Plasma miR-155, miR-203, and miR-205 are Biomarkers for Monitoring of Primary Cutaneous T-Cell Lymphomas
Abstract
:1. Introduction
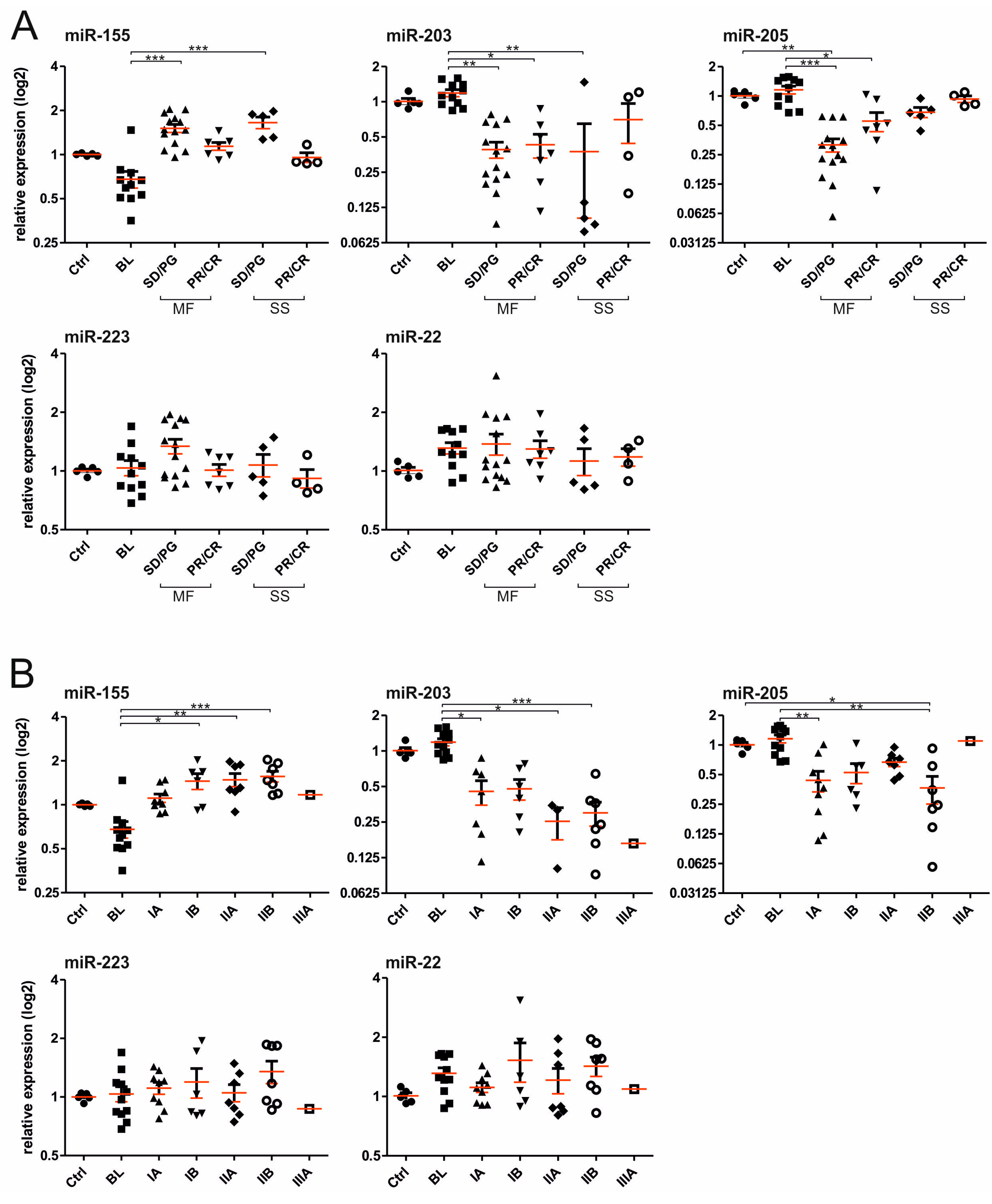
2. Results
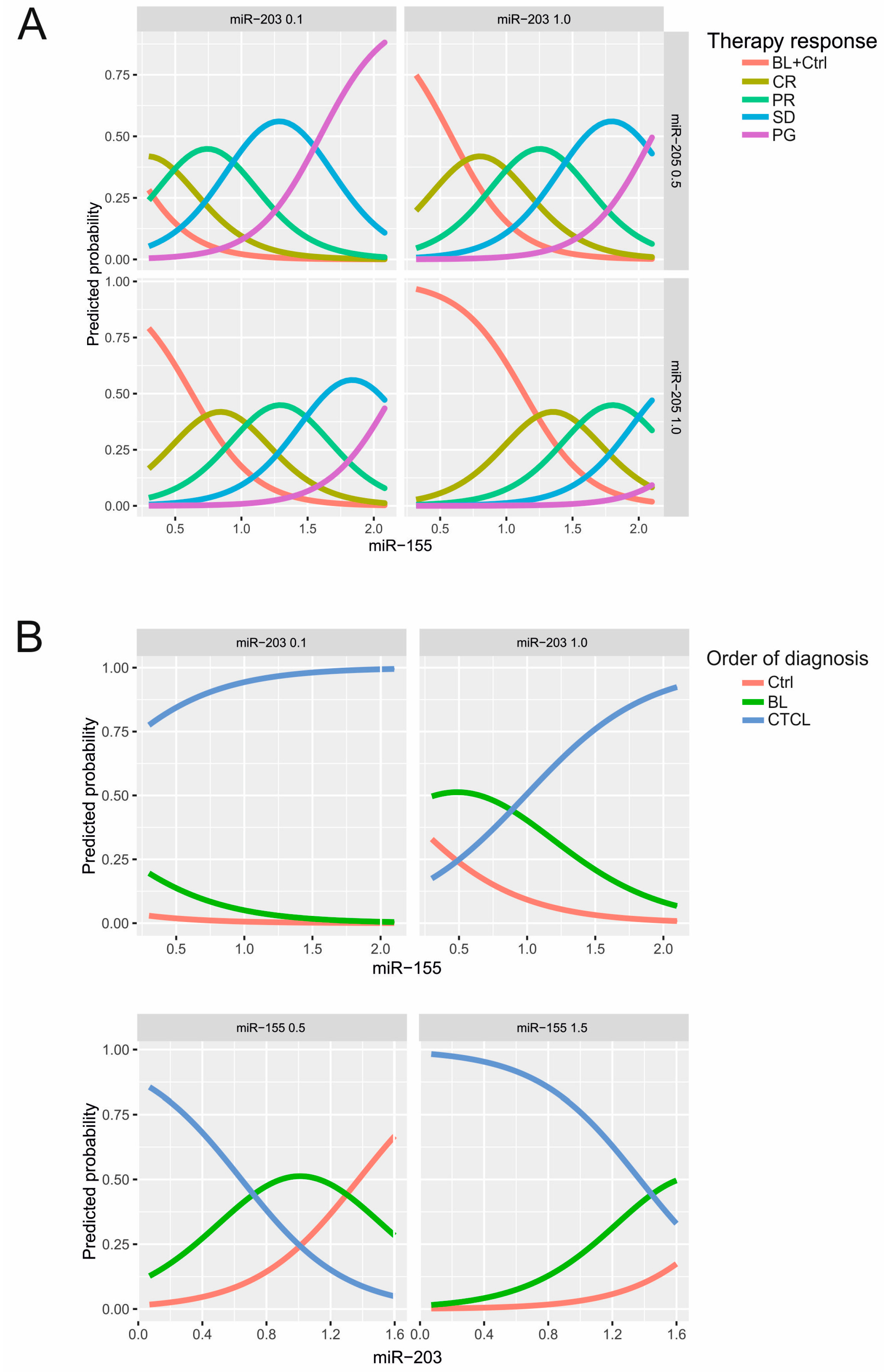
2.1. Establishment of the miRNA Classifier for Cutaneous T-Cell Lymphomas (CTCL) Plasma
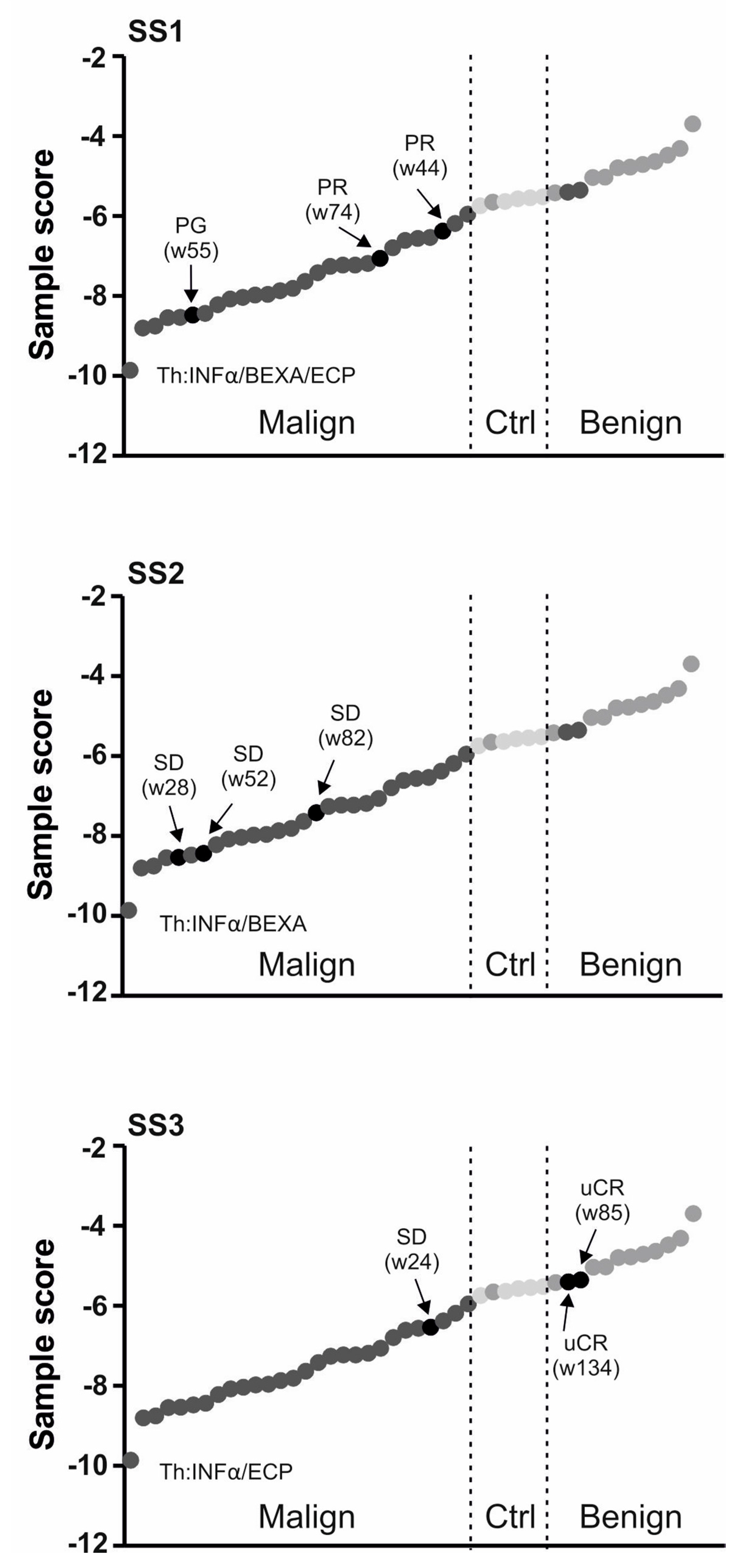
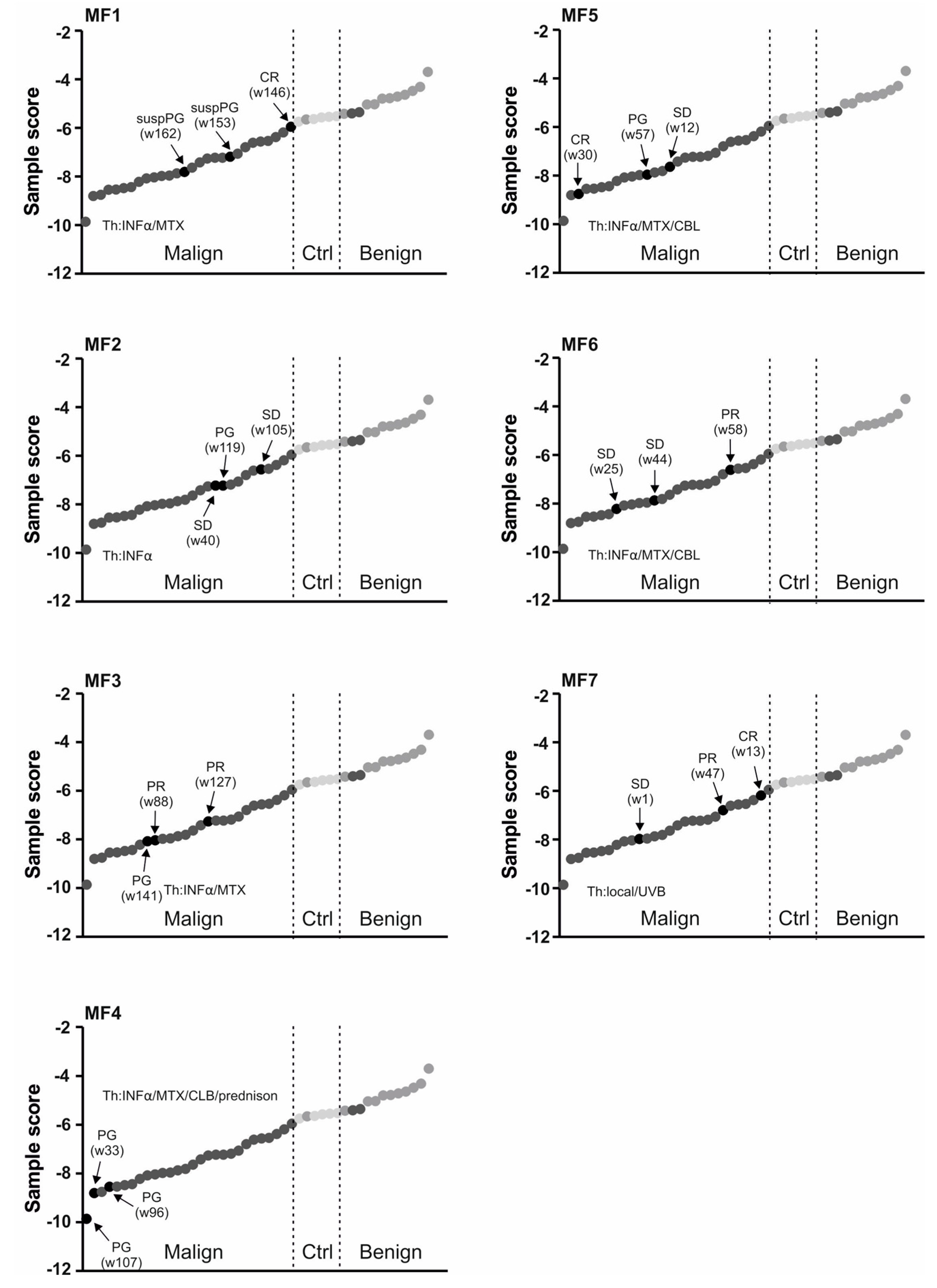
2.2. Use of miRNA Classifier for the CTCL Clinical Monitoring
3. Discussion
4. Material and Methods
4.1. Patients
4.2. MiR Extraction and Quantitation
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| miR | microRNA |
| CTCL | Cutaneous T-cell lymphomas |
| MF | Mycosis fungoides |
| SS | Sezary syndrome |
| BL | Benign lesion |
| PCA | Principal component analysis |
| Cp | Crossing point |
| CR | Complete Remission |
| PR | Partial Remission |
| SD | Stable Disease |
| PG | Progression |
References
- Wilcox, R.A. Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2016, 91, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Mirvish, J.J.; Pomerantz, R.G.; Falo, L.D., Jr.; Geskin, L.J. Role of infectious agents in cutaneous T-cell lymphoma: Facts and controversies. Clin. Dermatol. 2013, 31, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Ballegaard, V.; Ralfkiaer, U.; Pedersen, K.K.; Hove, M.; Koplev, S.; Braendstrup, P.; Ryder, L.P.; Madsen, H.O.; Gerstoft, J.; Gronbaek, K.; et al. MicroRNA-210, MicroRNA-331, and MicroRNA-7 are differentially regulated in treated HIV-1-infected individuals and are associated with markers of systemic inflammation. JAIDS 2017, 74, e104–e113. [Google Scholar] [CrossRef] [PubMed]
- Van der Fits, L.; van Kester, M.S.; Qin, Y.; Out-Luiting, J.J.; Smit, F.; Zoutman, W.H.; Willemze, R.; Tensen, C.P.; Vermeer, M.H. MicroRNA-21 expression in CD4+ T cells is regulated by STAT3 and is pathologically involved in Sezary syndrome. J. Investig. Dermatol. 2011, 131, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Ralfkiaer, U.; Hagedorn, P.H.; Bangsgaard, N.; Lovendorf, M.B.; Ahler, C.B.; Svensson, L.; Kopp, K.L.; Vennegaard, M.T.; Lauenborg, B.; Zibert, J.R.; et al. Diagnostic microRNA profiling in cutaneous T-cell lymphoma (CTCL). Blood 2011, 118, 5891–5900. [Google Scholar] [CrossRef] [PubMed]
- Sochor, M.; Basova, P.; Pesta, M.; Dusilkova, N.; Bartos, J.; Burda, P.; Pospisil, V.; Stopka, T. Oncogenic microRNAs: MiR-155, miR-19a, miR-181b, and miR-24 enable monitoring of early breast cancer in serum. BMC Cancer 2014, 14, 448. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.; Vonderheid, E.; Pimpinelli, N.; Willemze, R.; Kim, Y.; Knobler, R.; Zackheim, H.; Duvic, M.; Estrach, T.; Lamberg, S.; et al. Iscl/Eortc, Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007, 110, 1713–1722. [Google Scholar] [PubMed]
- Marstrand, T.; Ahler, C.B.; Ralfkiaer, U.; Clemmensen, A.; Kopp, K.L.; Sibbesen, N.A.; Krejsgaard, T.; Litman, T.; Wasik, M.A.; Bonefeld, C.M. Validation of a diagnostic microRNA classifier in cutaneous T-cell lymphomas. Leuk. Lymphoma 2014, 55, 957–958. [Google Scholar] [CrossRef] [PubMed]
- Ralfkiaer, U.; Lindahl, L.M.; Litman, T.; Gjerdrum, L.M.; Ahler, C.B.; Gniadecki, R.; Marstrand, T.; Fredholm, S.; Iversen, L.; Wasik, M.A.; et al. MicroRNA expression in early mycosis fungoides is distinctly different from atopic dermatitis and advanced cutaneous T-cell lymphoma. Anticancer Res. 2014, 34, 7207–7217. [Google Scholar] [PubMed]
- Ning, M.S.; Andl, T. Custodians of the transcriptome: How microRNAs guard stemness in squamous epithelia. Stem Cells 2015, 33, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Chim, C.S.; Wong, K.Y.; Leung, C.Y.; Chung, L.P.; Hui, P.K.; Chan, S.Y.; Yu, L. Epigenetic inactivation of the hsa-miR-203 in haematological malignancies. J. Cell. Mol. Med. 2011, 15, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Vargova, K.; Curik, N.; Burda, P.; Basova, P.; Kulvait, V.; Pospisil, V.; Savvulidi, F.; Kokavec, J.; Necas, E.; Berkova, A.; et al. MYB transcriptionally regulates the miR-155 host gene in chronic lymphocytic leukemia. Blood 2011, 117, 3816–3825. [Google Scholar] [CrossRef] [PubMed]
- Huskova, H.; Korecka, K.; Karban, J.; Vargova, J.; Vargova, K.; Dusilkova, N.; Trneny, M.; Stopka, T. Oncogenic microRNA-155 and its target PU.1: An integrative gene expression study in six of the most prevalent lymphomas. Int. J. Hematol. 2015, 102, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Kopp, K.L.; Ralfkiaer, U.; Gjerdrum, L.M.; Helvad, R.; Pedersen, I.H.; Litman, T.; Jonson, L.; Hagedorn, P.H.; Krejsgaard, T.; Gniadecki, R. STAT5-mediated expression of oncogenic miR-155 in cutaneous T-cell lymphoma. Cell Cycle 2013, 12, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Costinean, S.; Zanesi, N.; Pekarsky, Y.; Tili, E.; Volinia, S.; Heerema, N.; Croce, C.M. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc. Natl. Acad. Sci. USA 2006, 103, 7024–7029. [Google Scholar] [CrossRef] [PubMed]
- Kopp, K.L.; Ralfkiaer, U.; Nielsen, B.S.; Gniadecki, R.; Woetmann, A.; Odum, N.; Ralfkiaer, E. Expression of miR-155 and miR-126 in situ in cutaneous T-cell lymphoma. APMIS 2013, 121, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Krejsgaard, T.; Lindahl, L.M.; Mongan, N.P.; Wasik, M.A.; Litvinov, I.V.; Iversen, L.; Langhoff, E.; Woetmann, A.; Odum, N. Malignant inflammation in cutaneous T-cell lymphoma—A hostile takeover. Semin. Immunopathol. 2017, 39, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Moyal, L.; Barzilai, A.; Gorovitz, B.; Hirshberg, A.; Amariglio, N.; Jacob-Hirsch, J.; Maron, L.; Feinmesser, M.; Hodak, E. MiR-155 is involved in tumor progression of mycosis fungoides. Exp. Dermatol. 2013, 22, 431–433. [Google Scholar] [CrossRef] [PubMed]
- McGirt, L.Y.; Adams, C.M.; Baerenwald, D.A.; Zwerner, J.P.; Zic, J.A.; Eischen, C.M. MiR-223 regulates cell growth and targets proto-oncogenes in mycosis fungoides/cutaneous T-cell lymphoma. J. Investig. Dermatol. 2014, 134, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Sibbesen, N.A.; Kopp, K.L.; Litvinov, I.V.; Jonson, L.; Willerslev-Olsen, A.; Fredholm, S.; Petersen, D.L.; Nastasi, C.; Krejsgaard, T.; Lindahl, L.M.; et al. Jak3, STAT3, and STAT5 inhibit expression of miR-22, a novel tumor suppressor microRNA, in cutaneous T-Cell lymphoma. Oncotarget 2015, 6, 20555–20569. [Google Scholar] [CrossRef] [PubMed]
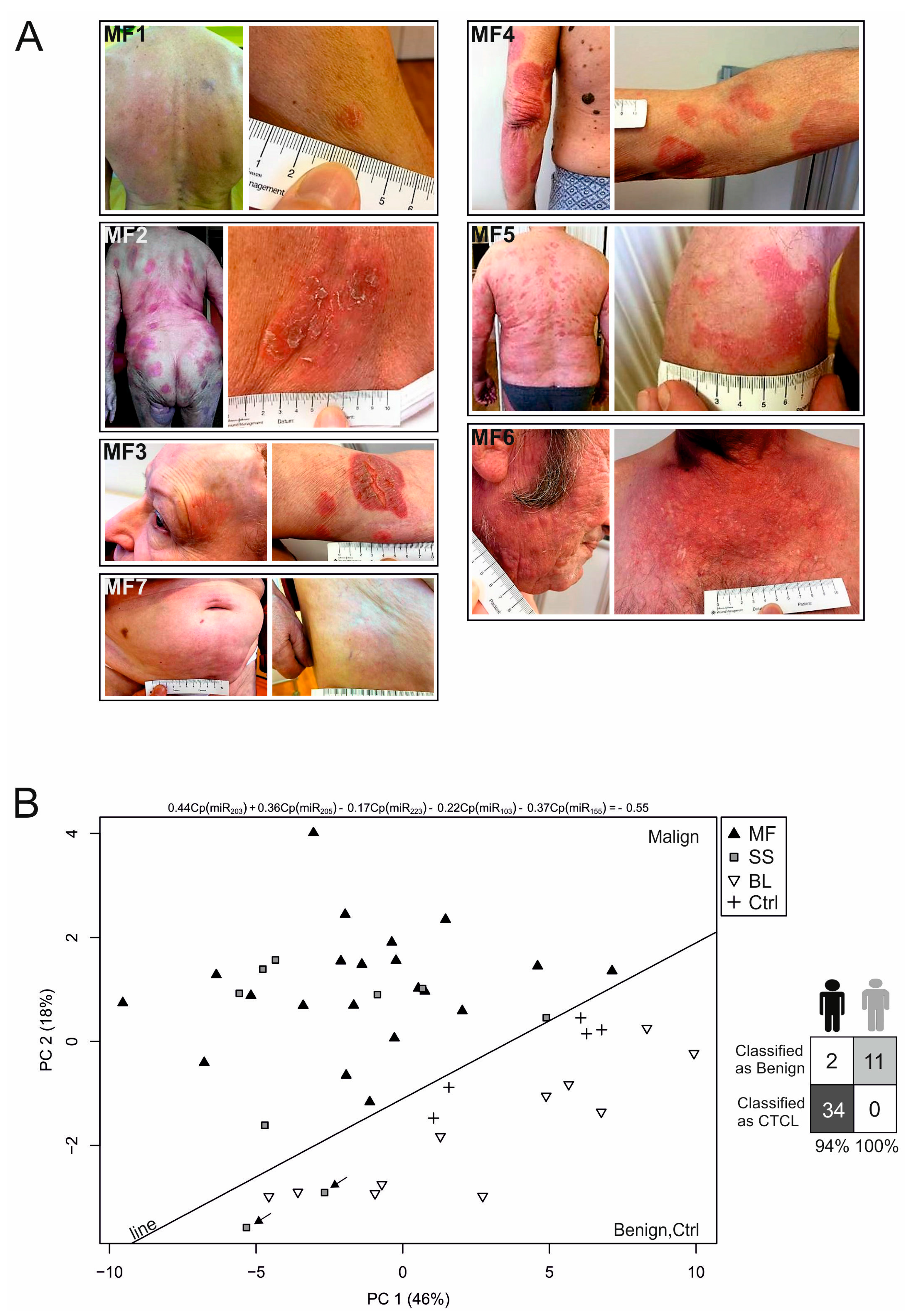
| ID | Age | Sex | DG | Stage S1 | OS | Alive | Therapy | Response |
|---|---|---|---|---|---|---|---|---|
| MF1 | 61 | M | MF | IB | 58 | Y | IFNα/LD MTX | CR |
| MF2 | 74 | F | MF | IB | 92 | Y | IFNα | SD |
| MF3 | 66 | F | MF | IB | 179 | Y | IFNα/LD MTX | PR |
| MF4 | 82 | M | MF | IIB | 68 | Y | IFNα/CLB/MTX/PRD | SD |
| MF5 | 62 | M | MF | IB | 28 | Y | IFNα/LD MTX/CLB | CR |
| MF6 | 61 | M | MF | IIB | 57 | Y | IFNα/LD MTX/CLB | PR |
| MF7 | 70 | F | MF | IA | 23 | Y | local Th/UVB | CR |
| SS1 | 72 | F | SS | IIIA | 94 | Y | IFNα/BEXA/ECP | PR |
| SS2 | 71 | F | SS | IIA | 40 | Y | IFNα/BEXA | SD |
| SS3 | 60 | M | SS | IIA | 44 | N | IFNα/ECP | uCR |
| Clinical Parameter | miR-155 | miR-203 | miR-205 | miR-223 | miR-22 |
|---|---|---|---|---|---|
| Th. Response | 0.00022 | 0.01758 | 0.00070 | 0.12323 | 0.08071 |
| Clinical Stage | 0.00404 | 0.00029 | 0.5580 | 0.5714 | 0.4340 |
| T (skin) | 0.0042 | 0.0004 | 0.7792 | 0.2813 | 0.8939 |
| Diagnosis | 0.0332 | 0.0034 | 0.234 | 0.684 | 0.071 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dusílková, N.; Bašová, P.; Polívka, J.; Kodet, O.; Kulvait, V.; Pešta, M.; Trněný, M.; Stopka, T. Plasma miR-155, miR-203, and miR-205 are Biomarkers for Monitoring of Primary Cutaneous T-Cell Lymphomas. Int. J. Mol. Sci. 2017, 18, 2136. https://doi.org/10.3390/ijms18102136
Dusílková N, Bašová P, Polívka J, Kodet O, Kulvait V, Pešta M, Trněný M, Stopka T. Plasma miR-155, miR-203, and miR-205 are Biomarkers for Monitoring of Primary Cutaneous T-Cell Lymphomas. International Journal of Molecular Sciences. 2017; 18(10):2136. https://doi.org/10.3390/ijms18102136
Chicago/Turabian StyleDusílková, Nina, Petra Bašová, Jindřich Polívka, Ondřej Kodet, Vojtěch Kulvait, Michal Pešta, Marek Trněný, and Tomáš Stopka. 2017. "Plasma miR-155, miR-203, and miR-205 are Biomarkers for Monitoring of Primary Cutaneous T-Cell Lymphomas" International Journal of Molecular Sciences 18, no. 10: 2136. https://doi.org/10.3390/ijms18102136