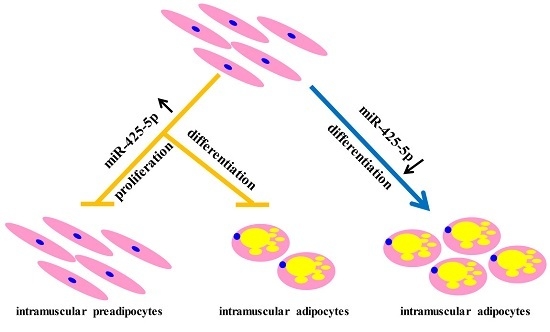
miR-425-5p Inhibits Differentiation and Proliferation in Porcine Intramuscular Preadipocytes
Abstract
:1. Introduction
2. Results
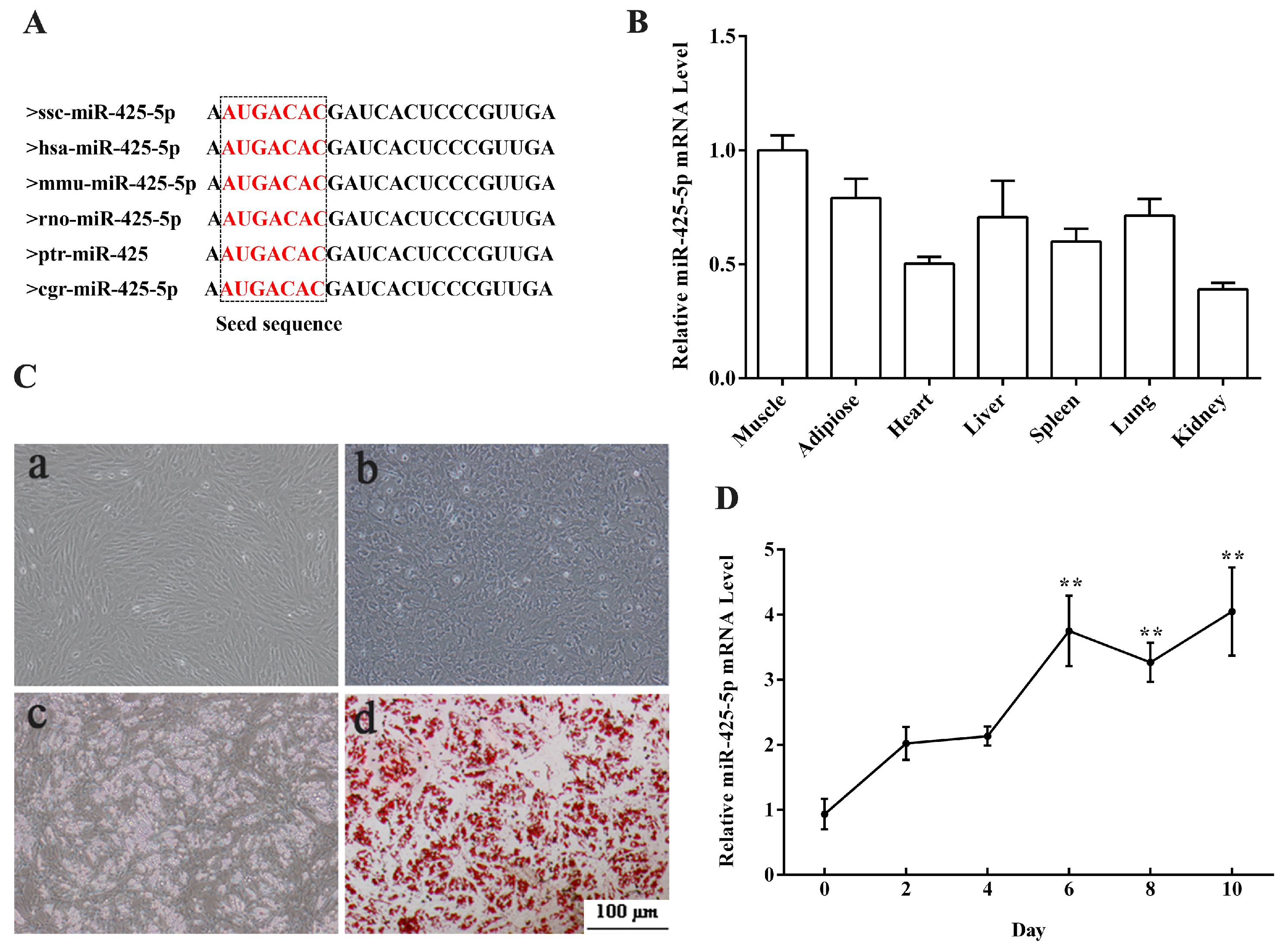
2.1. Expression Profiles of miR-425-5p in Porcine Tissues and during Intramuscular Preadipocytes Adipogenesis
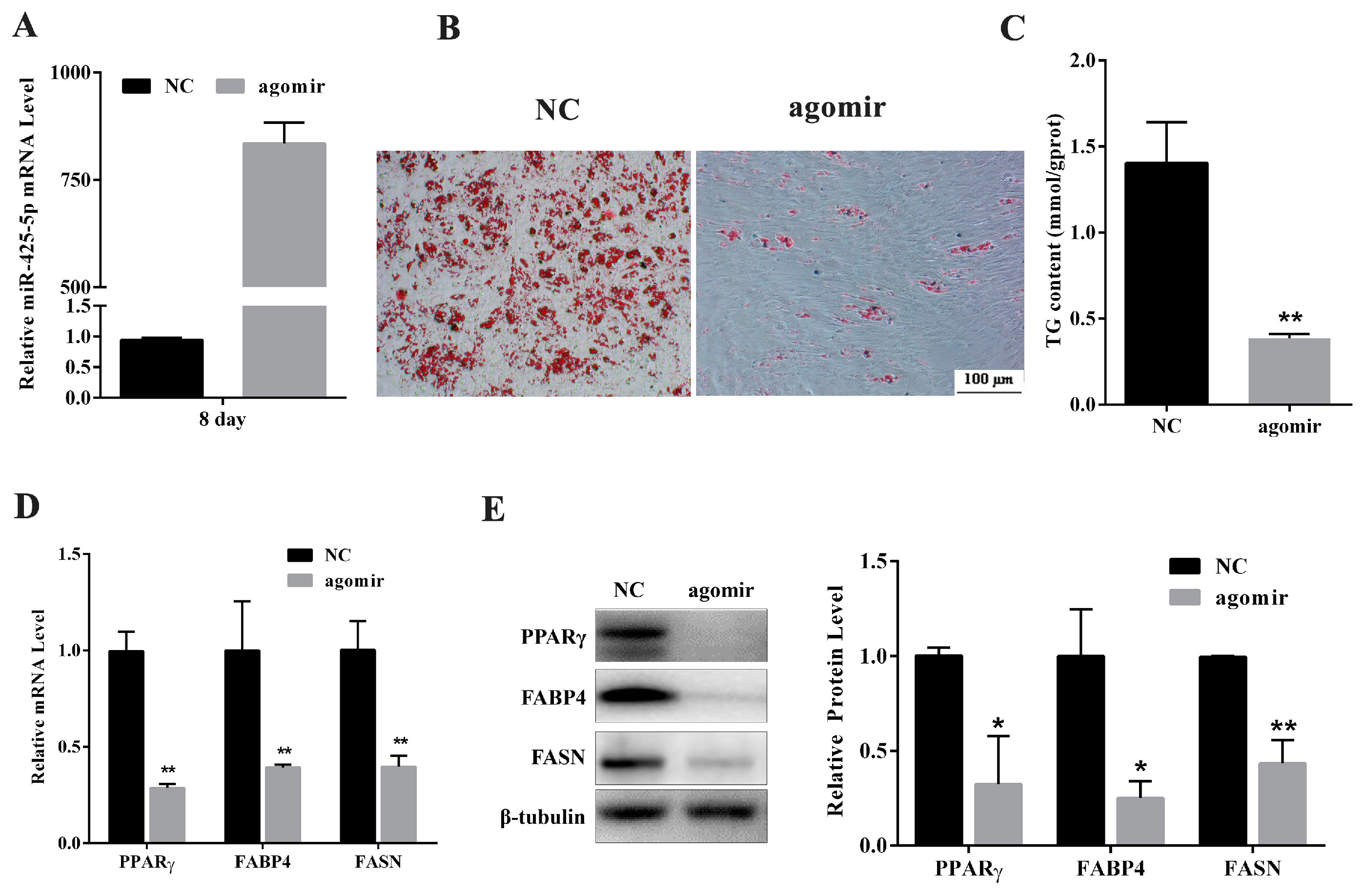
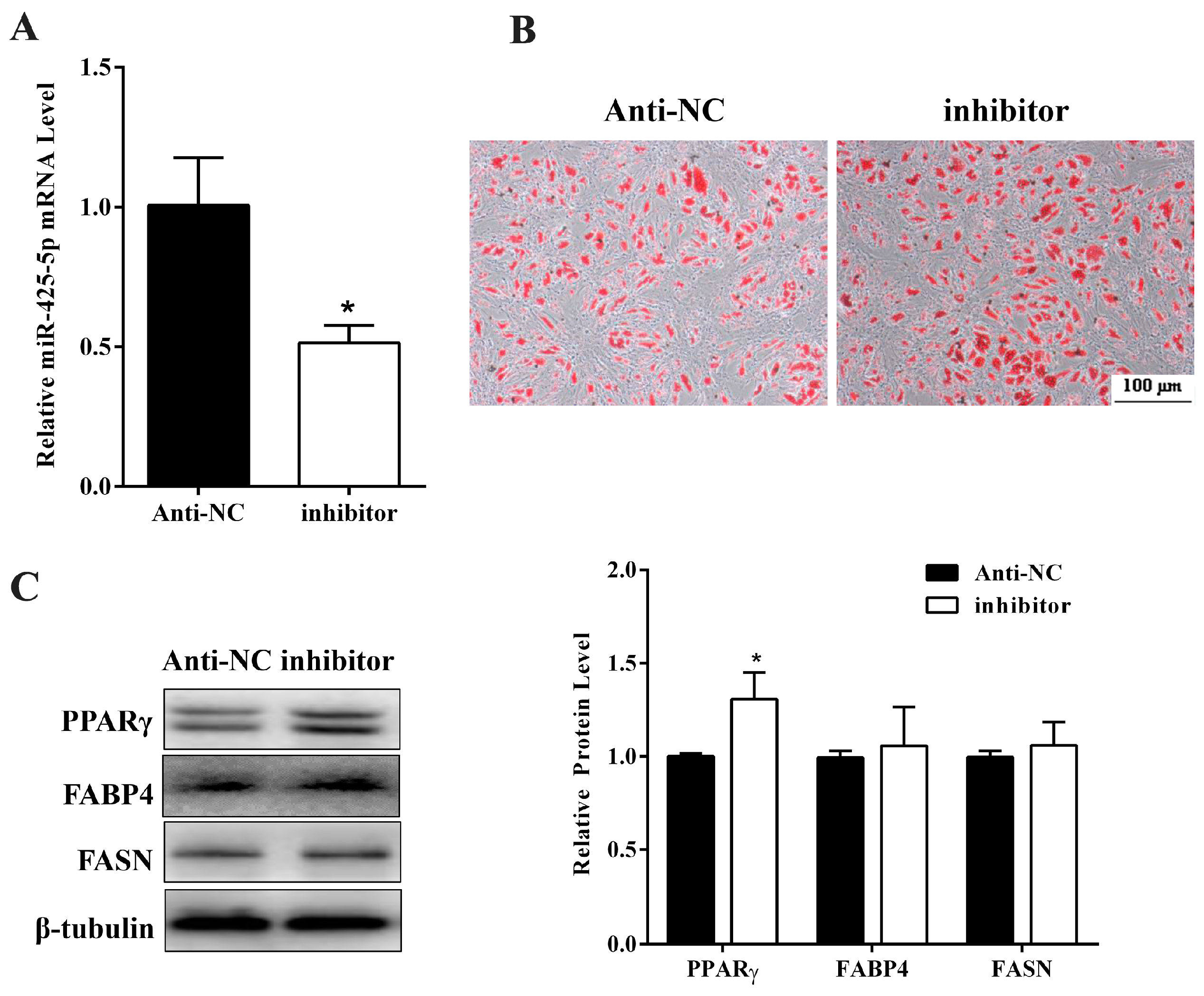
2.2. miR-425-5p Inhibits Differentiation of Porcine Intramuscular Preadipocytes
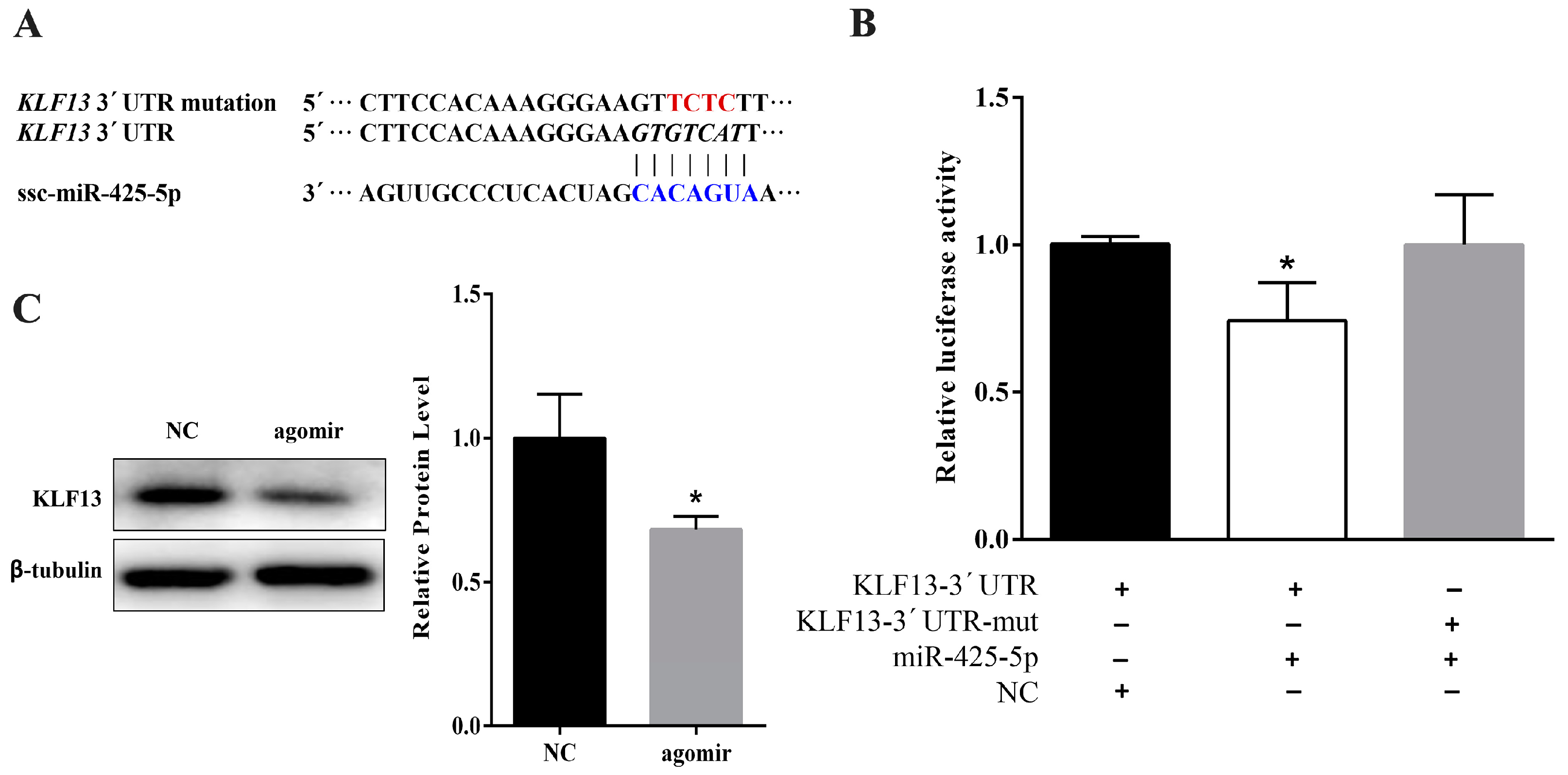
2.3. miR-425-5p Can Target KLF13 in Porcine Intramuscular Preadipocytes
2.4. miR-425-5p Inhibits Porcine Intramuscular Preadipocytes Proliferation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Porcine Intramuscular Preadipocytess Isolation and Induction
4.3. Transfection of miRNA Agomir and Inhibitor
4.4. RNA Extraction and RT-PCR
4.5. Western Blotting
4.6. Oil Red O Staining
4.7. Measurement TG
4.8. Luciferase Reporter Assay
4.9. Flow Cytometry
4.10. Cell Vitality Analysis
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PPARγ | Peroxisome proliferator activated receptor γ |
| FABP4 | Adipocyte fatty acid-binding protein 4 |
| FASN | Fatty acid synthase |
| KLF13 | Krüppel-like transcription factor 13 |
| Cyclin E | Cell cycle protein E |
| Cyclin B | Cell cycle protein B |
| Cyclin D | Cell cycle protein D |
| CDK4 | Cyclin-dependent kinases 4 |
| ssc | Susscrofa |
| hsa | Homo sapiens |
| mmu | Musmusculus |
| rno | Rattusnorvegicus |
| ptr | Pan troglodytes |
| cgr | Cricetulusgriseus |
References
- Hocquette, J.F.; Gondret, F.; Baeza, E.; Medale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Anim. Int. J. Anim. Biosci. 2010, 4, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Z.; Chen, X.C.; Wu, W.J.; Wang, W.S.; Pang, W.J.; Yang, G.S. Mat2b promotes adipogenesis by modulating same levels and activating akt/erk pathway during porcine intramuscular preadipocyte differentiation. Exp. Cell Res. 2016, 344, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.X.; Dodson, M.V.; Jiang, Z.H.; Yu, S.G.; Chu, W.W.; Chen, J. Myostatin inhibits porcine intramuscular preadipocyte differentiation in vitro. Domest. Anim. Endocrinol. 2016, 55, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; Jeffery, E.; Rodeheffer, M.S. Weighing in on adipocyte precursors. Cell Metab. 2014. [Google Scholar] [CrossRef] [PubMed]
- Poulos, S.P.; Dodson, M.V.; Hausman, G.J. Cell line models for differentiation: Preadipocytes and adipocytes. Exp. Biol. Med. 2010, 235, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Luo, Y.L.; Huang, Z.Q.; Jia, G.; Liu, G.M.; Zhao, H. Role of phosphotyrosine interaction domain containing 1 in porcine intramuscular preadipocyte proliferation and differentiation. Anim. Biotechnol. 2016, 27, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.V.; Allen, R.E.; Du, M.; Bergen, W.G.; Velleman, S.G.; Poulos, S.P.; Fernyhough-Culver, M.; Wheeler, M.B.; Duckett, S.K.; Young, M.R.; et al. Invited review: Evolution of meat animal growth research during the past 50 years: Adipose and muscle stem cells. J. Anim. Sci. 2015, 93, 457–481. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Micrornas: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. Micrornas: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Tufekci, K.U.; Meuwissen, R.L.; Genc, S. The role of micrornas in biological processes. Methods Mol. Biol. 2014, 1107, 15–31. [Google Scholar] [PubMed]
- Wang, Z.; Qiao, Y.; Zhang, J.; Shi, W.; Zhang, J. Genome wide identification of micrornas involved in fatty acid and lipid metabolism of brassica napus by small RNA and degradome sequencing. Gene 2017, 619, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qiu, Y.; Liu, S.; Dong, P.; Ning, X.; Li, Y.; Yang, G.; Sun, S. Over-expressed miR-103 promotes porcine adipocyte differentiation. Chin. J. Biotechnol. 2012, 28, 927–936. [Google Scholar]
- Li, H.; Chen, X.; Guan, L.; Qi, Q.; Shu, G.; Jiang, Q.; Yuan, L.; Xi, Q.; Zhang, Y. MiRNA-181a regulates adipogenesis by targeting tumor necrosis factor-α (TNF-α) in the porcine model. PLoS ONE 2013, 8, e71568. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Mai, Y.; Zhang, Z.; Mi, L.; Wu, G.; Chu, G.; Yang, G.; Sun, S. miR-15a/b promote adipogenesis in porcine pre-adipocyte via repressing foxo1. Acta Biochim. Biophys. Sin. 2014, 46, 565–571. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Ma, K.; Zhang, Z.; Zhao, T.; Zhang, X.; Tang, B.; Li, Z. miR-17, miR-21 and miR-143 enhance adipogenic differentiation from porcine bone marrow-derived mesenchymal stem cells. DNA Cell Biol. 2016, 35, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Liu, S.; Qiu, Y.; Li, G.; Li, Y.; Li, M.; Yang, G. Expression profiles and biological roles of miR-196a in swine. Genes 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xi, Q.Y.; Cheng, X.; Dong, T.; Zhu, X.T.; Shu, G.; Wang, L.N.; Jiang, Q.Y.; Zhang, Y.L. miR-146a-5p inhibits TNF-α-induced adipogenesis via targeting insulin receptor in primary porcine adipocytes. J. Lipid Res. 2016, 57, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Zhang, Y.; Zhang, Y.; Chen, L.; Mo, D. Up-regulated miR-145 expression inhibits porcine preadipocytes differentiation by targeting IRS1. Int. J. Biol. Sci. 2012, 8, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.L.; Song, C.C.; Li, Y.F.; He, J.J.; Li, Y.L.; Zheng, X.L.; Yang, G.S. miR-125a inhibits porcine preadipocytes differentiation by targeting erralpha. Mol. Cell. Biochem. 2014, 395, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.E.; Li, Y.F.; Jia, L.; Ji, H.L.; Song, Z.Y.; Cheng, J.; Wu, G.F.; Song, C.C.; Zhang, Q.L.; Zhu, J.Y.; et al. MicroRNA-199a-5p affects porcine preadipocyte proliferation and differentiation. Int. J. Mol. Sci. 2014, 15, 8526–8538. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, G.; Yuan, B.; Zhang, L.; Gao, Y.; Jiang, H.; Dai, L.; Zhang, J. miR-375 negatively regulates porcine preadipocyte differentiation by targeting BMPR2. FEBS Lett. 2016, 590, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, F.F.; Ge, J.; Zhu, J.Y.; Shi, X.E.; Li, X.; Yu, T.Y.; Chu, G.Y.; Yang, G.S. miR-429 inhibits differentiation and promotes proliferation in porcine preadipocytes. Int. J. Mol. Sci. 2016, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Li, W.; Bao, L.; Han, P.; Yang, W.; Ma, L.; Meng, F.; Cao, B. Pu.1 promotes miR-191 to inhibit adipogenesis in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2014, 451, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, T.; Zhang, N.; Yang, X.; Wang, Z.; Ma, J.; Gu, X.; Fan, Y.; Cai, D. miR-425 up-regulation induced by interleukin-1beta promotes the proliferation of gastric cancer cell ags. Zhonghua Yi Xue Za Zhi 2014, 94, 1889–1893. (In Chinese) [Google Scholar] [PubMed]
- Liu, L.; Zhao, Z.; Zhou, W.; Fan, X.; Zhan, Q.; Song, Y. Enhanced expression of miR-425 promotes esophageal squamous cell carcinoma tumorigenesis by targeting SMAD2. J. Genet. Genom. 2015, 42, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yin, Y.; Xing, X.; Zhao, Z.; Lu, Y.; Sun, Y.; Zhuang, Z.; Wang, M.; Ji, W.; He, Y. Arsenic-induced anti-angiogenesis via miR-425-5p-regulated CCM3. Toxicol. Lett. 2016, 254, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Dong, H.; Liu, S.; Yu, L.; Yan, D.; Yao, X.; Sun, W.; Han, D.; Gao, G. Long noncoding RNA mhencr promotes melanoma progression via regulating miR-425/489-mediated pi3k-akt pathway. Am. J. Transl. Res. 2017, 9, 90–102. [Google Scholar] [PubMed]
- Nakagawa, R.; Muroyama, R.; Saeki, C.; Goto, K.; Kaise, Y.; Koike, K.; Nakano, M.; Matsubara, Y.; Takano, K.; Ito, S.; et al. miR-425 regulates inflammatory cytokine production in CD4+ T cells via n-Ras upregulation in primary biliary cholangitis. J. Hepatol. 2017, 66, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wen, M.; Guo, J.; Shi, J.; Wang, Z.; Tan, B.; Zhang, G.; Zheng, X.; Zhang, A. Clinical value of miR-425-5p detection and its association with cell proliferation and apoptosis of gastric cancer. Pathol. Res. Pract. 2017, 213, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hu, Y.; Ma, L.; Du, M.; Xia, L.; Hu, Z. MiR-425 inhibits melanoma metastasis through repression of pi3k-akt pathway by targeting IGF-1. Biomed. Pharmacother. 2015, 75, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wei, H.; Song, T.; Yang, Y.; Zhang, F.; Zhou, Y.; Peng, J.; Jiang, S. Klf13 promotes porcine adipocyte differentiation through ppargamma activation. Cell Biosci. 2015, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.; Fleetwood, J.; Eaton, S.; Crossley, M.; Bao, S. Kruppel-like transcription factors: A functional family. Int. J. Biochem. Cell Biol. 2008, 40, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Li, T.; Guan, L.; Liu, F.E.; Chen, X.M.; Zhao, J.; Lin, S.; Liu, Z.Z.; Zhang, H.Q. Ctnna3 is a tumor suppressor in hepatocellular carcinomas and is inhibited by miR-425. Oncotarget 2016, 7, 8078–8089. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Feve, B. Adipogenesis: Cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Q.; Zhu, L.; Ma, M.L.; Chen, X.C.; Gao, Y.; Yu, T.Y.; Yang, G.S.; Pang, W.J. Mulberry 1-deoxynojirimycin inhibits adipogenesis by repression of the Erk/ppargamma signaling pathway in porcine intramuscular adipocytes. J. Agric. Food Chem. 2015, 63, 6212–6220. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Wang, Y.; Wei, N.; Xu, R.; Xiong, Y.; Wang, P.; Shen, Q.; Yang, G. Sirt1 inhibits AKT2-mediated porcine adipogenesis potentially by direct protein-protein interaction. PLoS ONE 2013, 8, e71576. [Google Scholar] [CrossRef] [PubMed]

| Gene | Accession Number | Orientation | Primer Sequences (5′-3′) | Production Length (bp) |
|---|---|---|---|---|
| PPARγ | NM_214379 | Forward | AGGACTACCAAAGTGCCATCAAA | 142 |
| Reverse | GAGGCTTTATCCCCACAGACAC | |||
| FABP4 | HM_453202 | Forward | GAGCACCATAACCTTAGATGGA | 121 |
| Reverse | AAATTCTGGTAGCCGTGACA | |||
| FASN | EF589048.1 | Forward | AGCCTAACTCCTCGCTGCAAT | 196 |
| Reverse | TCCTTGGAACCGTCTGTGTTC | |||
| Cyclin B | NM_001170768 | Forward | AATCCCTTCTTGTGGTTA | 104 |
| Reverse | CTTAGATGTGGCATACTTG | |||
| CDK4 | NM_001123097 | Forward | ATCAGCACGGTTCGTGAAGT | 133 |
| Reverse | GCTCAAACACCAGGGTCACT | |||
| GAPDH | KJ786424 | Forward | AGGTCGGAGTGAACGGATTTG | 118 |
| Reverse | ACCATGTAGTGGAGGTCAATGAAG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.-F.; Xiong, Y.; Peng, Y.; Gao, Y.; Qin, J.; Chu, G.-Y.; Pang, W.-J.; Yang, G.-S. miR-425-5p Inhibits Differentiation and Proliferation in Porcine Intramuscular Preadipocytes. Int. J. Mol. Sci. 2017, 18, 2101. https://doi.org/10.3390/ijms18102101
Chen F-F, Xiong Y, Peng Y, Gao Y, Qin J, Chu G-Y, Pang W-J, Yang G-S. miR-425-5p Inhibits Differentiation and Proliferation in Porcine Intramuscular Preadipocytes. International Journal of Molecular Sciences. 2017; 18(10):2101. https://doi.org/10.3390/ijms18102101
Chicago/Turabian StyleChen, Fen-Fen, Yan Xiong, Ying Peng, Yun Gao, Jin Qin, Gui-Yan Chu, Wei-Jun Pang, and Gong-She Yang. 2017. "miR-425-5p Inhibits Differentiation and Proliferation in Porcine Intramuscular Preadipocytes" International Journal of Molecular Sciences 18, no. 10: 2101. https://doi.org/10.3390/ijms18102101