New Pyrazole-Hydrazone Derivatives: X-ray Analysis, Molecular Structure Investigation via Density Functional Theory (DFT) and Their High In-Situ Catecholase Activity
Abstract
:1. Introduction
2. Results
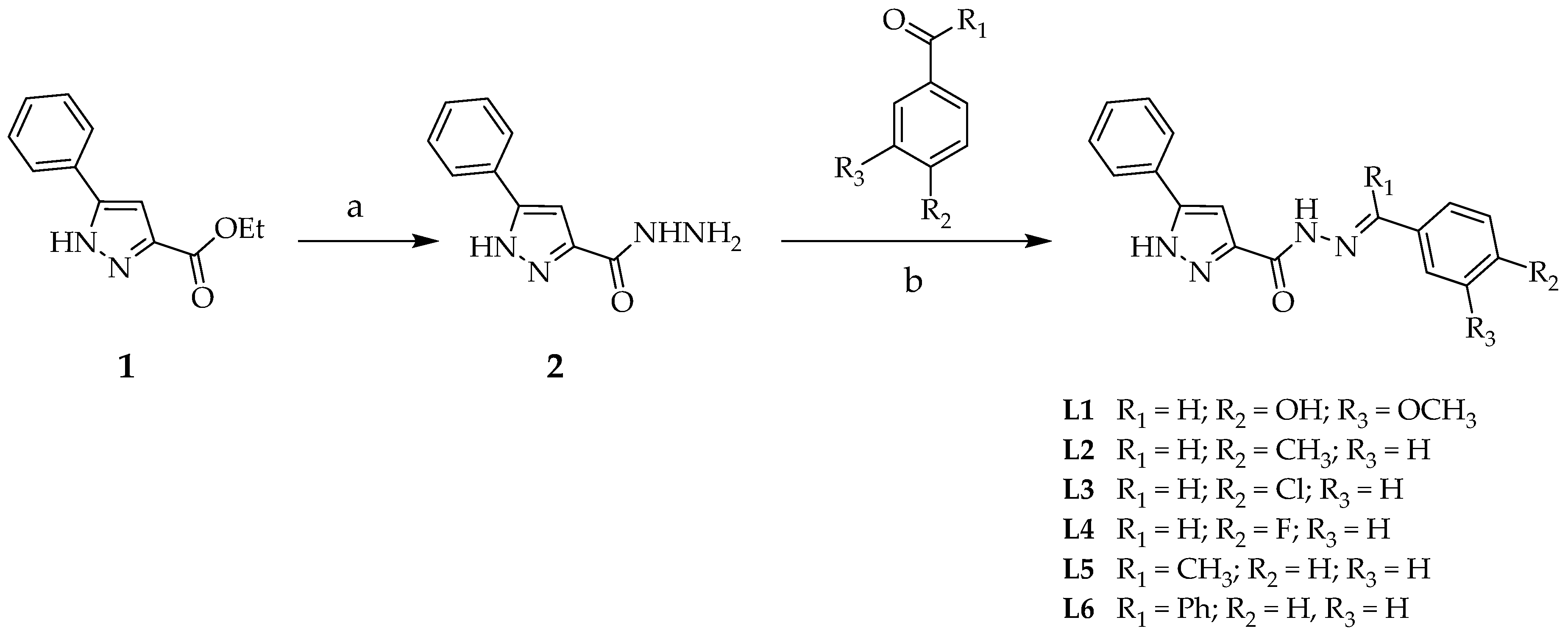
2.1. Synthesis
2.2. X-Ray Crystal Structures Description
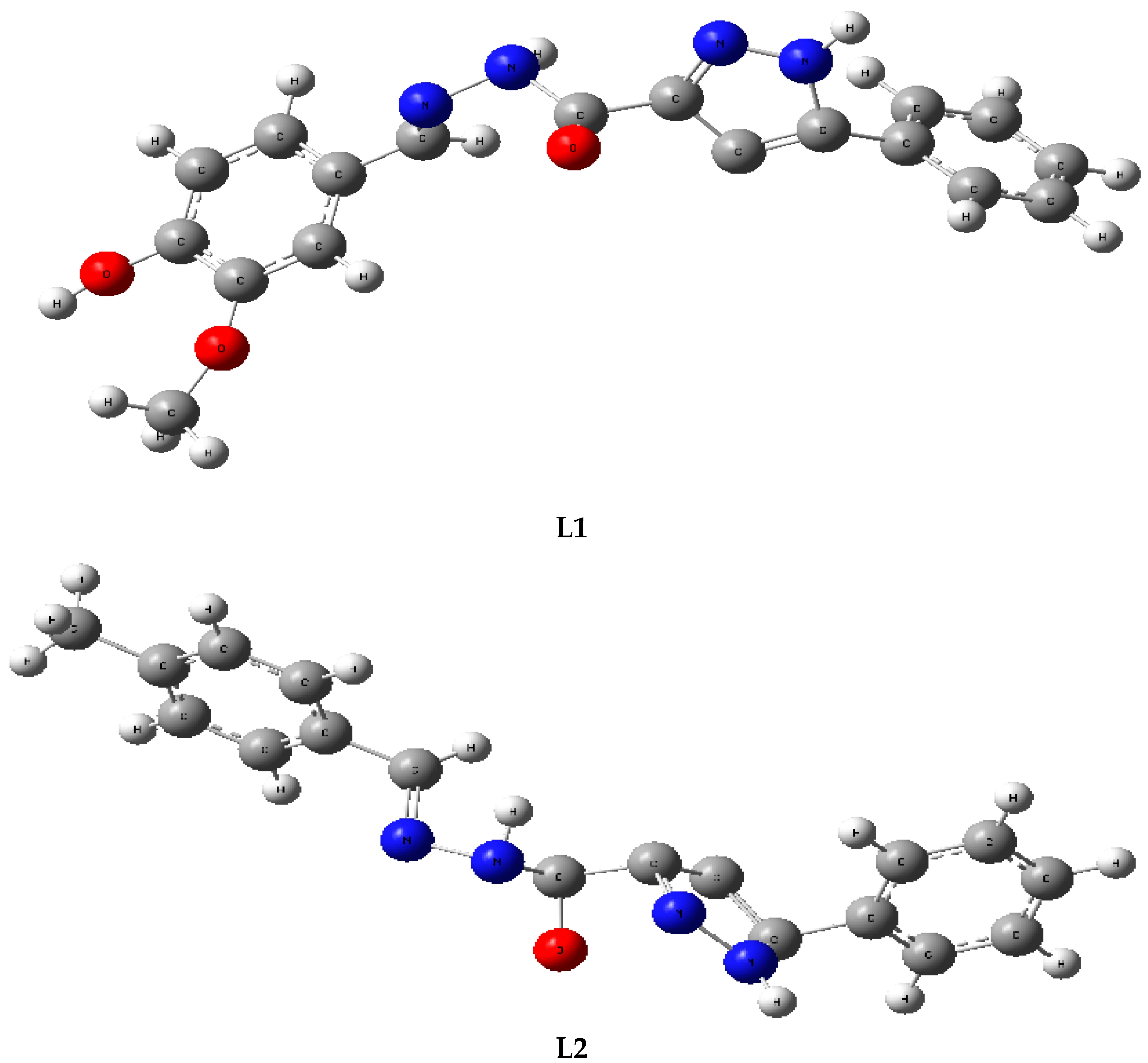
2.3. Computational Studies
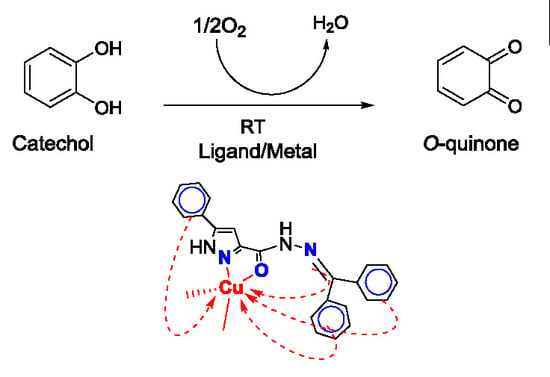
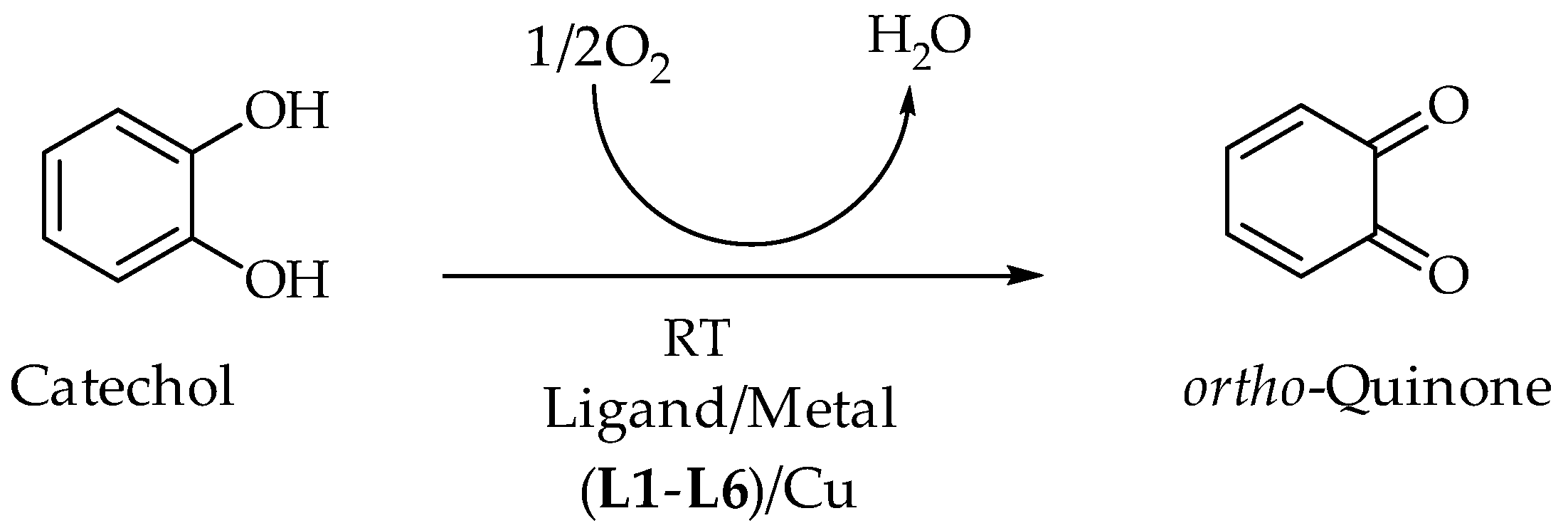
2.4. Catecholase Activity: Spectrophotometric Study
2.4.1. Effect of Ligand Concentration on the Catecholase Activity
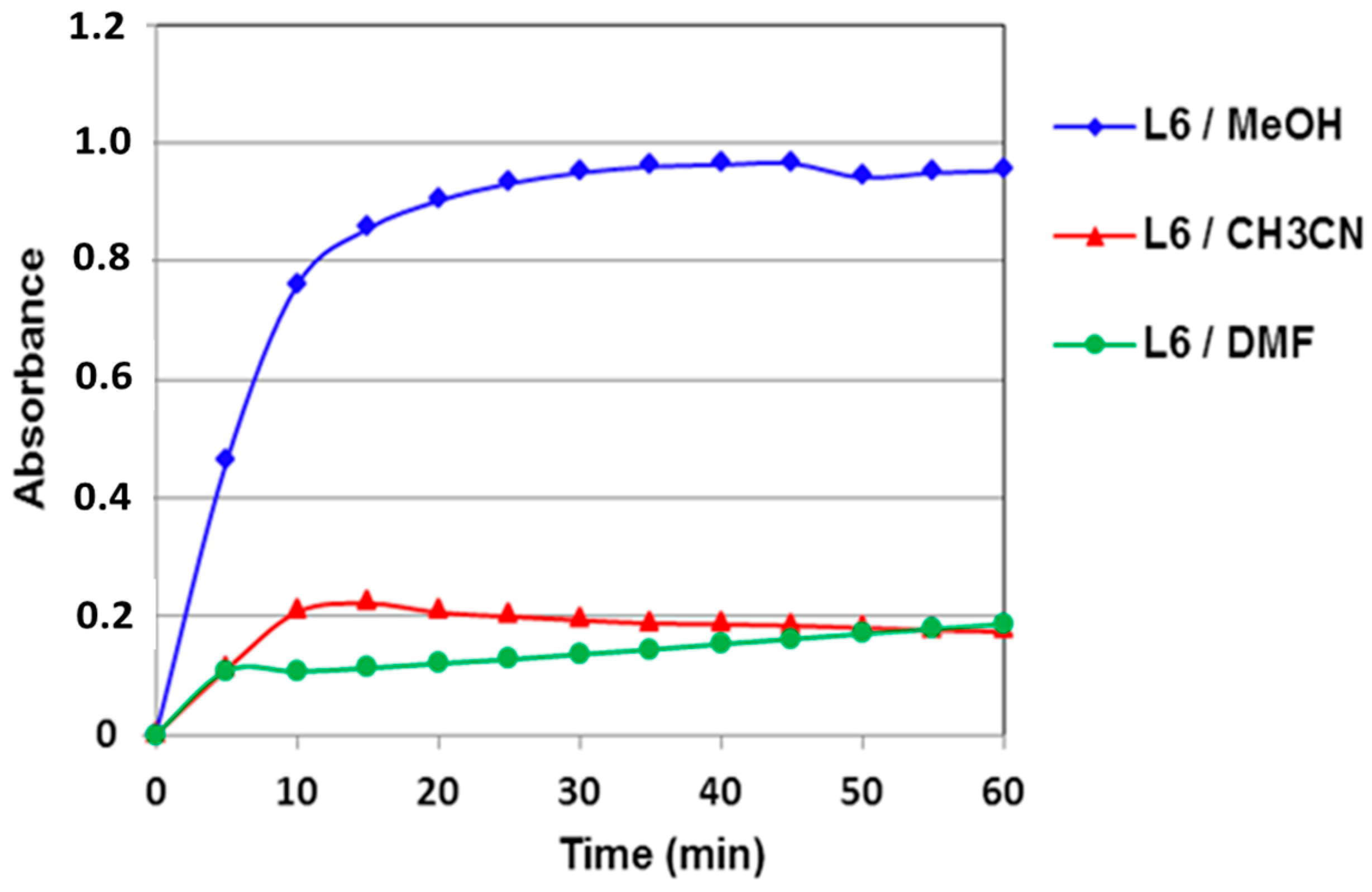
2.4.2. Solvent Effect
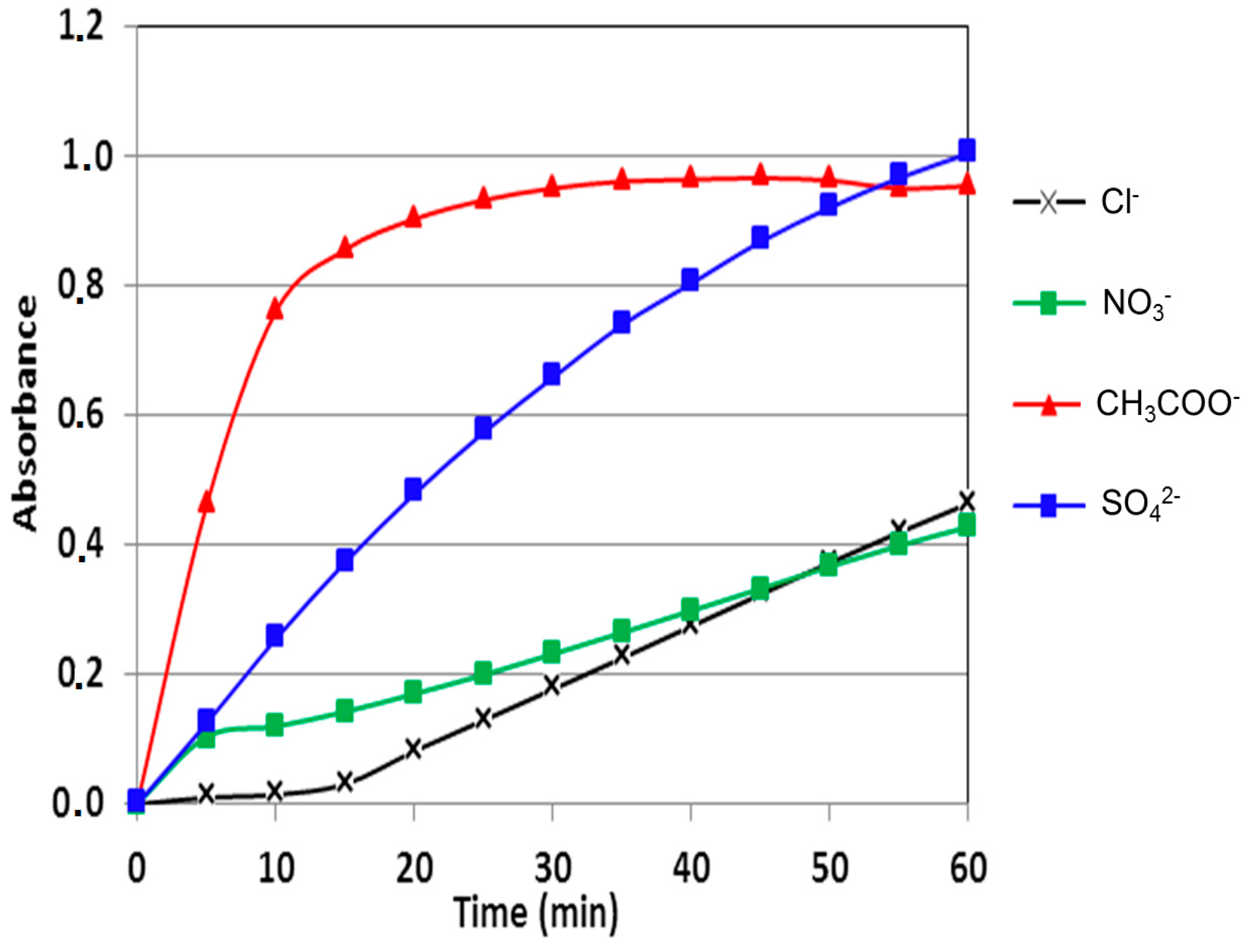
2.4.3. Comparison with Alternative Catalysts
3. Experimental
3.1. General Methods
3.2. Synthesis
3.2.1. Synthesis of 3-phenyl-1H-pyrazole-4-carbohydrazides (2)
3.2.2. General Procedure for the Synthesis of Ligands (L1–L6)
3.2.3. N’-(4-hydroxy-3-methoxybenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide (L1)
3.2.4. N’-(4-methylbenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide (L2)
3.2.5. N’-(4-chlorobenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide (L3)
3.2.6. N’-(4-fluorobenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide (L4)
3.2.7. 5-phenyl-N’-(1-phenylethylidene)-1H-pyrazole-3-carbohydrazide (L5)
3.2.8. N’-(diphenylmethylene)-5-phenyl-1H-pyrazole-3-carbohydrazide (L6)
3.3. X-Ray Crystallographic Analysis
3.4. DFT Computational Method
3.5. Catecholase Activity Measurement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hartinger, C.G.; Dyson, P.J. Bioorganometallic chemistry—from teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009, 38, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Gasser, G. Organometallic Compounds: An Opportunity for Chemical Biology? ChemBioChem 2012, 13, 1232–1252. [Google Scholar] [CrossRef] [PubMed]
- Daumann, L.J.; Schenk, G.; Ollis, D.L.; Gahan, L.R. Spectroscopic and mechanistic studies of dinuclear metallohydrolases and their biomimetic complexes. Dalton Trans. 2014, 43, 910–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalle, K.E.; Meyer, F. Modelling binuclear metallobiosites: Insights from pyrazole-supported biomimetic and bioinspired complexes. Eur. J. Inorg. Chem. 2015, 2015, 3391–3405. [Google Scholar] [CrossRef]
- Mistri, S.; Paul, A.; Bhunia, A.; Manne, R.K.; Santra, M.K.; Puschmann, H.; Manna, S.C. A combined experimental and theoretical investigation on the Cu(II) sensing behavior of a piperazinyl moiety based ligand, and catecholase and biological activities of its Cu(II) complex in combination with pyridine 2,5-dicarboxylate. Polyhedron 2016, 104, 63–72. [Google Scholar] [CrossRef]
- Rosenzweig, A.C.; Sazinsky, M.H. Structural insights into dioxygen-activating copper enzymes. Curr. Opin. Struct. Biol. 2006, 16, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Mirica, L.M.; Ottenwaelder, X.; Stack, T.D.P. Structure and Spectroscopy of Copper−Dioxygen Complexes. Chem. Rev. 2004, 104, 1013–1046. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Fukuzumi, S. Monooxygenase Activity of Type 3 Copper Proteins. Acc. Chem. Res. 2007, 40, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Mutti, F.G.; Zoppellaro, G.; Gullotti, M.; Santagostini, L.; Pagliarin, R.; Andersson, K.K.; Casella, L. Biomimetic Modelling of Copper Enzymes: Synthesis, Characterization, EPR Analysis and Enantioselective Catalytic Oxidations by a New Chiral Trinuclear Copper(II) Complex. Eur. J. Inorg. Chem. 2009, 2009, 554–566. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Ezhevskaya, M.; Moons, H.; Neuburger, M.; Cristescu, C.; Van Doorslaer, S.; Palivan, C. Structural characterization of a highly active superoxide-dismutase mimic. Phys. Chem. Chem. Phys. 2009, 11, 6778–6787. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, B. Diphenoxo-Bridged Copper(II) Complexes of Reduced Schiff Base Ligands as Functional Models for Catechol Oxidase. Aust. J. Chem. 2009, 62, 968–979. [Google Scholar] [CrossRef]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef] [PubMed]
- Eicken, C.; Krebs, B.; Sacchettini, J.C. Catechol oxidase—structure and activity. Curr. Opin. Struct. Biol. 1999, 9, 677–683. [Google Scholar] [CrossRef]
- Beyazit, N.; Çatıkkaş, B.; Bayraktar, Ş.; Demetgül, C.J. Synthesis, characterization and catecholase-like activity of new Schiff base metal complexes derived from visnagin: Theoretical and experimental study. J. Mol. Struct. 2016, 1119, 124–132. [Google Scholar] [CrossRef]
- Shaban, S.Y.; Ramadan, A.E.-M.M.; Ibrahim, M.M.; Mohamed, M.A.; van Eldik, R. Spectroscopic, thermodynamic, kinetic studies and oxidase/antioxidant biomimetic catalytic activities of tris(3,5-dimethylpyrazolyl)borate Cu(II) complexes. Daltan Trans. 2015, 44, 14110–14121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comba, P.; Martin, B.; Muruganantham, A.; Straub, J. Structure, Bonding, and Catecholase Mechanism of Copper Bispidine Complexes. Inorg. Chem. 2012, 51, 9214–9225. [Google Scholar] [CrossRef] [PubMed]
- Ackermanna, J.; Buchlerb, S.; Meyer, F. Structure–activity correlations in highly preorganized dicopper catechol oxidase model systems. C. R. Chim. 2007, 10, 421–432. [Google Scholar] [CrossRef]
- Belle, C.; Selmeczi, K.; Torelli, S.; Pierre, J.-L. Chemical tools for mechanistic studies related to catechol oxidase activity. C. R. Chim. 2007, 10, 271–283. [Google Scholar] [CrossRef]
- Matoga, D.; Szklarzewicz, J.; Stadnicka, K.; Shongwe, M.S. Iron (III) complexes with a biologically relevant aroylhydrazone: crystallographic evidence for coordination versatility. Inorg. Chem. 2007, 46, 9042–9044. [Google Scholar] [CrossRef] [PubMed]
- Shongwe, M.S.; Al-Rahbi, S.H.; Al-Azani, M.A.; Al-Muharbi, A.A.; Al-Mjeni, F.; Matoga, D.; Gismelseed, A.; Al-Omari, I.A.; Yousif, A.; Adams, H.; et al. Coordination versatility of tridentate pyridyl aroylhydrazones towards iron: tracking down the elusive aroylhydrazono-based ferric spin-crossover molecular materials. Dalton Trans. 2012, 41, 2500–2514. [Google Scholar] [CrossRef] [PubMed]
- Anbu, S.; Paul, A.; Ribeiro, A.P.C.; da Silva, M.F.C.G.; Kuznetsov, M.L.; Pombeiro, A.J.L. Biomolecular interaction, catecholase like activity and alkane oxidation in ionic liquids of a phenylcarbohydrazone-based monocopper(II) complex. Inorg. Chim. Acta 2016, 450, 426–436. [Google Scholar] [CrossRef]
- Ruben, M.; Lehn, J.-M.; Vaughan, G. Synthesis of ionisable [2 × 2] grid-type metallo-arrays and reversible protonic modulation of the optical properties of the [CoII4L4]8+ species. Chem. Commun. 2003, 12, 1338–1339. [Google Scholar] [CrossRef]
- Mondal, S.; Pakhira, B.; Blake, A.J.; Drew, M.G.B.; Chattopadhyay, S.K. Co(III) and Ni(II) complexes of an anthracene appended aroyl hydrazone: Synthesis, crystal structures, DNA binding and catecholase activity. Polyhedron 2016, 117, 327–337. [Google Scholar] [CrossRef]
- Katyal, M.; Dutt, Y. Analytical applications of hydrazones. Talanta 1975, 22, 151–166. [Google Scholar] [CrossRef]
- Mohan, M.; Gupta, M.P.; Chandra, L.; Jha, N.K. Synthesis, characterization and antitumour properties of some metal (II) complexes of 2-pyridinecarboxaldehyde 2′-pyridylhydrazone and related compounds. Inorg. Chim. Acta 1988, 151, 61–68. [Google Scholar] [CrossRef]
- Karrouchi, K.; Charkaoui, Y.; Benlafya, K.; Ramli, Y.; Taoufik, J.; Radi, S.; Ansar, M. Synthesis, characterization and preliminary biological activity of some new pyrazole carbohydrazide derivatives. J. Chem. Pharm. Res. 2013, 5, 1–6. [Google Scholar]
- Karrouchi, K.; Radi, S.; Taoufik, J.; Ghabbour, H.A.; Mabkhot, Y.N. Crystal structure of N′-(4-nitrobenzylidene)-5-phenyl-1H-pyrazole-3-carbohydrazide, C17H13N5O3. Z. Krist. New Cryst. Struct. 2016, 231, 839–841. [Google Scholar] [CrossRef]
- Karrouchi, K.; Ansar, M.; Radi, S.; Saadi, M.; El Ammari, L. Crystal structure of N′-diphenylmethylidene-5-methyl-1H-pyrazole-3-carbohydrazide. Acta Crystallogr. E Crystallogr. Commun. 2015, 71, o890–o891. [Google Scholar] [CrossRef] [PubMed]
- El Kodadi, M.; Malek, F.; Touzani, R.; Ramdani, A. Synthesis of new tripodal ligand 5-(bis(3,5-dimethyl-1H-pyrazol-1-ylmethyl)amino)pentan-1-ol, catecholase activities studies of three functional tripodal pyrazolyl N-donor ligands, with different copper (II) salts. Catal. Commun. 2008, 9, 966–969. [Google Scholar] [CrossRef]
- Boussaleh, N.; Touzani, R.; Bouabdallah, I.; El Kadiri, S.; Ghalem, S. Synthesis, structure and catalytic properties of tripodal amino-acid derivatized pyrazole-based ligands. J. Mol. Catal. A Chem. 2009, 306, 113–117. [Google Scholar] [CrossRef]
- Bouabdallah, I.; Touzani, R.; Zidane, I.; Ramdani, A. Synthesis of new tripodal ligand: N,N-bis[(1,5-dimethylpyrazol-3-yl)methyl]benzylamine.: Catecholase activity of two series of tripodal ligands with some copper (II) salts. Catal. Commun. 2007, 8, 707–712. [Google Scholar] [CrossRef]
- Zerrouki, A.; Touzani, R.; El Kadiri, S. Synthesis of new derivatized pyrazole based ligands and their catecholase activity studies. Arab. J. Chem. 2011, 4, 459–464. [Google Scholar] [CrossRef]
- Mouadili, A.; Attayibat, A.; El Kadiri, S.; Radi, S.; Touzani, R. Catecholase activity investigations using in situ copper complexes with pyrazole and pyridine based ligands. Appl. Catal. A Gen. 2013, 454, 93–99. [Google Scholar] [CrossRef]
- Thabti, S.; Djedouani, A.; Rahmouni, S.; Touzani, R.; Bendaas, A.; Mousser, H.; Mousser, A. Synthesis, X-ray crystal structures and catecholase activity investigation of new chalcone ligands. J. Mol. Struct. 2015, 1102, 295–301. [Google Scholar] [CrossRef]
- Mouadili, A.; Zerrouki, A.; Herrag, L.; Hammouti, B.; El Kadiri, S.; Touzani, R. Catechol oxidation: activity studies using electron-rich nitrogen-based ligands. Res. Chem. Intermed. 2012, 38, 2427–2433. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Program SHELXTL97; Siemens Analytical X-ray Instruments, Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford CT, England, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. 1988, 38, 3098. [Google Scholar] [CrossRef]

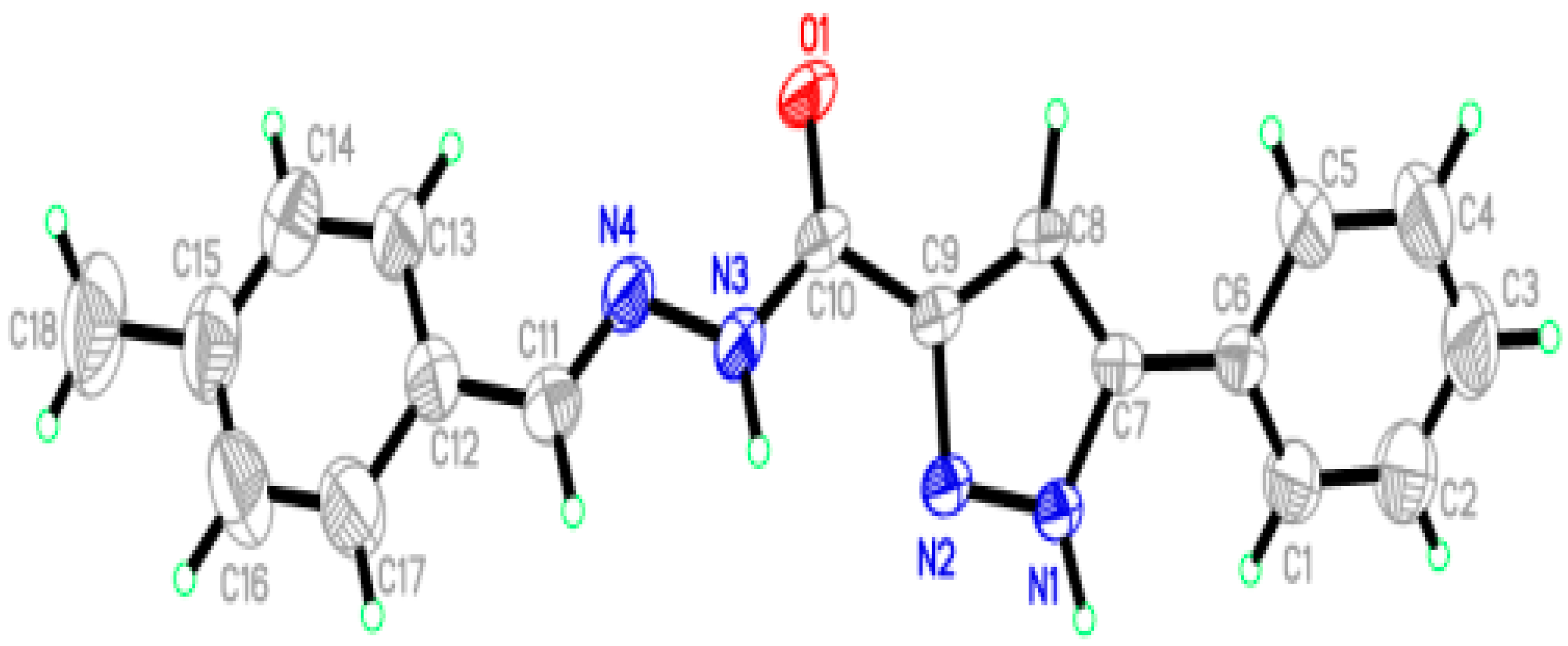



| Bond Length (Å) | Experimental Bond Lengths | Calculated Bond Lengths | Bond Angle (°) | Experimental Bond Angles | Calculated Bond Angles |
|---|---|---|---|---|---|
| O1–C10 | 1.22(3) | 1.24 | N2–C9–C8 | 106.0(2) | 109.2 |
| O2–C14 | 1.36(3) | 1.39 | O1–C10–N3 | 122.8(2) | 125.0 |
| O2–C18 | 1.41(4) | 1.45 | N3–C10–C9 | 116.0(2) | 112.7 |
| O3–C15 | 1.36(3) | 1.38 | O1–C10–C9 | 121.3(2) | 122.2 |
| N1–N2 | 1.33(3) | 1.37 | N4–C11–C12 | 122.9(2) | 121.3 |
| N1–C7 | 1.33(3) | 1.38 | O2–C14–C13 | 125.7(2) | 126.0 |
| N2–C9 | 1.34(3) | 1.36 | C14–O2–C18 | 118.3(2) | 118.5 |
| N3–N4 | 1.38(3) | 1.37 | N2–N1–C7 | 105.3(2) | 113.2 |
| N3–C10 | 1.34(3) | 1.38 | N1–N2–C9 | 112.6(2) | 105.0 |
| N4–C11 | 1.27(3) | 1.29 | N4–N3–C10 | 119.5(2) | 121.0 |
| Bond Length (Å) | Experimental Bond Lengths | Calculated Bond Lengths | Bond Angle (°) | Experimental Bond Angles | Calculated Bond Angles |
|---|---|---|---|---|---|
| O1–C10 | 1.22(2) | 1.21 | N2–N1–C7 | 113.4(15) | 113.6 |
| N1–N2 | 1.34(2) | 1.34 | N1–N2–C9 | 103.9(15) | 105.4 |
| N1–C7 | 1.34(3) | 1.37 | N4–N3–C10 | 119.2(17) | 117.2 |
| N2–C9 | 1.33(2) | 1.34 | N3–N4–C11 | 115.6(18) | 114.2 |
| N3–N4 | 1.38(2) | 1.41 | N1–C7–C6 | 122.7(17) | 122.39 |
| N3–C10 | 1.33(3) | 1.36 | N1–C7–C8 | 105.3(18) | 103.59 |
| N4–C11 | 1.26(3) | 1.27 | N2–C9–C8 | 111.4(16) | 109.21 |
| C15–C18 | 1.512(4) | 1.50 | N2–C9–C10 | 119.4(17) | 119.83 |
| N1–H | 0.86(2) | 1.00 | N3–C10–C9 | 114.9(16) | 111.05 |
| Molecular Energy (a.u.) (eV) | L1 | L2 |
|---|---|---|
| TE | −31008.6 | −26911.4 |
| EHOMO | −5.8186 | −6.4850 |
| ELUMO | −1.0152 | −0.7349 |
| Gap ΔE | 4.8034 | 5.7500 |
| Chemical potential µ (D) | 5.8871 | 6.3122 |
| Ionization potential (IP) | 5.8186 | 6.4850 |
| Electron affinity (EA) | 1.0152 | 0.7349 |
| Electron negatiity (χ) | 3.4169 | 3.6100 |
| Global hardness (η) | 2.4017 | 2.875 |
| Global electrophilicity (ω) | 2.4306 | 2.2664 |
| Ligand/Metallic Salt | Cu(NO3)2 | CuCl2 | Cu(CH3COO)2 | CuSO4 |
|---|---|---|---|---|
| L1 | 10.57 | 10.28 | 9.80 | 9.27 |
| L2 | 15.52 | 0.05 | 22.92 | 16.06 |
| L3 | 37.89 | 9.19 | 24.58 | 21.01 |
| L4 | 17.71 | 11.43 | 15.02 | 6.57 |
| L5 | 3.10 | 8.07 | 19.62 | 10.61 |
| L6 | 27.77 | 40.27 | 60.50 | 72.92 |
| Cu(II)-Ligands | Cu(II) Salt Used | Oxidation Rate (µmol·L−1·min−1) | Ref. |
|---|---|---|---|
| ligand L6 | CuSO4 | 72.920 | - |
| ligand L6 | Cu(CH3COO)2 | 60.500 | - |
| ligand L6 | CuCl2 | 40.270 | - |
| C,N-bipyrazole | Cu(CH3COO)2 | 4.440 | [29] |
| bipyrazolic tripode-prop-2-ylacetate | Cu(CH3COO)2 | 11.825 | [30] |
| bipyrazolic tripode-4-hydroxyphenyl | CuCl2 | 1.458 | [31] |
| bipyrazolic tripode-3-hydroxypropyl | CuSO4 | 28.990 | [32] |
| bipyrazolic tripode-3-hydroxypropyl | CuCl2 | 4.378 | [33] |
| indole-3-chalcone | Cu(CH3COO)2 | 31.780 | [34] |
| [(3,5-dimethyl-pyrazol-1-ylmethyl)-amino]-propionitrile | CuSO4 | 8.710 | [35] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karrouchi, K.; Yousfi, E.B.; Sebbar, N.K.; Ramli, Y.; Taoufik, J.; Ouzidan, Y.; Ansar, M.; Mabkhot, Y.N.; Ghabbour, H.A.; Radi, S. New Pyrazole-Hydrazone Derivatives: X-ray Analysis, Molecular Structure Investigation via Density Functional Theory (DFT) and Their High In-Situ Catecholase Activity. Int. J. Mol. Sci. 2017, 18, 2215. https://doi.org/10.3390/ijms18112215
Karrouchi K, Yousfi EB, Sebbar NK, Ramli Y, Taoufik J, Ouzidan Y, Ansar M, Mabkhot YN, Ghabbour HA, Radi S. New Pyrazole-Hydrazone Derivatives: X-ray Analysis, Molecular Structure Investigation via Density Functional Theory (DFT) and Their High In-Situ Catecholase Activity. International Journal of Molecular Sciences. 2017; 18(11):2215. https://doi.org/10.3390/ijms18112215
Chicago/Turabian StyleKarrouchi, Khalid, El Bekkaye Yousfi, Nada Kheira Sebbar, Youssef Ramli, Jamal Taoufik, Younes Ouzidan, M’hammed Ansar, Yahia N. Mabkhot, Hazem A. Ghabbour, and Smaail Radi. 2017. "New Pyrazole-Hydrazone Derivatives: X-ray Analysis, Molecular Structure Investigation via Density Functional Theory (DFT) and Their High In-Situ Catecholase Activity" International Journal of Molecular Sciences 18, no. 11: 2215. https://doi.org/10.3390/ijms18112215