Sequence Alignment and Genetic Variation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Scientific Name (M = Male, F = Female) | Specimen No. | Individual Code | Date Collected | Host | Locality | Coordinates | Collector | GenBank Accessions–KT885 |
|---|---|---|---|---|---|---|---|---|
| Scirtothrips dorsalis (F) | CLM9.13 | SD-1 | 7.Aug. 2007 | Indian Hawthorne | USA, Florida-Apopka | 28.63 N, 81.55 W | Lance Osborne | 200, 212 |
| Scirtothrips dorsalis (F) | CLM9.14 | SD-2 | 7 Aug. 2007 | Indian Hawthorne | USA, Florida-Apopka | 28.63 N, 81.55 W | Lance Osborne | 201, 213 |
| Scirtothrips dorsalis (M) | CLM9.15 | SD-3 | 7 Aug. 2007 | Indian Hawthorne | USA, Florida-Apopka | 28.63 N, 81.55 W | Lance Osborne | 202, 214 |
| Scirtothrips dorsalis (M) | CLM9.16 | SD-4 | 7 Aug. 2007 | Indian Hawthorne | USA, Florida-Apopka | 28.63 N, 81.55 W | Lance Osborne | 203, 215 |
| Thrips palmi (F) | CLM85.5 | TP-1 | 11 Mar. 2010 | Vlaspek cucumber | USA, Florida-Homestead | 25.50 N, 80.49 W | Vivek Kumar | 204, 216 |
| Thrips palmi (F) | CLM85.6 | TP-2 | 11 Mar. 2010 | Vlaspek cucumber | USA, Florida-Homestead | 25.50 N, 80.49 W | Vivek Kumar | 205, 217 |
| Thrips palmi (M) | CLM85.9 | TP-3 | 11 Mar. 2010 | Vlaspek cucumber | USA, Florida-Homestead | 25.50 N, 80.49 W | Vivek Kumar | 206, 218 |
| Thrips palmi (M) | CLM85.10 | TP-4 | 11 Mar. 2010 | Vlaspek cucumber | USA, Florida-Homestead | 25.50 N, 80.49 W | Vivek Kumar | 207, 219 |
| Frankliniella occidentalis (F) | CLM87.20 | FO-1 | 16 Apr. 2011 | Green beans | USA, Florida-Tallahassee | 30.48 N, 84.17 W | Stuart Reitz | 208, 220 |
| Frankliniella occidentalis (F) | CLM87.22 | FO-2 | 16 Apr. 2011 | Green beans | USA, Florida-Tallahassee | 30.48 N, 84.17 W | Stuart Reitz | 209, 221 |
| Frankliniella occidentalis (M) | CLM87.25 | FO-3 | 16 Apr. 2011 | Green beans | USA, Florida-Tallahassee | 30.48 N, 84.17 W | Stuart Reitz | 210, 222 |
| Frankliniella occidentalis (M) | CLM87.30 | FO-4 | 16 Apr. 2011 | Green beans | USA, Florida-Tallahassee | 30.48 N, 84.17 W | Stuart Reitz | 211, 223 |
| PCR Primer Set | PCR Amplification Conditions (25 µL Reactions) |
|---|---|
| mtCO1 primers LCO1490:5′-GGTCAACAAATCATAAAGATATTGG-3′ HCO2198: 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ * mt D-7.2F: 5′-ATTAGGAGCHCCHGAYATAGCATT-3′ * mt D9.2R: 5′-CAGGCAAGATTAAAATATAAACTTCTG-3′ | 94 °C 2 min 35 cycles of 94 °C 30 s 54 °C for 30 s 72 °C for 1 min 72 °C for 10 min |
| ITS2 primers ITSF: 5′-TGTGAACTGCAGGACACATG-3′ ITSR: 5′-AATGCTTAAATTTAGGGGGTA-3′ | 94 °C 2 min 35 cycles of 94 °C 30 s 52 °C for 1 min 72 °C for 1 min 72 °C for 10 min |
References
- Murai, T.; Toda, S. Variation of Thrips tabaci in colour and size. In Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera; CSIRO Entomology: Reggio Calabria, Italy, 2001; pp. 377–378. [Google Scholar]
- Rugman-Jones, P.F.; Hoddle, M.S.; Stouthamer, R. Nuclear-mitochondrial barcoding exposes the global pest western flower thrips (Thysanoptera: Thripidae) as two sympatric cryptic species in its native California. J. Econ. Entomol. 2010, 103, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, G.; Seal, D.R.; Kumar, V. Assessing abundance and distribution of an invasive thrips Frankliniella schultzei (Trybom) (Thysanoptera: Thripidae) in South Florida. Bull. Entomol. Res. 2012, 102, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Mehle, N.; Trdan, S. Traditional and modern methods for the identification of thrips (Thysanoptera) species. J. Pest Sci. 2012, 85, 179–190. [Google Scholar] [CrossRef]
- Brunner, P.C.; Fleming, C.; Frey, J.E. A molecular identification key for economically important thrips species (Thysanoptera: Thripidae) using direct sequencing and a PCR-RFLP-based approach. Agric. For. Entomol. 2002, 4, 127–136. [Google Scholar] [CrossRef]
- Kumar, V. Characterizing Phenotypic and Genetic Variations in the Invasive Chilli Thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae). Ph.D. Dissertation, University of Florida, Gainesville, FL, USA, 2012. [Google Scholar]
- Kumar, V.; Seal, D.R.; Osborne, L.; McKenzie, C.L. Coupling SEM with DNA barcoding: A novel approach for thrips identification. Appl. Entomol. Zool. 2014, 49, 403–409. [Google Scholar] [CrossRef]
- Przybylska, A.; Fiedler, Ż.; Kucharczyk, H.; Obrępalska-Stęplowska, A. Detection of the quarantine species Thrips palmi by loop-mediated isothermal amplification. PLoS ONE 2015, 10, e0122033. [Google Scholar] [CrossRef] [PubMed]
- Asokan, R.; Kumar, K.; Kumar, N.K.V.; Ranganath, H.R. Molecular differences in the mitochondrial cytochrome oxidase I (mtCOI) gene and development of a species-specific marker for onion thrips, Thrips tabaci Lindeman, and melon thrips, T. palmi Karny (Thysanoptera: Thripidae), vectors of tospoviruses (Bunyaviridae). Bull. Entomol. Res. 2007, 97, 461–470. [Google Scholar] [PubMed]
- Hoddle, M.S.; Heraty, J.M.; Rugman-Jones, P.F.; Mound, L.A.; Stouthamer, R. Relationships among species of Scirtothrips (Thysanoptera: Thripidae, Thripinae) using molecular and morphological data. Ann. Entomol. Soc. Am. 2008, 101, 491–500. [Google Scholar] [CrossRef]
- Dickey, A.M.; Kumar, V.; Hoddle, M.S.; Funderburk, J.E.; Morgan, J.K.; Jara-Cavieres, A.; Shatters, R.G., Jr.; Osborne, L.S.; McKenzie, C.L. The Scirtothrips dorsalis species complex: Endemism and invasion in a global pest. PLoS ONE 2015, 10, e0123747. [Google Scholar] [CrossRef] [PubMed]
- Rubinoff, D.; Cameron, S.; Will, K. A genomic perspective on the shortcomings of mitochondrial DNA for “barcoding” identification. J. Hered. 2006, 97, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Barr, N.B.; Gui, L.; McPheron, B.A. Molecular systematic of nuclear gene period in genus Anastrapha (Tephritidae). Ann. Entomol. Soc. Am. 2005, 98, 173–180. [Google Scholar] [CrossRef]
- Chanbusarakum, L.; Ullman, D. Characterization of bacterial symbionts in Frankliniella occidentalis (Pergande), western flower thrips. J. Invert. Pathol. 2008, 99, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Buckman, R.S.; Mound, L.A.; Whiting, M.F. Phylogeny of thrips (Insecta: Thysanoptera) based on five molecular loci. Syst. Entomol. 2013, 38, 123–133. [Google Scholar] [CrossRef]
- Rugman-Jones, P.F.; Hoddle, M.S.; Mound, L.A.; Stouthamer, R. Molecular identification key for pest species of Scirtothrips (Thysanoptera: Thripidae). J. Econ. Entomol. 2006, 99, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Buhay, E.; Whiting, M.F.; Crandall, K.A. Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc. Natl. Acad. Sci. USA 2008, 105, 13486–13491. [Google Scholar] [CrossRef] [PubMed]
- Frey, J.E.; Frey, B. Origin of intra-individual variation in PCR amplified mitochondrial cytochrome oxidase I of Thrips tabaci (Thysanoptera: Thripidae): Mitochondrial heteroplasmy or nuclear integration? Hereditas 2004, 140, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.D.D.; Jiggins, F.M. Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: The effects of inherited symbionts. Proc. Biol. Sci. 2005, 272, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Brower, A.V.Z. Problems with DNA barcodes for species delimitation: “ten species” of Astraptes fulgerator reassessed (Lepidoptera: Hesperiidae). Syst. Biodivers. 2006, 4, 127–132. [Google Scholar] [CrossRef]
- Meier, R.; Shiyang, K.; Vaidya, G.; Ng, P.K.L. DNA barcoding and taxonomy in Diptera: A tale of high intraspecific variability and low identification success. Syst. Biol. 2006, 55, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Cognato, A.I. Standard percent DNA sequence difference for insects does not predict species boundaries. J. Econ. Entomol. 2006, 99, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.; Hill, R.I.; Willmott, K.R.; Dashmahapatra, K.K.; Brower, A.V.Z.; Mallet, J.; Jigins, H.D. Limited performance of DNA barcoding in a diverse community of tropical butterflies. Proc. Biol. Sci. 2007, 274, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, T.L.; Dawson, R.D.; Magalon, H.; Baudry, E. DNA barcoding cannot reliably identify species of the blowfly genus Protocalliphora (Diptera: Calliphoridae). Proc. Biol. Sci. 2007, 274, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Navajas, M.; Lagnel, J.; Gutierrez, J.; Boursot, P. Species-wide homogeneity of nuclear ITS2 sequences in the spider mite Tetranychus urticae contrasts with extensive mitochondrial COI polymorphism. Heredity 1998, 80, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Fairley, T.L.; Kilpatrick, C.W.; Conn, J.E. Intragenomic heterogeneity of internal transcribed spacer rDNA in neotropical malaria vector, Anopheles aquasalis (Diptera: Culicidae). J. Med. Entomol. 2005, 42, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Liao, D. Concerted evolution: Molecular mechanism and biological implications. Am. J. Hum. Genet. 1999, 64, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wörheide, G.; Nichols, S.A.; Goldberg, J. Intragenomic variation of the rDNA internal transcribed spacers in sponges (phylum Porifera): Impliations for phylogenetic studies. Mol. Phylogenet. Evol. 2004, 33, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, E.A.; Martin, S.L.; Beverley, S.M.; Kan, Y.W.; Wilson, A.C. Rapid duplication and loss of genes coding for the alphachains of hemoglobin. Proc. Natl. Acad. Sci. USA 1980, 77, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- Leo, N.P.; Barker, S.C. Intragenomic variation in ITS2 rDNA in the louse of humans, Pediculus humanus: ITS2 is not a suitable marker for population studies in this species. Insect Mol. Biol. 2002, 11, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wilkerson, R.C. Intragenomic rDNA ITS2 variation in the neotropical Anopheles (Nyssorhynchus) albistarsis complex (Diptera: Culicidae). J. Hered. 2007, 98, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Vesgueiro, F.T.; Demari-Silva, B.; Malafronte, R.D.S.; Sallum, M.A.M.; Marrelli, M.T. Intragenomic variation in the second internal transcribed spacer of the ribosomal DNA of species of the genera Culex and Lutzia (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz. 2011, 106, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.G.; Hoddle, M.S. Invasion biology of thrips. Annu. Rev. Entomol. 2006, 51, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Bergant, K.; Trdan, S.; Znidarcic, D.; Crepinsek, Z.; Kajfez-Bogataj, L. Impact of climate change on developmental dynamics of Thrips tabaci (Thysanoptera: Thripidae): Can it be quantified? Environ. Entomol. 2005, 34, 755–766. [Google Scholar] [CrossRef]
- Cannon, R.J.C.; Matthews, L.; Collins, D.W. A review of the pest status and control options for Thrips palmi. Crop Prot. 2007, 26, 1089–1098. [Google Scholar] [CrossRef]
- Trdan, S.; Znidarèiè, D.; Vidrih, M. Control of Frankliniella occidentalis on glasshouse-grown cucumbers: An efficacy comparison of foliar application of Steinernema feltiae and spraying with abamectin. Russ. J. Nematol. 2007, 15, 25–34. [Google Scholar]
- Lin, C.N.; Wei, M.Y.; Chang, N.T.; Chuang, Y.Y. The occurrence of Scirtothrips dorsalis hood in mango orchards and factors influencing its population dynamics in Taiwan. J. Asia-Pac. Entomol. 2015, 18, 361–367. [Google Scholar] [CrossRef]
- Seal, D.R.; Kumar, V. Biological responses of chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), to various regimes of chemical and biorational insecticides. Crop Prot. 2010, 29, 1241–1247. [Google Scholar] [CrossRef]
- Reitz, S.R.; GAO, Y-lin.; LEI, Z-ren. Thrips: Pests of concern to China and the United States. Agric. Sci. China 2011, 10, 867–892. [Google Scholar]
- Demirozer, O.; Tyler-Julian, K.; Funderburk, J.; Reitz, S.; Leppla, N. Integrated pest management program for thrips and Tospoviruses in fruiting vegetables. Pest. Manag. Sci. 2012, 68, 1537–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seal, D.R.; Kumar, V.; Kakkar, G.; Mello, S.C. Abundance of adventive Thrips palmi (Thysanoptera:Thripidae) populations in Florida during the first sixteen years. Fla. Entomol. 2013, 96, 789–796. [Google Scholar] [CrossRef]
- Kumar, V.; Kakkar, G.; Seal, D.R.; McKenzie, C.L.; Colee, J.; Osborne, L. Temporal and spatial distribution of an invasive thrips species Scirtothrips dorsalis (Thysanoptera: Thripidae). Crop Prot. 2014, 55, 80–90. [Google Scholar] [CrossRef]
- Cluever, J.D.; Smith, H.A.; Funderburk, J.E.; Frantz, G. Western Flower Thrips. Featured Creatures. EDIS- EENY-883. Entomology and Nematology Department, IFAS, University of Florida, 2015. Available online: http://edis.ifas.ufl.edu/pdffiles/IN/IN108900.pdf (accessed on 22 June 2015).
- Kumar, V.; Kakkar, G.; Seal, D.; McKenzie, C.L.; Osborne, L.S. Evaluation of insecticides for curative, prophylactic and rotational use on Scirtothrips dorsalis South Asia 1. Fla. Entomol. 2017, 100, 634–646. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Ullman, D.E.; German, T.L. Tospovirus-thrips interactions. Annu. Rev. Phytopathol. 2005, 43, 451–4889. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.H.; Paskewitz, S.M.; Finnerty, V. Ribosomal RNA genes of the Anopheles gambiae species complex. Adv. Dis. Vector Res. 1989, 6, 1–28. [Google Scholar]
- Lohe, A.R.; Roberts, P.A. Evolution of DNA in heterochromatin: The Drosophila melanogaster sibling species subgroup as a resource. Genetica 2000, 109, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Brianti, M.T.; Ananina, G.; Recco-Pimentel, S.M.; Klaczko, L.B. Comparative analysis of the chromosomal positions of rDNA genes in species of the tripunctata radiation of Drosophila. cytogenet. Genome Res. 2009, 125, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Panzera, Y.; Pita, S.; Ferreiro, M.J.; Ferrandis, I.; Lages, C.; Pérez, R.; Silva, A.E.; Guerra, M.; Panzera, F. High dynamics of rDNA cluster location in kissing bug holocentric chromosomes (Triatominae, Heteroptera) cytogenet. Genome Res. 2012, 138, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Guo, F.Z.; Riley, D.; Diffie, S.; Gitaitis, R.; Sparks, A.; Jeyaprakash, A. Assessment of variation among Thrips tabaci populations from Georgia and Peru based on polymorphisms in mitochondrial cytochrome oxidase I and ribosomal ITS2 sequences. J. Entomol. Sci. 2011, 46, 191–203. [Google Scholar] [CrossRef]
- Barnes, W.M. PCR amplification of up tp 35-kb DNA with high fidelity and high yield from Lambda bacteriophage templates. Proc. Natl. Acad. Sci. USA 1994, 91, 2216–2220. [Google Scholar] [CrossRef] [PubMed]
- Eckert, K.A.; Kunkela, T.A. DNA polymerase fidelity and the polymerase chain reaction. PCR Methods Appl. 1991, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pray, L. DNA replication and causes of mutation. Nat. Edu. 2008, 1, 214. Available online: http://www.nature.com/scitable/topicpage/dna-replication-and-causes-of-mutation-409 (accessed on 15 January 2014).
- Campbell, N.J.H.; Barker, S.C. The novel mitochondrial gene arrangement of the cattle tick, Boophilus microplus: Five-fold tandem repetition of a coding region. Mol. Biol. Evol. 1999, 16, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Magnacca, K.N.; Brown, M.J.F. Mitochondrial heteroplasmy and DNA barcoding in Hawaiian Hylaeus (Nesoprosopis) bees (Hymenoptera: Colletidae). BMC Evol. Biol. 2010, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Bensasson, D.; Zhang, D-X.; Hartl, D.L.; Hewitt, G.M. Mitochondrial pseudogenes: Evolution’s misplaced witnesses. Trends Ecol. Evol. 2001, 16, 314–321. [Google Scholar] [CrossRef]
- Bensasson, D.; Zhang, D.-X.; Hewitt, G.M. Frequent assimilation of mitochondrial DNA by grasshopper nuclear genomes. Mol. Biol. Evol. 2000, 17, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Gellissen, G.; Bradfield, J.Y.; White, B.N.; Wyatt, G.R. Mitochondrial DNA sequences in the nuclear genome of a locust. Nature 1983, 301, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-X.; Hewitt, G.M. Challenge for mitochondrial DNA markers. Trends Ecol. Evol. 1996, 11, 247–251. [Google Scholar]
- Sunnucks, P.; England, P.E.; Taylor, A.C.; Hales, D.F. Microsatellite and chromosome evolution of parthenogenetic sitobion aphids in Australia. Genetics 1996, 144, 747–756. [Google Scholar] [PubMed]
- Wolff, J.N.; Shearman, D.C.; Brooks, R.C.; Ballard, J.W. Selective enrichment and sequencing of whole mitochondrial genomes in the presence of nuclear encoded mitochondrial pseudogenes (numts). PLoS ONE 2012, 7, e37142. [Google Scholar] [CrossRef] [PubMed]
- Boyce, T.M.; Zwick, M.E.; Aquadro, C.F. Mitochondrial DNA of the bark weevils: Size, structure and heteroplasmy. Genetics 1989, 123, 825–836. [Google Scholar] [PubMed]
- Brunner, A.M.; Schimenti, J.C.; Duncan, C.H. Dual evolutionary modes in the bovine globin locus. Biochemistry 1986, 25, 5028–5035. [Google Scholar] [CrossRef] [PubMed]
- Murti, J.R.; Bumbulis, M.; Schimenti, J.C. High-frequency germ line gene conversion in transgenic mice. Mol. Cell Biol. 1992, 12, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Mayol, M.; Rossello, J.A. Why nuclear ribosomal DNA spacers (ITS) tell different stories in Quercus. Mol. Phylogenet. Evol. 2001, 19, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Fritz, G.N.; Conn, J.; Cockburn, A.; Seawright, J. Sequence analysis of the ribosomal DNA internal transcribed spacer 2 from a population of Anopheles nuneztovari (Diptera: Culicidae). Mol. Biol. Evol. 1994, 11, 406–416. [Google Scholar] [PubMed]
- Tautz, D.; Hancock, J.M.; Webb, D.A.; Tautz, C.; Dover, G.A. Complete sequences of the ribosomal RNA genes of Drosophilla mealogaster. Mol. Biol. Evol. 1988, 5, 366–376. [Google Scholar] [PubMed]
- Tang, J.M.; Toe, L.; Back, C.; Unnasch, T.R. Intraspecific heterogeneity of the rDNA internal transcribed spacer in the Simulium damnsoum (Diptera: Simuliidae) complex. Mol. Biol. Evol. 1996, 13, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Gasser, R.B.; Zhu, X.; Chilton, N.B.; Newton, L.A.; Nedegaard, T.; Guldberg, P. Analysis of sequence homogenisation in rDNA arrays of Haemonchus contortus by denaturing gradient gel electrophoresis. Electrophoresis 1998, 19, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Harpke, D.; Peterson, A. Non-concerted ITS evolution in Mammillaria (Cactaceae). Mol. Phylogenet. Evol. 2006, 41, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Benevolenskaya, E.V.; Kogan, G.L.; Tulin, A.V.; Phillip, D.; Gvozdev, V.A. Segmented gene conversion as a mechanism of correction of 18s rRNA pseudogene located outside of rDNA cluster in D. melanogaster. J. Mol. Evol. 1997, 44, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Brownell, E.; Krystal, M.; Arnheim, N. Structure and evolution of human and African Ape rDNA pseudogenes. Mol. Biol. Evol. 1983, 1, 29–37. [Google Scholar] [PubMed]
- Razafimandimbison, S.G.; Kellogg, E.A.; Bremer, B. Recent origin and phylogenetic utility of divergent ITS putative pseudogenes: A case study from Naucleeae (Rubiaceae). Syst. Biol. 2004, 53, 177–192. [Google Scholar] [CrossRef]
- Scholin, C.A.; Anderson, D.M.; Sogin, M.L. Two distinct small subunit ribosomal RNA genes in the North American toxic dinoflagellates Alexandrium fundyense (Dinophyceae). J. Phycol. 1993, 29, 209–216. [Google Scholar] [CrossRef]
- Santos, S.R.; Kinzie, R.A.; Sakai, K.; Coffroth, M.A. Molecular characterization of nuclear small subunit (18S)-rDNA pseudogenes in a symbiotic dinoflagellate (Symbiodinium, Dinophyta). J. Eukar. Microbiol. 2003, 50, 417–421. [Google Scholar] [CrossRef]
- Bailey, C.D.; Carr, T.G.; Harris, S.A.; Hughes, C.E. Characterization of agiosperm nrDNA polymorphism, paralogy, and pseudogenes. Mol. Phylogenet. Evol. 2003, 29, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Capinera, J.L. Melon Thrips. Featured Creatures. EDIS- EENY-135. Entomology and Nematology Department, IFAS, University of Florida, 2000 (Updated 2013). Available online: http://entnemdept.ufl.edu/creatures/veg/melon_thrips.htm (accessed on 11 March 2016).
- Kakkar, G.; Seal, D.R.; Stansly, P.A.; Liburd, O.E.; Kumar, V. Abundance of Frankliniella schultzei (Thysanoptera: Thripdae) in flowers on major vegetable crops of south Florida. Florida Entomol. 2012, 95, 468–475. [Google Scholar] [CrossRef]
- Hoddle, M.S.; Mound, L.A.; Paris, D.L. Scirtothrips Dorsalis. Thrips of California. University of California: California, USA, 2009. Available online: http://keys.lucidcentral.org/keys/v3/thrips_of_california/data/key/thysanoptera/Media/Html/browse_species/Scirtothrips_dorsalis.htm (accessed on 11 July 2016).
- Folmer, O.; Black, M.; Hoehm, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Campbell, B.C.; Steffen-Campbell, J.D.; Werren, J.H. Phylogeny of the Nasonia species complex (Hymenoptera: Pteromalidae) inferred from an internal transcribed spacer (ITS2) and 28S rDNA sequences. Insect Mol. Biol. 1993, 2, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. ClustalW and ClustalX version 2. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 2.75. 2011. Available online: http://mesquiteproject.org (accessed on 12 October 2014).
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A. Tracer Version 1.6. 2007. Available online: http://tree.bio.ed.ac.uk/software/tracer/ (accessed on 16 October 2014).
- Sukumaran, J.; Holder, M.T. DendroPy: A Python library for phylogenetic computing. Bioinformatics 2010, 26, 1569–1571. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree, a Graphical Viewer of Phylogenetic Trees. Version 1.4. 2007. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 25 October 2014).
- S.A.S. Institute. Version 8.0.1. SAS Institute: Cary, NC, USA, 2009. [Google Scholar]
| Cytochrome Oxidase 1 (mtCO1) | Internal Transcribed Spacer 2 (ITS2) | |||||
|---|---|---|---|---|---|---|
| Individual a | No. of Clones Sequenced | No. of Different Haplotypes | Freq. of Most Common Haplotype (%) | No. of Clones Sequenced | No. of Different Haplotypes | Freq. of Most Common Haplotype (%) |
| SD-1 | 33 | 4 | 90.9 | 23 | 17 | 21.7 |
| SD-2 | 44 | 11 | 77.2 | 26 | 19 | 23.0 |
| SD-3 | 24 | 4 | 87.5 | 42 | 21 | 33.3 |
| SD-4 | 31 | 5 | 87.0 | 46 | 19 | 26.0 |
| All Scirtothrips dorsalis clones | 132 | 21 | 84.8 | 137 | 71 | 10.9 |
| TP-1 | 42 | 5 | 90.4 | 41 | 23 | 24.3 |
| TP-2 | 24 | 3 | 91.6 | 38 | 18 | 18.4 |
| TP-3 | 24 | 1 | 100 | 31 | 16 | 22.5 |
| TP-4 | 30 | 4 | 90 | 39 | 28 | 15.3 |
| All Thrips palmi clones | 120 | 11 | 60.8 | 149 | 76 | 14.7 |
| FO-1 | 42 | 6 | 85.7 | 20 | 4 | 85 |
| FO-2 | 31 | 4 | 87.0 | 17 | 2 | 94.1 |
| FO-3 | 46 | 5 | 86.9 | 36 | 7 | 83.3 |
| FO-4 | 31 | 2 | 96.7 | 32 | 3 | 93.7 |
| All Frankliniella occidentalis clones | 150 | 14 | 88.6 | 105 | 14 | 72.3 |
| Thrips Species | Cytochrome Oxidase 1 (mtCO1) | Internal Transcribed Spacer 2 (ITS2) | ||||
|---|---|---|---|---|---|---|
| Intragenomic Divergence | Intergenomic Divergence | Intragenomic > Intergenomic Var. (%) | Intragenomic Divergence | Intergenomic Divergence | Intragenomic > Intergenomic Var. (%) | |
| Scirtothrips dorsalis | 0.59 ± 0.02 | 0.61 ± 0.01 | 25 | 1.45 ± 0.03 | 1.83 ± 0.02 | 25 |
| Thrips palmi | 0.41 ± 0.04 | 0.81 ± 0.04 | 16.6 | 1.26 ± 0.02 | 1.37 ± 0.01 | 41.6 |
| Frankliniella occidentalis | 0.66 ± 0.05 | 0.78 ± 0.04 | 33.3 | 0.74 ± 0.05 | 0.98 ± 0.05 | 25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Dickey, A.M.; Seal, D.R.; Shatters, R.G.; Osborne, L.S.; McKenzie, C.L. Unexpected High Intragenomic Variation in Two of Three Major Pest Thrips Species Does Not Affect Ribosomal Internal Transcribed Spacer 2 (ITS2) Utility for Thrips Identification. Int. J. Mol. Sci. 2017, 18, 2100. https://doi.org/10.3390/ijms18102100
Kumar V, Dickey AM, Seal DR, Shatters RG, Osborne LS, McKenzie CL. Unexpected High Intragenomic Variation in Two of Three Major Pest Thrips Species Does Not Affect Ribosomal Internal Transcribed Spacer 2 (ITS2) Utility for Thrips Identification. International Journal of Molecular Sciences. 2017; 18(10):2100. https://doi.org/10.3390/ijms18102100
Chicago/Turabian StyleKumar, Vivek, Aaron M. Dickey, Dakshina R. Seal, Robert G. Shatters, Lance S. Osborne, and Cindy L. McKenzie. 2017. "Unexpected High Intragenomic Variation in Two of Three Major Pest Thrips Species Does Not Affect Ribosomal Internal Transcribed Spacer 2 (ITS2) Utility for Thrips Identification" International Journal of Molecular Sciences 18, no. 10: 2100. https://doi.org/10.3390/ijms18102100