New Strategy for the Immobilization of Lipases on Glyoxyl–Agarose Supports: Production of Robust Biocatalysts for Natural Oil Transformation
Abstract
:1. Introduction
2. Results and Discussion
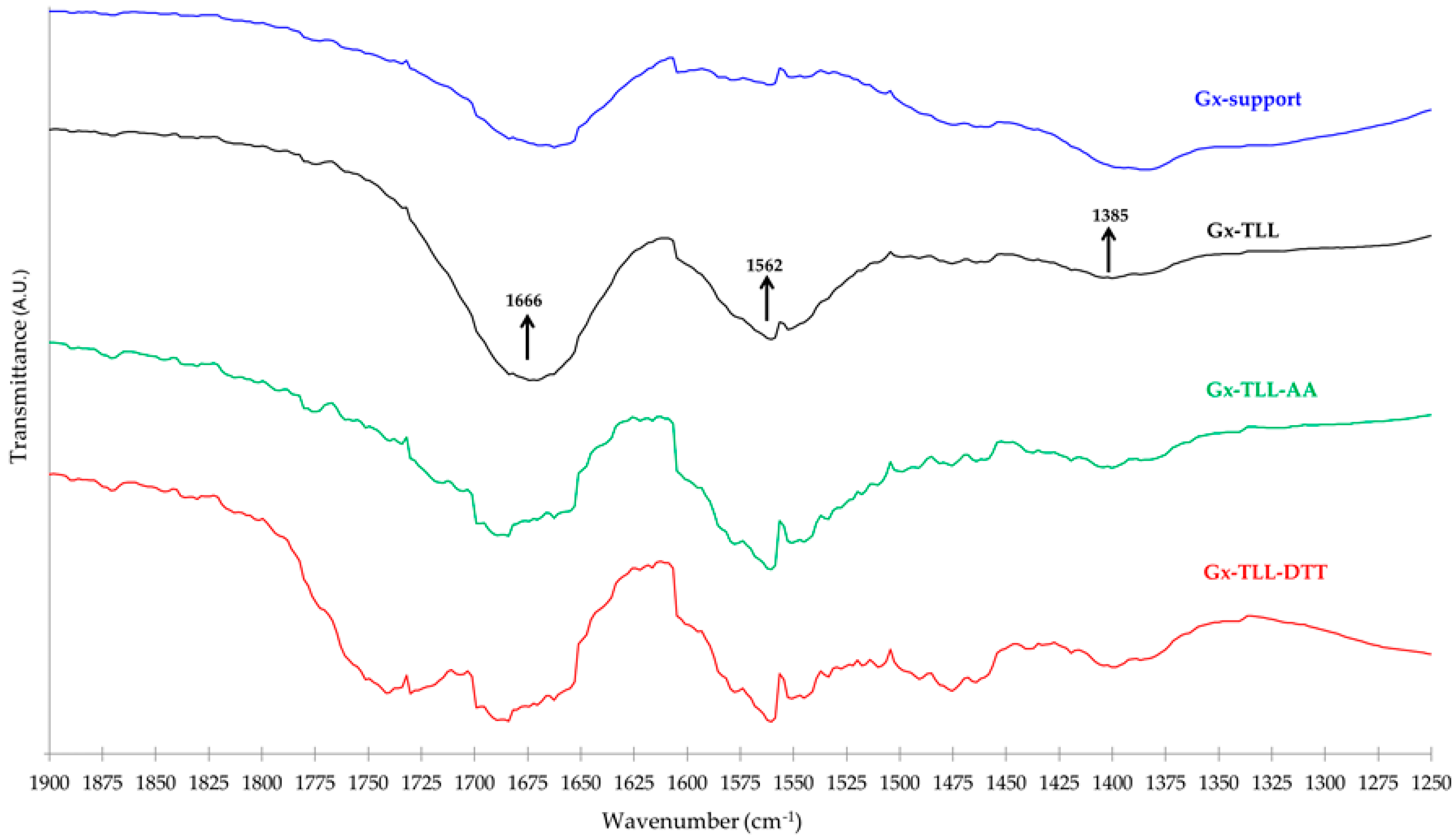
2.1. Immobilization of Enzymes at Neutral pH on Gx: Suggested Mechanism of Gx Modification and Enzyme Immobilization with Additives
2.2. Effect of the Initial and Final pH on Derivative Recovered Activity and Stability: Production of a Highly Stabilized Biocatalyst
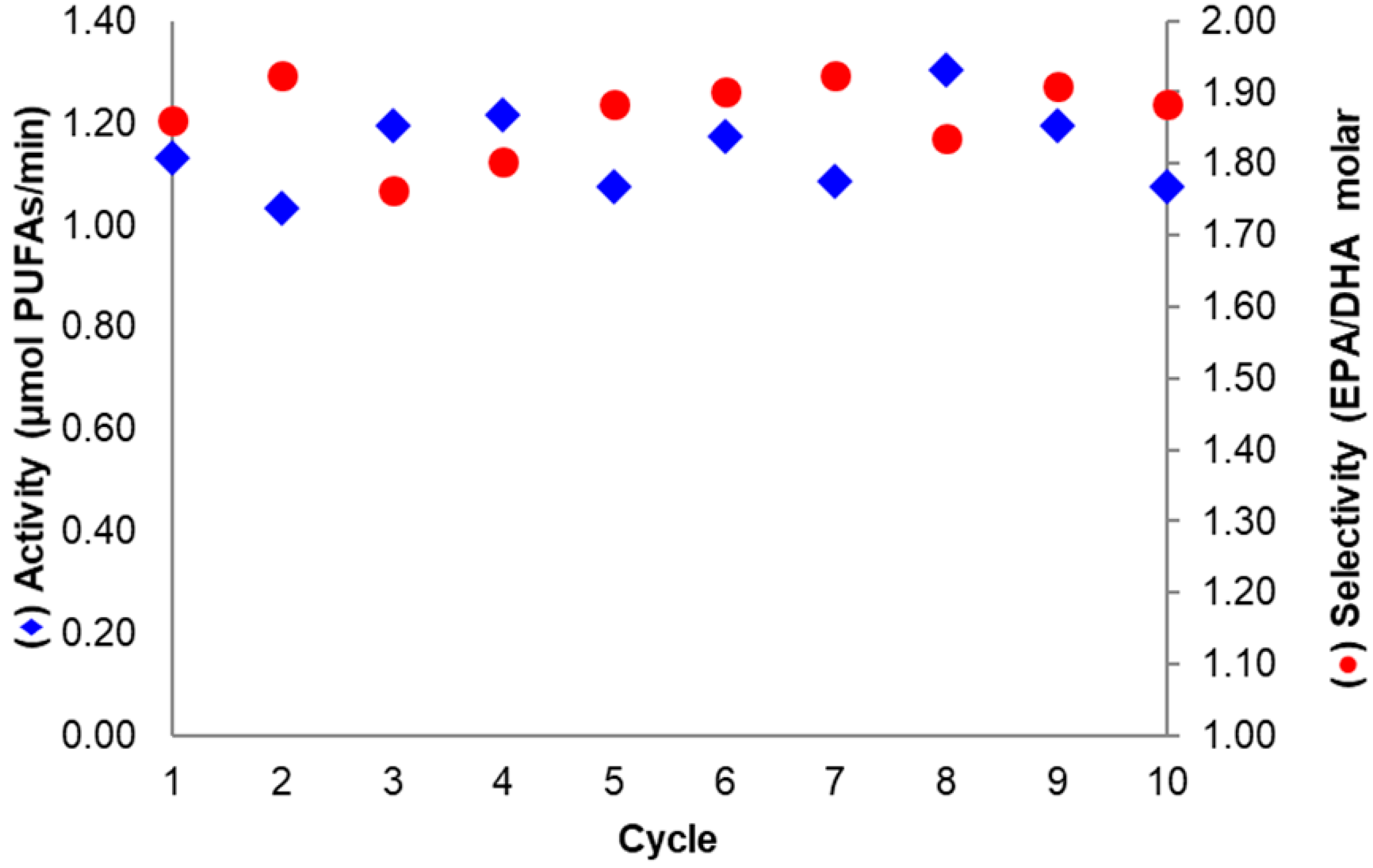
2.3. Hydrolysis of Fish Oil
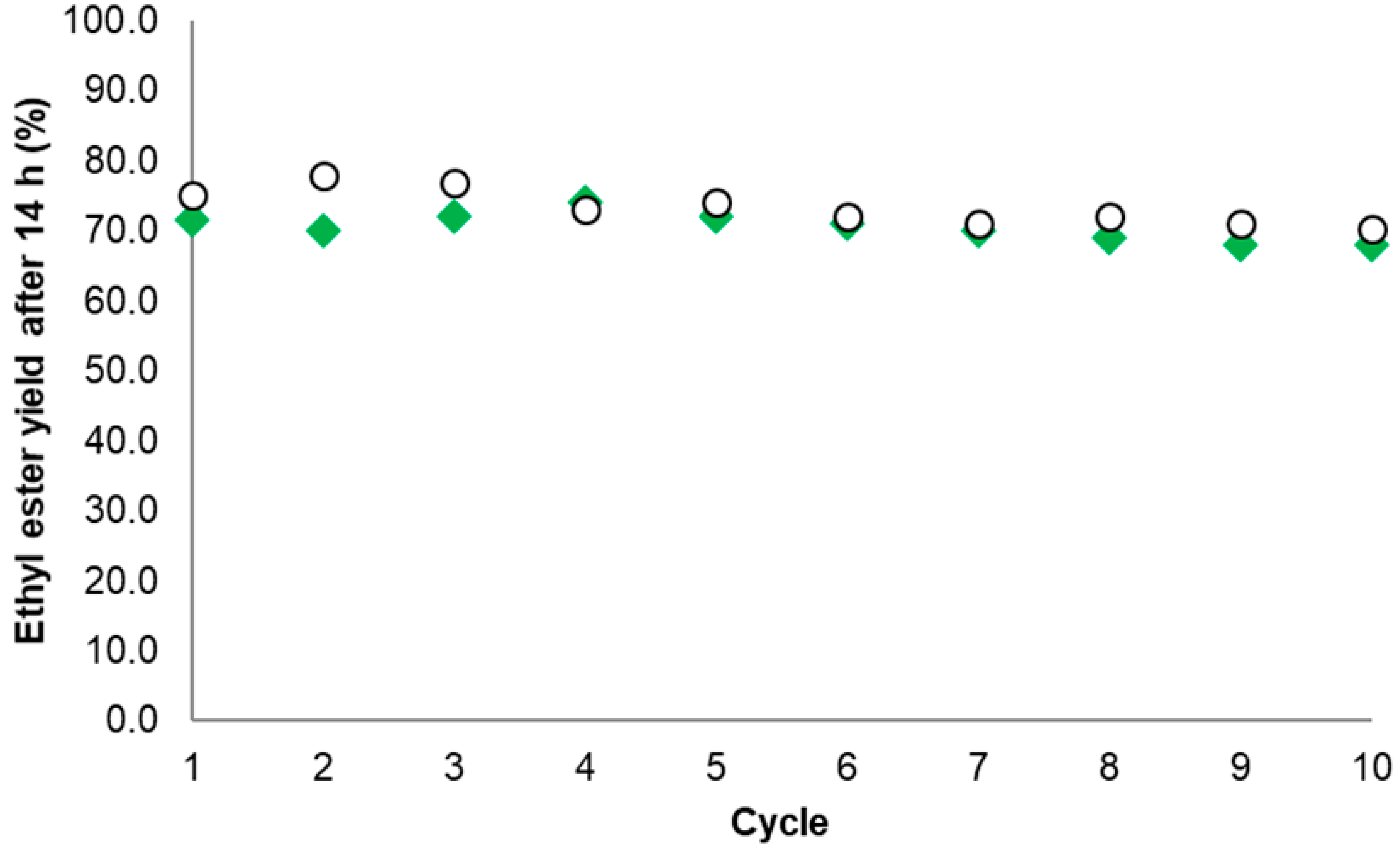
2.4. Ethyl Ester (EE) Production from Palm Olein
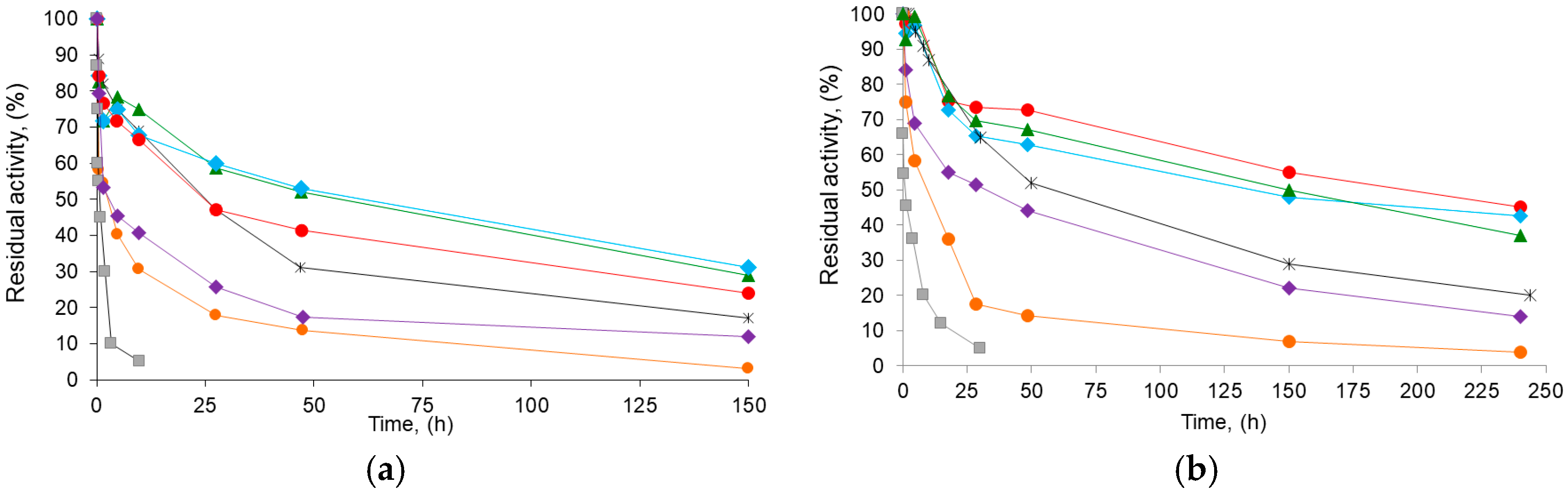
2.5. Operational Stability of Selected Biocatalysts
3. Materials and Methods
3.1. Materials
3.2. Production of Derivatives
3.2.1. Immobilizations on Glyoxyl–Agarose Support (Gx)
3.2.2. Basification: Incubation at Higher pH of DTT-Immobilized BTL2 on Gx
3.3. Derivative Stability
3.4. Hydrolytic Activity and Protein Determination
3.5. One-Step Solvent-Free Ethyl Ester Production from Palm Olein
3.6. Operational Stability of Selected Derivatives
3.7. Spectroscopic Measures of Modified Gx Supports
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2016. [Google Scholar] [CrossRef]
- Gricajeva, A.; Kalediene, L. Lipase-secreting bacillus species: From soil bacteria to promising strains for a variety of applications in biotechnology. J. Biotechnol. 2016, 231, S55. [Google Scholar] [CrossRef]
- Mendes, A.A.; de Castro, H.F.; Giordano, R.L.C. Covalent attachment of lipases on glyoxyl-agarose beads: Application in fruit flavor and biodiesel synthesis. Int. J. Biol. Macromol. 2014, 70, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Soler, L.; Illanes, A.; Wilson, L. Immobilization of Alcaligenes sp. lipase as catalyst for the transesterification of vegetable oils to produce biodiesel. Catal. Today 2016, 259, 177–182. [Google Scholar] [CrossRef]
- Zdarta, J.; Wysokowski, M.; Norman, M.; Kołodziejczak-Radzimska, A.; Moszyński, D.; Maciejewski, H.; Ehrlich, H.; Jesionowski, T. Candida antarctica Lipase B Immobilized onto Chitin Conjugated with POSS® Compounds: Useful Tool for Rapeseed Oil Conversion. Int. J. Mol. Sci. 2016, 17, 1581. [Google Scholar] [CrossRef] [PubMed]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.L.; Tonetto, G.M. Enzymatic Synthesis of Structured Triglycerides: From Laboratory to Industry; Springer: Basel, Switzerland, 2017; ISBN 978-3-319-51574-8. [Google Scholar]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, R.; Show, P.L.; Chang, J.-S. Biodiesel production using immobilized lipase: Feasibility and challenges. Biofuels Bioprod. Biorefining 2016, 10, 896–916. [Google Scholar] [CrossRef]
- Rajendran, A.; Palanisamy, A.; Thangavelu, V. Lipase catalyzed ester synthesis for food processing industries. Braz. Arch. Biol. Technol. 2009, 52, 207–219. [Google Scholar] [CrossRef]
- Ferreira-Dias, S.; Sandoval, G.; Plou, F.; Valero, F. The potential use of lipases in the production of fatty acid derivatives for the food and nutraceutical industries. Electron. J. Biotechnol. 2013, 16. [Google Scholar] [CrossRef]
- Andualema, B.; Gessesse, A. Microbial lipases and their industrial applications: Review. Biotechnology 2012, 11, 100–118. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Trono, D. Recombinant Lipases and Phospholipases and Their Use as Biocatalysts for Industrial Applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef] [PubMed]
- De Godoy Daiha, K.; Angeli, R.; de Oliveira, S.D.; Almeida, R.V. Are Lipases Still Important Biocatalysts? A Study of Scientific Publications and Patents for Technological Forecasting. PLoS ONE 2015, 10, e0131624. [Google Scholar] [CrossRef]
- Norjannah, B.; Ong, H.C.; Masjuki, H.H.; Juan, J.C.; Chong, W.T. Enzymatic transesterification for biodiesel production: A comprehensive review. RSC Adv. 2016, 6, 60034–60055. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Guisan, J.M.; Bastida, A.; Blanco, R.M.; Fernández-Lafuente, R.; García-Junceda, E. Immobilization of Enzymes on Glyoxyl Agarose: Strategies for Enzyme Stabilization by Multipoint ATTACHMENT. In Immobilization of Enzymes and Cells; Humana Press: Clifton, NJ, USA, 1996; Volume 1, pp. 277–288. ISBN 0-89603-386-4. [Google Scholar]
- Godoy, C.A.; de las Rivas, B.; Bezbradica, D.; Bolivar, J.M.; López-Gallego, F.; Fernandez-Lorente, G.; Guisan, J.M. Reactivation of a thermostable lipase by solid phase unfolding/refolding: Effect of cysteine residues on refolding efficiency. Enzym. Microb. Technol. 2011, 49, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C. Improved reactivation of immobilized-stabilized lipase from thermomyces lanuginosus by its coating with highly hydrophilic polymers. J. Biotechnol. 2009, 144, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lorente, G. Immobilization of Proteins on Glyoxyl Activated Supports: Dramatic Stabilization of Enzymes by Multipoint Covalent Attachment on Pre-Existing Supports. Curr. Org. Chem. 2015, 19, 1–13. [Google Scholar] [CrossRef]
- Talley, K.; Alexov, E. On the pH-optimum of activity and stability of proteins. Proteins Struct. Funct. Bioinform. 2010, 78, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Godoy, C.A.; de las Rivas, B.; Guisán, J.M. Site-directing an intense multipoint covalent attachment (MCA) of mutants of the Geobacillus thermocatenulatus lipase 2 (BTL2): Genetic and chemical amination plus immobilization on a tailor-made support. Process Biochem. 2014, 49, 1324–1331. [Google Scholar] [CrossRef]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and Its Derivatives as Supports for Enzyme Immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M. Complete reactivation of immobilized derivatives of a trimeric glutamate dehydrogenase from thermus thermophillus. Process Biochem. 2010, 45, 107–113. [Google Scholar] [CrossRef]
- Marques, D.; Pessela, B.C.; Betancor, L.; Monti, R.; Carrascosa, A.V.; Rocha-Martin, J.; Guisán, J.M.; Fernandez-Lorente, G. Protein hydrolysis by immobilized and stabilized trypsin. Biotechnol. Prog. 2011, 27, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Lorente, G. Solid-Phase Chemical Amination of a Lipase from Bacillus thermocatenulatus to Improve Its Stabilization via Covalent Immobilization on Highly Activated Glyoxyl-Agarose. Biomacromolecules 2008, 9, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Godoy, C.A.; Volpato, G.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization–stabilization of the lipase from Thermomyces lanuginosus: Critical role of chemical amination. Process Biochem. 2009, 44, 963–968. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernandez-Lafuente, R. Amination of enzymes to improve biocatalyst performance: Coupling genetic modification and physicochemical tools. RSC Adv. 2014, 4, 38350–38374. [Google Scholar] [CrossRef]
- Mateo, C. Glyoxyl agarose: A fully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzym. Microb. Technol. 2006, 39, 274–280. [Google Scholar] [CrossRef]
- Bolivar, J.M.; López-Gallego, F.; Godoy, C.; Rodrigues, D.S.; Rodrigues, R.C.; Batalla, P.; Rocha-Martín, J.; Mateo, C.; Giordano, R.L.C.; Guisán, J.M. The presence of thiolated compounds allows the immobilization of enzymes on glyoxyl agarose at mild pH values: New strategies of stabilization by multipoint covalent attachment. Enzym. Microb. Technol. 2009, 45, 477–483. [Google Scholar] [CrossRef]
- Kim, H.-W.; Ishikawa, K. The role of disulfide bond in hyperthermophilic endocellulase. Extremophiles 2013, 17, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Nossoni, Z.; Toth, M.; Stewart, N.K.; Frase, H.; Vakulenko, S.B. Role of the Conserved Disulfide Bridge in Class A Carbapenemases. J. Biol. Chem. 2016, 291, 22196–22206. [Google Scholar] [CrossRef] [PubMed]
- Crisalli, P.; Kool, E.T. Water-soluble Organocatalysts for Hydrazone and Oxime Formation. J. Org. Chem. 2013, 78, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gurav, D.; Oommen, O.P.; Varghese, O.P. Insights into the Mechanism and Catalysis of Oxime Coupling Chemistry at Physiological pH. Chem. Eur. J. 2015, 21, 5980–5985. [Google Scholar] [CrossRef] [PubMed]
- Godoy, C.A.; de las Rivas, B.; Grazú, V.; Montes, T.; Guisán, J.M.; López-Gallego, F. Glyoxyl-disulfide agarose: A tailor-made support for site-directed rigidification of proteins. Biomacromolecules 2011, 12, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich Co. LLC. Anthranilic Acid|Sigma-Aldrich. Available online: http://www.sigmaaldrich.com/catalog/substance/anthranilicacid1371411892311?lang=en®ion=CO (accessed on 27 April 2017).
- Blank, K. Site-specific immobilization of genetically engineered variants of candida antarctica lipase B. Chembiochem 2006, 7, 1349–1351. [Google Scholar] [CrossRef] [PubMed]
- Hedin, E.M.; Patkar, S.A.; Vind, J.; Svendsen, A.; Hult, K.; Berglund, P. Selective reduction and chemical modification of oxidized lipase cysteine mutants. Can. J. Chem. 2002, 80, 529–539. [Google Scholar] [CrossRef]
- Bernal, C.; Illanes, A.; Wilson, L. Heterofunctional Hydrophilic–Hydrophobic Porous Silica as Support for Multipoint Covalent Immobilization of Lipases: Application to Lactulose Palmitate Synthesis. Langmuir 2014, 30, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, J.W. Infrared spectra of N-aryl imines of o-hydroxybenzaldehyde between 2000 and 1500 cm−1. J. Phys. Chem. 1977, 81, 54–59. [Google Scholar] [CrossRef]
- Han, D.; Song, J.; Ding, X.; Xu, X.; Niu, L. Fabrication and characterization of self-doped poly(aniline-co-anthranilic acid) nanorods in bundles. Mater. Chem. Phys. 2007, 105, 380–384. [Google Scholar] [CrossRef]
- Cortelazzo, Â.L.; de Campos Vidal, B.; Mello, M.L.S. Basic fuchsins and the Schiff-aldehyde reaction. Acta Histochem. 1983, 73, 121–133. [Google Scholar] [CrossRef]
- Mehta, G.K.; Kondaveeti, S.; Siddhanta, A.K. Facile synthesis of agarose-l-phenylalanine ester hydrogels. Polym. Chem. 2011, 2, 2334–2340. [Google Scholar] [CrossRef]
- Hu, Z.; Hong, P.; Liao, M.; Kong, S.; Huang, N.; Ou, C.; Li, S. Preparation and Characterization of Chitosan—Agarose Composite Films. Materials 2016, 9, 816. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Cruz, J.; Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Villalonga, R.; Fernandez-Lafuente, R. Inactivation of immobilized trypsin under dissimilar conditions produces trypsin molecules with different structures. RSC Adv. 2016, 6, 27329–27334. [Google Scholar] [CrossRef]
- Ulbrich, R.; Schellenberger, A.; Damerau, W. Studies on the thermal inactivation of immobilized enzymes. Biotechnol. Bioeng. 1986, 28, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C. Some special features of glyoxyl supports to immobilize proteins. Enzym. Microb. Technol. 2005, 37, 456–462. [Google Scholar] [CrossRef]
- Knezevic, Z.; Milosavic, N.; Bezbradica, D.; Jakovljevic, Z.; Prodanovic, R. Immobilization of lipase from Candida rugosa on Eupergit® C supports by covalent attachment. Biochem. Eng. J. 2006, 30, 269–278. [Google Scholar] [CrossRef]
- Godoy, C.A.; Romero, O.; de las Rivas, B.; Mateo, C.; Fernandez-Lorente, G.; Guisan, J.M.; Palomo, J.M. Changes on enantioselectivity of a genetically modified thermophilic lipase by site-directed oriented immobilization. J. Mol. Catal. B Enzym. 2013, 87, 121–127. [Google Scholar] [CrossRef]
- Pizarro, C.; Brañes, M.C.; Markovits, A.; Fernández-Lorente, G.; Guisán, J.M.; Chamy, R.; Wilson, L. Influence of different immobilization techniques for Candida cylindracea lipase on its stability and fish oil hydrolysis. J. Mol. Catal. B Enzym. 2012, 78, 111–118. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Pizarro, C.; López-Vela, D.; Betancor, L.; Carrascosa, A.V.; Pessela, B.; Guisan, J.M. Hydrolysis of Fish Oil by Lipases Immobilized Inside Porous Supports. J. Am. Oil Chem. Soc. 2011, 88, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Mendes, A.A.; Oliveira, P.C.; Vélez, A.M.; Giordano, R.C.; de Giordano, R.L.C.; de Castro, H.F. Evaluation of immobilized lipases on poly-hydroxybutyrate beads to catalyze biodiesel synthesis. Int. J. Biol. Macromol. 2012, 50, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-W.; Wu, W.-T. Regeneration of immobilized Candida antarctica lipase for transesterification. J. Biosci. Bioeng. 2003, 95, 466–469. [Google Scholar] [CrossRef]
- Lifshitz, C.; Laskin, J. Principles of Mass Spectrometry Applied to Biomolecules; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-0-470-05041-5. [Google Scholar]
- Deng, H.; Callender, R.; Zhu, J.; Nguyen, K.T.; Pei, D. Determination of the Ionization State and Catalytic Function of Glu-133 in Peptide Deformylase by Difference FTIR Spectroscopy. Biochemistry (Mosc.) 2002, 41, 10563–10569. [Google Scholar] [CrossRef]
- Galvis, M.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Effect of solid-phase chemical modification on the features of the lipase from Thermomyces lanuginosus. Process Biochem. 2012, 47, 460–466. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R.; Torres, R. Chemical amination of lipases improves their immobilization on octyl-glyoxyl agarose beads. Catal. Today 2016, 259 Pt 1, 107–118. [Google Scholar] [CrossRef]
- Zagonel, G.F.; Peralta-Zamora, P.; Ramos, L.P. Multivariate monitoring of soybean oil ethanolysis by FTIR. Talanta 2004, 63, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- De Melo, R.R.; Alnoch, R.C.; Vilela, A.F.L.; de Souza, E.M.; Krieger, N.; Ruller, R.; Sato, H.H.; Mateo, C. New Heterofunctional Supports Based on Glutaraldehyde-Activation: A Tool for Enzyme Immobilization at Neutral pH. Molecules 2017, 22, 1088. [Google Scholar] [CrossRef] [PubMed]
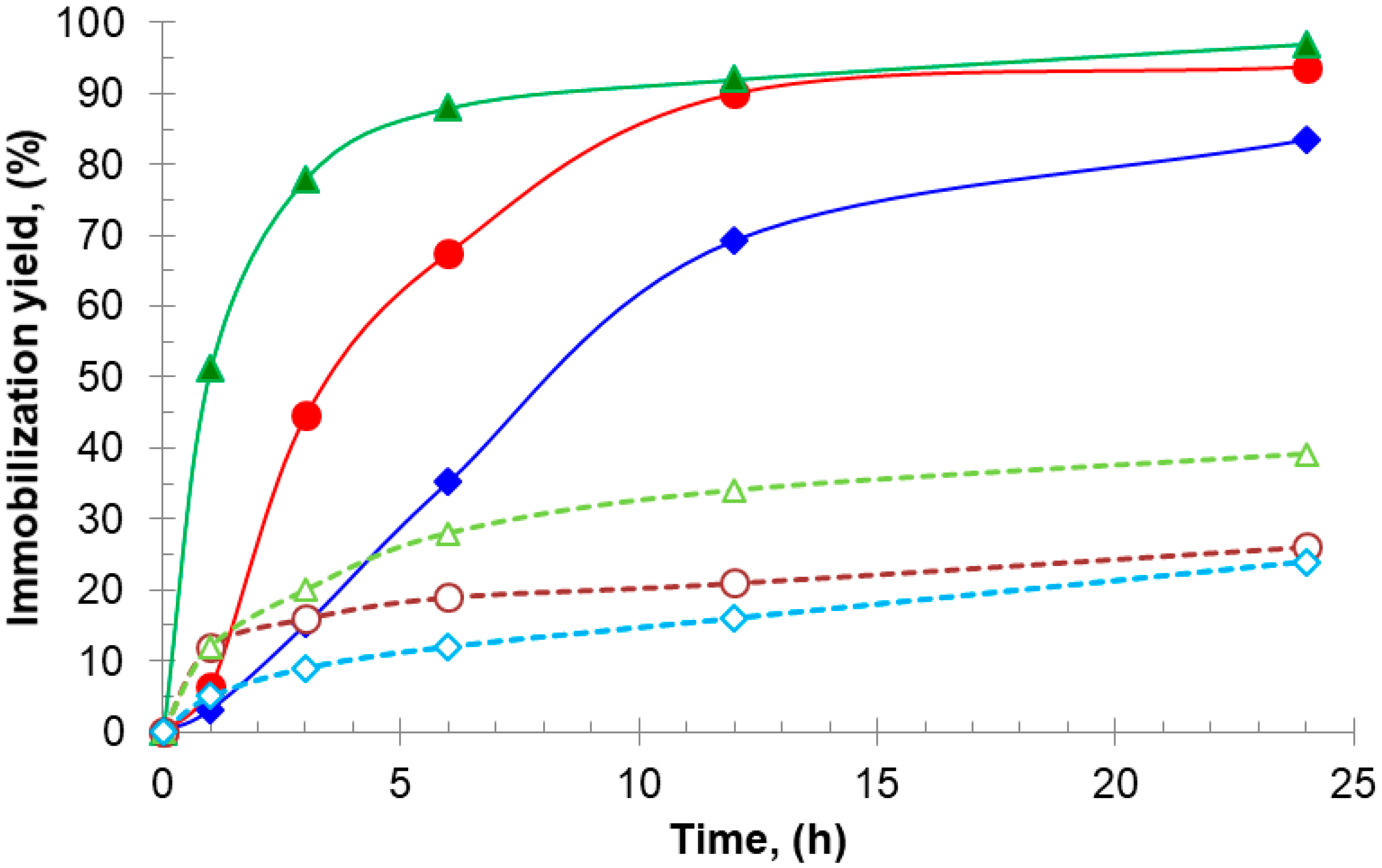
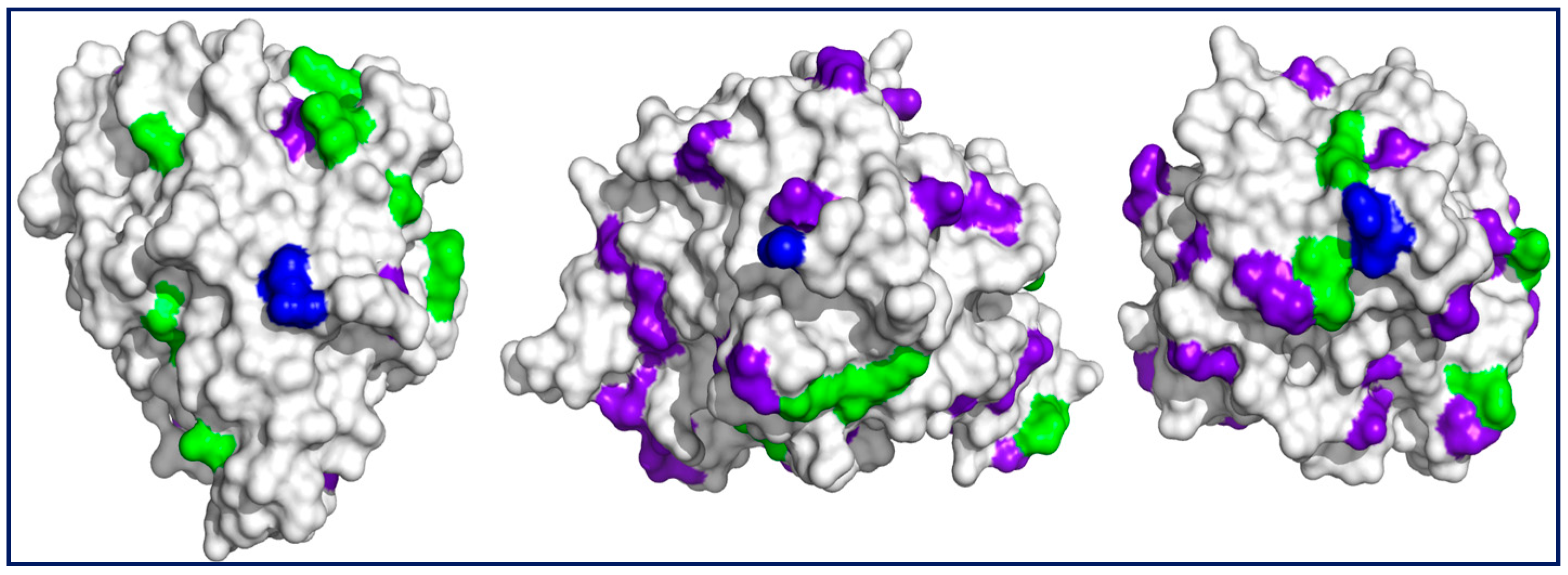
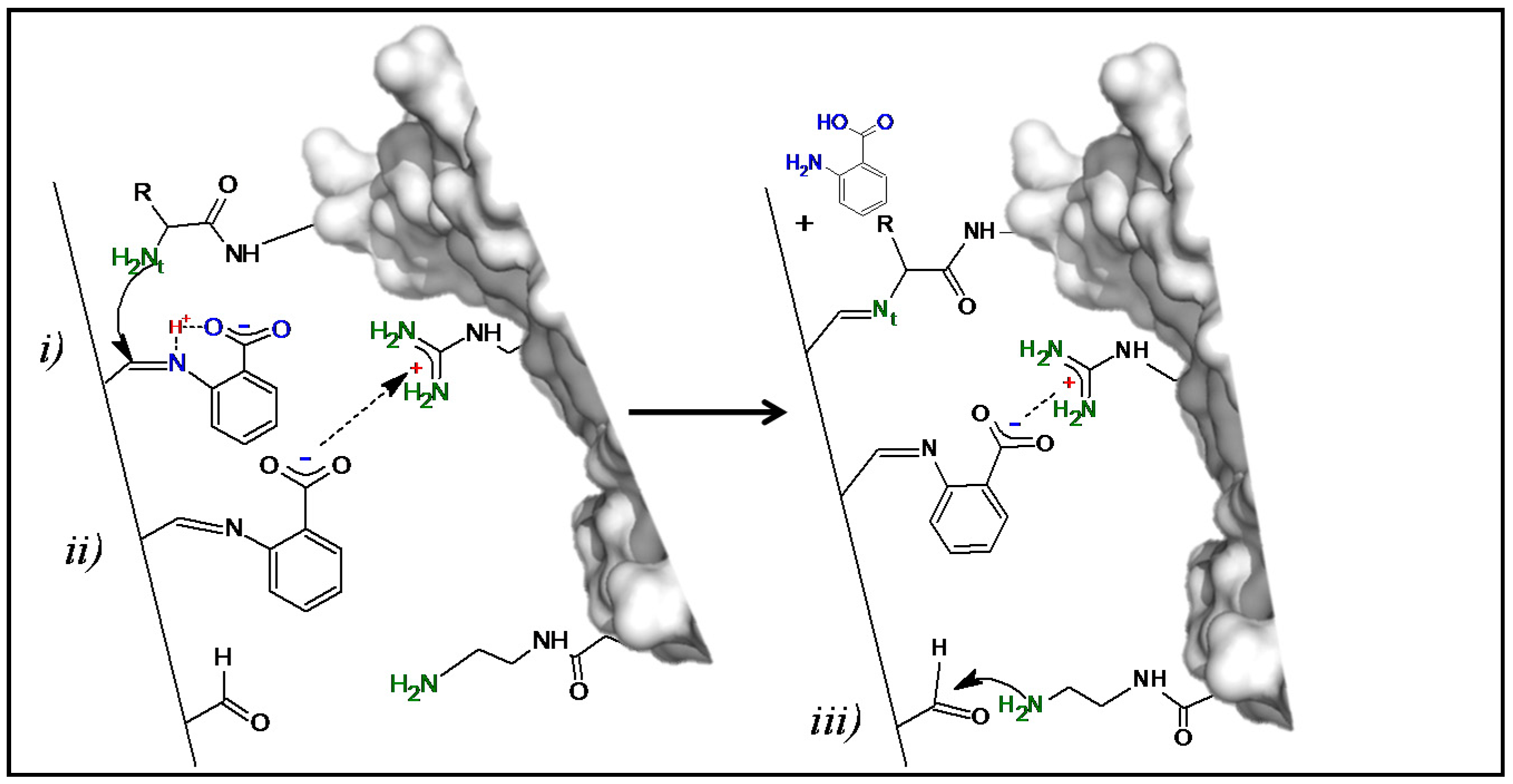
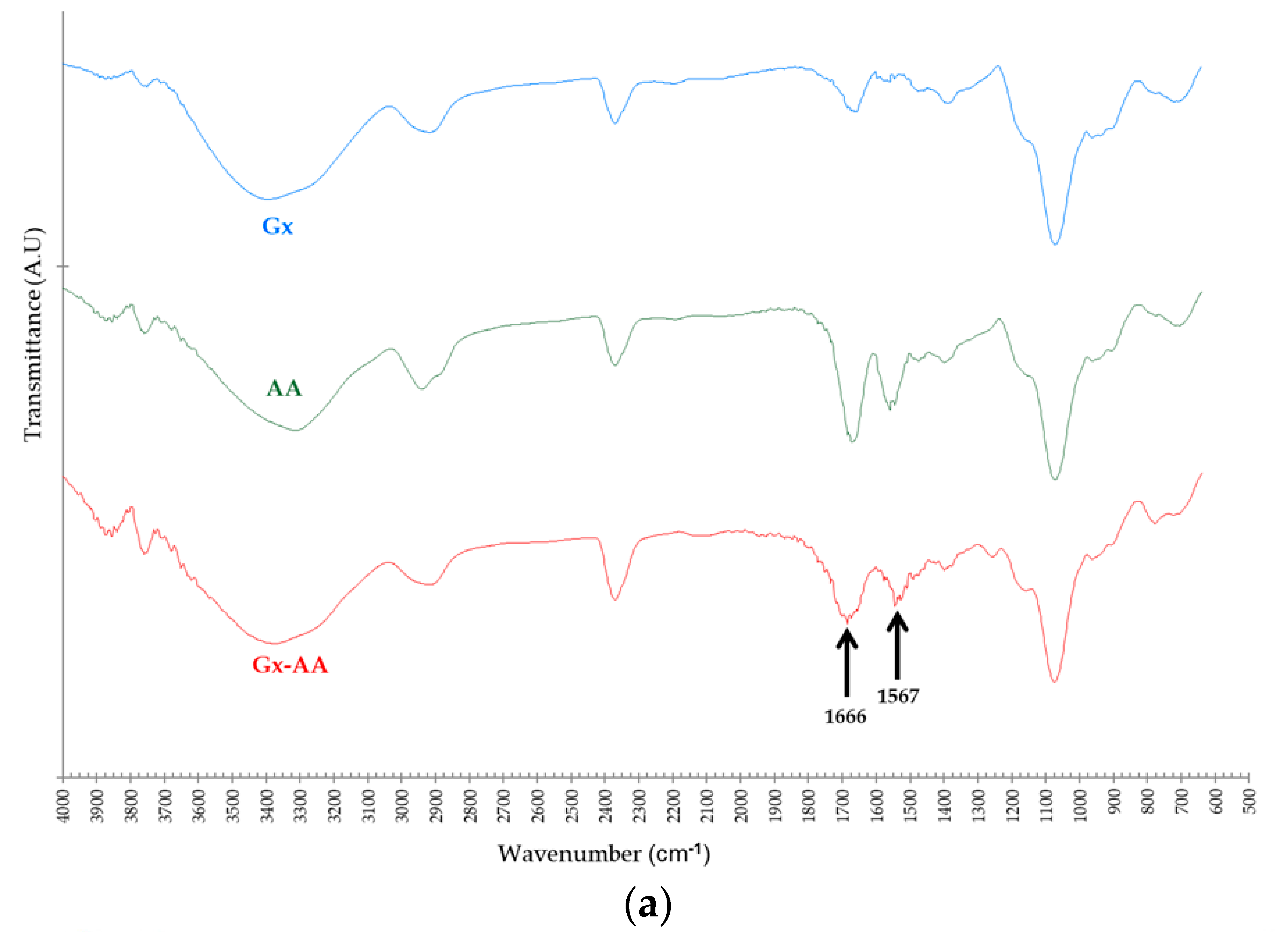

| Enzyme | Additive (20 mM) | Yield at 24 h (%) a |
|---|---|---|
| TLL | - | 39.1 ± 2.2 |
| DTT | 99.7 ± 3.6 | |
| AA | 96.8 ± 2.7 | |
| MA | 44.3 ± 1.1 | |
| AN | 95.6 ± 3.0 | |
| CAL | - | 23.9 ± 1.1 |
| DTT | 88.5 ± 2.5 | |
| AA | 83.5 ± 2.3 | |
| MA | 32.0 ± 1.0 | |
| AN | 90.3 ± 3.1 | |
| BTL2 | - | 26.1 ± 0.9 |
| DTT | 93.0 ± 4.1 | |
| AA | 69.2 ± 2.4 | |
| MA | 28.0 ± 0.4 | |
| AN | 72.0 ± 2.3 |
| Gx-Derivative | Immobilization Parameters | ||
|---|---|---|---|
| Enzyme | Additive (20 mM) | Residual Control Activity (%) a | Recovered Activity (%) b |
| TLL | - | 96.3 ± 3.1 | - |
| DTT | 5.1 ± 0.3 | 10.5 ± 0.4 | |
| AA | 55.2 ± 1.5 | 58.9 ± 1.7 | |
| CAL | - | 94.7 ± 2.7 | - |
| DTT | 64.4 ± 1.8 | 39.7 ± 1.1 | |
| AA | 83.2 ± 2.3 | 50.2 ± 2.0 | |
| BTL2 | - | 95.0 ± 2.0 | - |
| DTT | 91.1 ± 1.6 | 76.7 ± 3.3 | |
| AA | 95.4 ± 3.0 | 72.1 ± 2.8 | |
| Derivative Production Conditions | Immobilization Parameters | Stability Parameters c | Stabilization Factor d | ||||
|---|---|---|---|---|---|---|---|
| Immobilization Step (pH) | Incubation Step (pH) | Yield (%) a | Recovered Activity (%) b | Half-Life at 70 °C (h) | Half-Life in 80% Dioxane (h) | At 70 °C | In 80% Dioxane |
| 7.0 | 7.0 | 90 | 76 | 2.1 | 10.2 | 42 | 167 |
| 7.0 | 8.0 | 90 | 74 | 50.0 | 130 | 1000 | 2131 |
| 7.0 | 10.1 | 90 | 72 | 54.5 | 140 | 1090 | 2295 |
| 8.0 | 8.0 | 96 | 65 | 3.7 | 8.1 | 74 | 133 |
| 8.0 | 10.1 | 99 | 62 | 53.7 | 195 | 1074 | 3197 |
| Reference Gx derivative (pH 9.0) | 10.1 | 96 | 64 | 24.1 | 74.2 | 482 | 1216 |
| Reference CNBr derivative (pH 7.0) | - | 99 | 78 | 0.050 | 0.061 | 1 | 1 |
| Derivative Production Conditions | Hydrolysis of Sardine Oil | ||
|---|---|---|---|
| Immobilization Step (pH) | Incubation Step (pH) | Activity a | EPA/DHA Ratio b |
| 7.0 | 7.0 | 1.38 ± 0.12 | 1.90 ± 0.10 |
| 7.0 | 8.0 | 1.22 ± 0.05 | 1.83 ± 0.05 |
| 7.0 | 10.1 | 1.13 ± 0.08 | 1.86 ± 0.03 |
| 8.0 | 8.0 | 1.01 ± 0.08 | 2.11 ± 0.02 |
| 8.0 | 10.1 | 1.04 ± 0.04 | 2.32 ± 0.15 |
| Reference Gx derivative (pH 9.0) c | 10.1 | 1.10 ± 0.07 | 1.70 ± 0.07 |
| Reference CNBr derivative (pH 7.0) d | - | 1.88 ± 0.09 | 1.97 ± 0.10 |
| Derivative | Ethyl Ester (EE) | ||||
|---|---|---|---|---|---|
| Enzyme | Immobilization Condition | Yield at 14 h (%) a | Yield at 44 h (%) a | ||
| Non-Dried | Dried | Non-Dried | Dried | ||
| TLL | Reference | 11.7 ± 1.2 | 51.6 ± 2.2 | 30.3 ± 1.1 | 69.8 ± 1.8 |
| DTT | 5.3 ± 0.3 | 6.3 ± 0.5 | 5.7 ± 0.3 | 7.4 ± 0.3 | |
| AA | 14.3 ± 0.8 | 71.5 ± 2.8 | 23.2 ± 1.8 | 74.8 ± 2.8 | |
| CAL | Reference | 8.0 ± 0.1 | 13.0 ± 0.9 | 8.5 ± 0.4 | 22.0 ± 1.7 |
| DTT | 5.9 ± 0.5 | 5.3 ± 0.7 | 6.1 ± 0.3 | 7.2 ± 0.5 | |
| AA | 6.4 ± 0.4 | 5.6 ± 0.3 | 8.5 ± 0.6 | 7.7 ± 0.2 | |
| BTL2 | Reference | 0.9 ± 0.2 | 8.8 ± 0.2 | 2.0 ± 0.5 | 16.1 ± 0.1 |
| DTT | 2.1 ± 0.1 | 9.5 ± 0.7 | 3.1 ± 0.8 | 15.0 ± 0.8 | |
| AA | 1.9 ± 0.2 | 10.3 ± 0.6 | 2.8 ± 0.1 | 11.2 ± 0.5 | |
| Novozyme® 435 (industrial reference [7,14,15]) | 47.2 ± 0.2 | 75.1 ± 2.9 | 78.0 ± 3.5 | 79.4 ± 1.9 | |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy, C.A. New Strategy for the Immobilization of Lipases on Glyoxyl–Agarose Supports: Production of Robust Biocatalysts for Natural Oil Transformation. Int. J. Mol. Sci. 2017, 18, 2130. https://doi.org/10.3390/ijms18102130
Godoy CA. New Strategy for the Immobilization of Lipases on Glyoxyl–Agarose Supports: Production of Robust Biocatalysts for Natural Oil Transformation. International Journal of Molecular Sciences. 2017; 18(10):2130. https://doi.org/10.3390/ijms18102130
Chicago/Turabian StyleGodoy, César A. 2017. "New Strategy for the Immobilization of Lipases on Glyoxyl–Agarose Supports: Production of Robust Biocatalysts for Natural Oil Transformation" International Journal of Molecular Sciences 18, no. 10: 2130. https://doi.org/10.3390/ijms18102130






