FGF-2b and h-PL Transform Duct and Non-Endocrine Human Pancreatic Cells into Endocrine Insulin Secreting Cells by Modulating Differentiating Genes
Abstract
:1. Introduction
2. Results
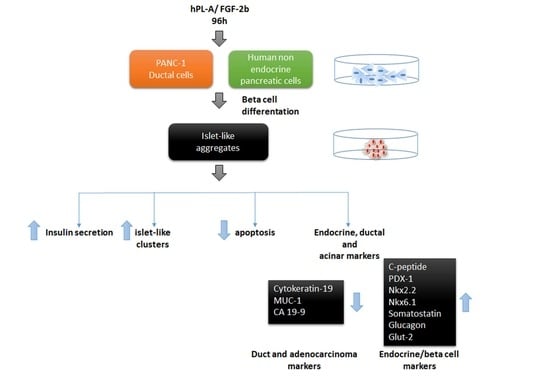
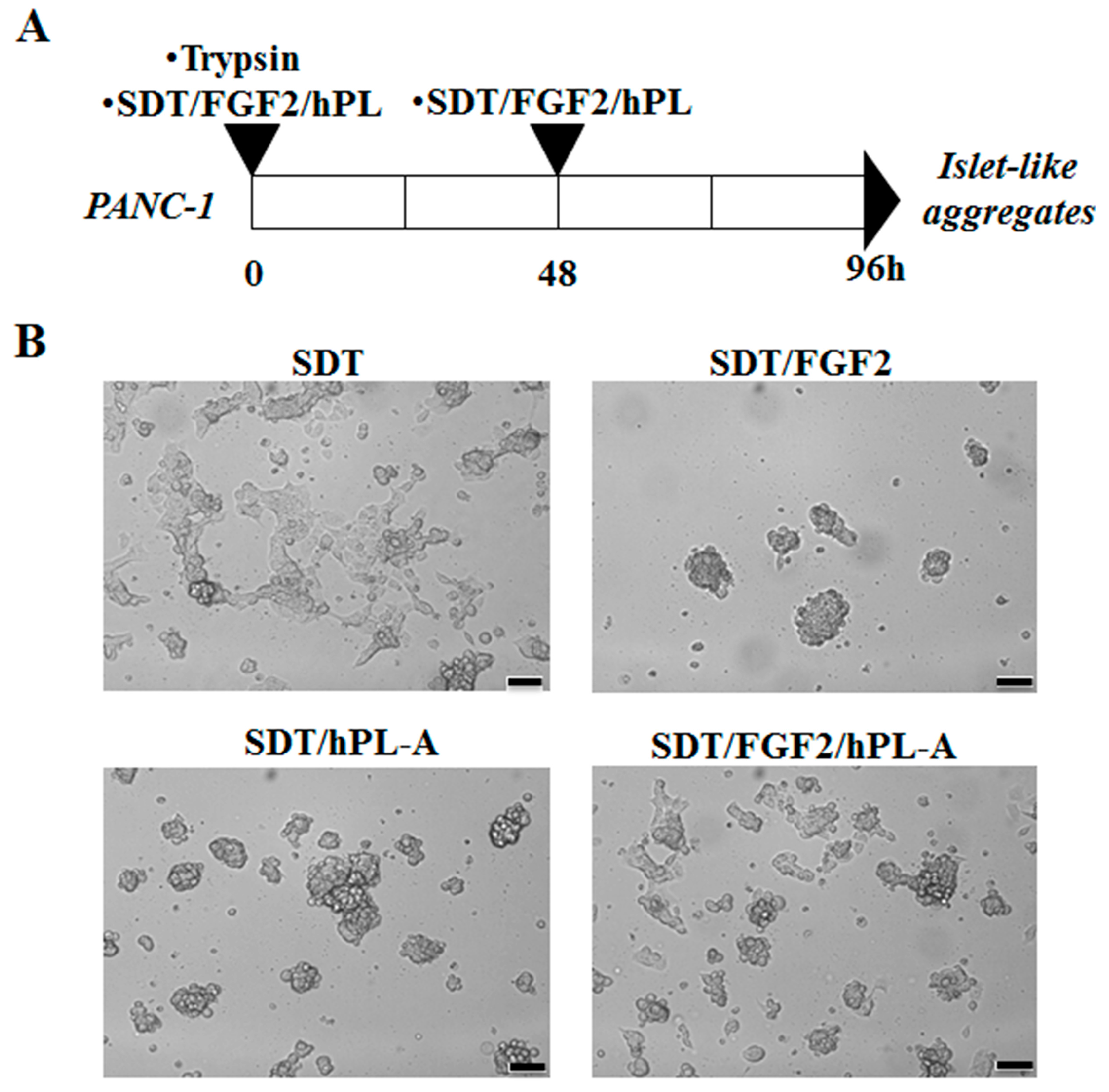
2.1. Treatment with Human Fibroblast Growth Factor (FGF)-2b and Human Placental Lactogen (hPL)-A Promote Differentiation of Human Pancreatic Ductal-Cells (PANC-1) into Islet-Like Cell Aggregates
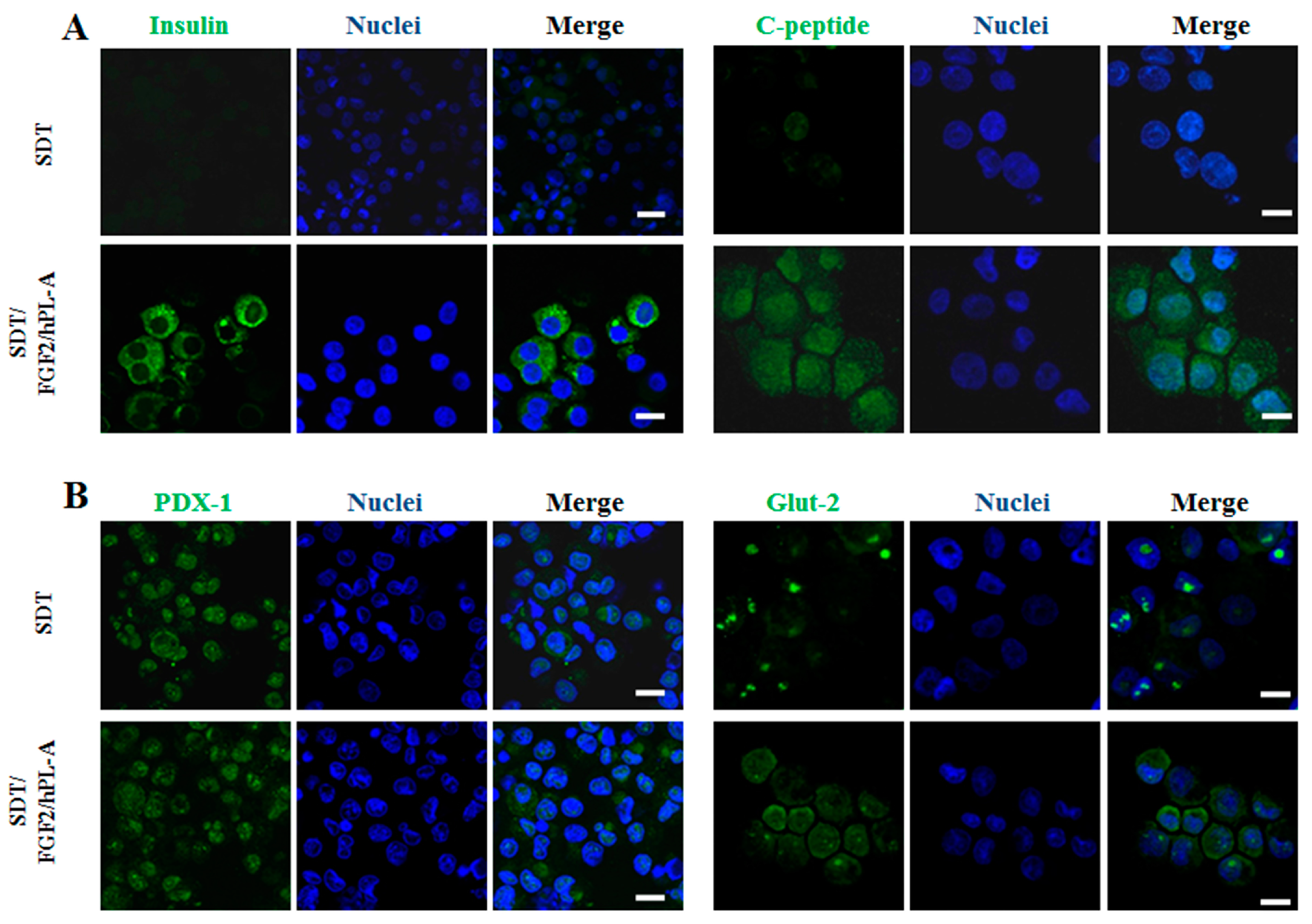
2.2. Markers of Endocrine β Cells Are Modulated in Transformed Islet-Like Cell Aggregates after FGF-2b/hPL-A Treatment
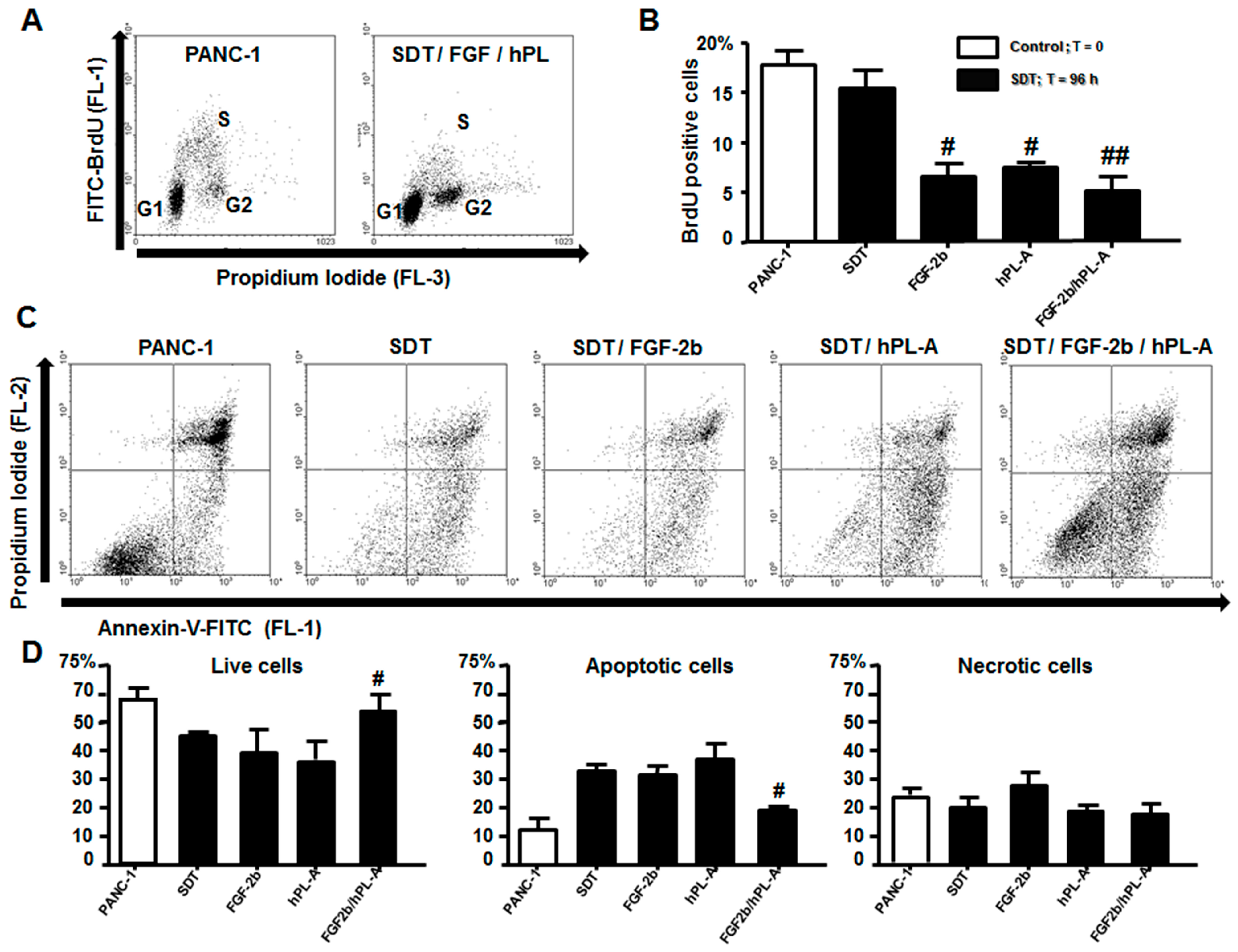
2.3. Treatment with FGF-2b and hPL-A Inhibits Cellular Growth and Reduces Apoptosis in Islet-Like Aggregates
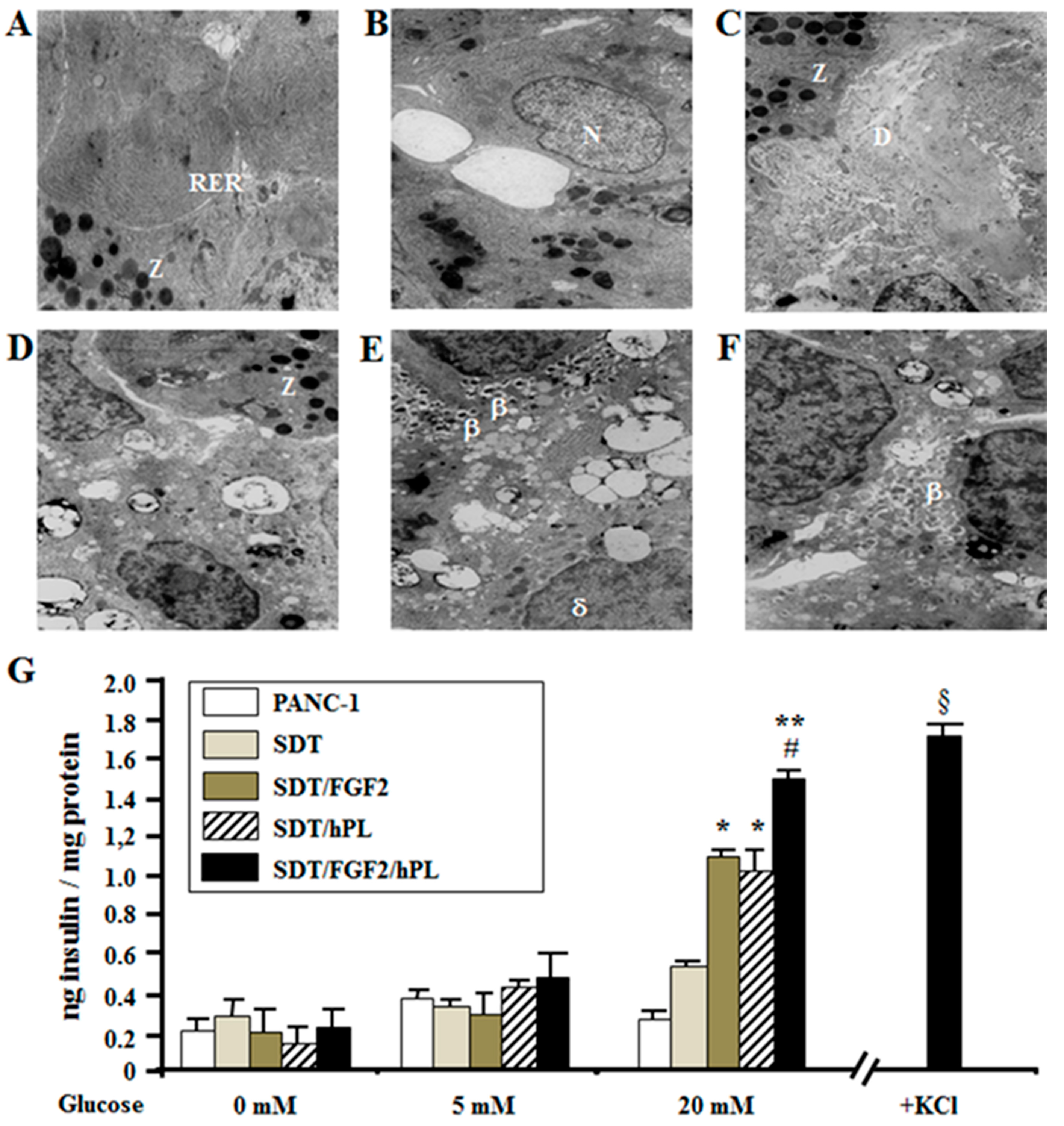
2.4. FGF-2b Plus hPL-A Treatment Changes Ultrastructure and Biological Activity of PANC-1 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Differentiation Protocol
4.2. Isolation of Human Non-Endocrine Pancreatic Cells
4.3. Immunofluorescence Analysis
4.4. Cytofluorimetric Analysis
4.5. Cell Growth and Apoptosis
4.6. Insulin Secretion Assay
4.7. Electron Microscopy Analysis
4.8. Statistical Analysis
4.9. Ethical Approval
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic cardiovascular disease induced by oxidative stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.A.; Gavin Iii, J.R.; Aguilar, R.B.; Herman, M.E. A unified pathophysiological construct of diabetes and its complications. Trends Endocrinol. Metab. 2017, 28, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Antosik, K.; Borowiec, M. Genetic factors of diabetes. Arch. Immunol. Ther. Exp. 2016, 64, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Nigi, L.; Grieco, G.E.; Mancarella, F.; Ventriglia, G.; Dotta, F. Circulating micrornas and diabetes mellitus: A novel tool for disease prediction, diagnosis, and staging? J. Endocrinol. Investig. 2017, 40, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Type 2 diabetes-a matter of β-cell life and death? Science 2005, 307, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J.; Bhushan, A.; Butler, A.E.; Rizza, R.A.; Butler, P.C. Sustained β cell apoptosis in patients with long-standing type 1 diabetes: Indirect evidence for islet regeneration? Diabetologia 2005, 48, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.H.; Kaur, S.; Pociot, F. Long non-coding rnas as novel players in β cell function and type 1 diabetes. Hum. Genom. 2017, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.F.; Hakonarson, H. Genome-wide association studies in type 1 diabetes. Curr. Diabetes Rep. 2009, 9, 157–163. [Google Scholar] [CrossRef]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. B-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.; Guiot, Y.; Goebbels, R.M.; Sempoux, C.; Henquin, J.C. Pancreatic β-cell mass in european subjects with type 2 diabetes. Diabetes Obes. Metab. 2008, 10 (Suppl. S4), 32–42. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, N.; Khetan, S.; Ucar, D.; Stitzel, M.L. Genomics of islet (DYS)function and type 2 diabetes. Trends Genet. 2017, 33, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.S.; Stein, R.W. Evidence for loss in identity, de-differentiation, and trans-differentiation of islet β-cells in type 2 diabetes. Front. Genet. 2017, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Fekete, A.; Vannay, A. [importance of diabetic nephropathy in childhood. Clinical findings and basic research in recent decades]. Orv. Hetil. 2014, 155, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Shi, A.; Zhao, J. Epidemiological perspectives of diabetes. Cell Biochem. Biophys. 2015, 73, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E.F.; Tzanakakis, E.S. Human pluripotent stem cell differentiation to functional pancreatic cells for diabetes therapies: Innovations, challenges and future directions. J. Biol. Eng. 2017, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bendala, J.; Ricordi, C. Present and future cell therapies for pancreatic β cell replenishment. World J. Gastroenterol. 2012, 18, 6876–6884. [Google Scholar] [CrossRef] [PubMed]
- Afelik, S.; Rovira, M. Pancreatic β-cell regeneration: Facultative or dedicated progenitors? Mol. Cell. Endocrinol. 2017, 445, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gysemans, C.; Mathieu, C. B-cell differentiation and regeneration in type 1 diabetes. Diabetes Obes. Metab. 2013, 15 (Suppl. S3), 98–104. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Hong, T. Lineage reprogramming: A promising road for pancreatic β cell regeneration. Trends Endocrinol. Metab. 2016, 27, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Calafiore, R.; Montanucci, P.; Basta, G. Stem cells for pancreatic β-cell replacement in diabetes mellitus: Actual perspectives. Curr. Opin. Organ Transplant. 2014, 19, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Trucco, M. Regeneration of the pancreatic β cell. J. Clin. Investig. 2005, 115, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Toyoda, T.; Inagaki, N.; Osafune, K. Ipsc technology-based regenerative therapy for diabetes. J. Diabetes Investig. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dabkowski, K.; Labedz-Maslowska, A.; Zuba-Surma, E.; Starzynska, T. [role of the bone marrow derived stem cells in pancreatic inflammatory disorders]. Pol. Merkur. Lekarski 2017, 42, 137–141. [Google Scholar] [PubMed]
- Aghazadeh, Y.; Nostro, M.C. Cell therapy for type 1 diabetes: Current and future strategies. Curr. Diabetes Rep. 2017, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Afelik, S.; Rovira, M. Pancreatic β-cell regeneration: Advances in understanding the genes and signaling pathways involved. Genome Med. 2017, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Cao-Minh, L.; Galasso, R.; Rizza, R.A.; Corradin, A.; Cobelli, C.; Butler, P.C. Adaptive changes in pancreatic β cell fractional area and β cell turnover in human pregnancy. Diabetologia 2010, 53, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, L.; Hindi, S.; Sorenson, R.L.; German, M.S. B-cell adaptation in pregnancy. Diabetes Obes Metab 2016, 18 (Suppl. S1), 63–70. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.; Powers, A.C. Replicative capacity of β-cells and type 1 diabetes. J. Autoimmun. 2016, 71, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, A.; Rutter, G.A.; Latreille, M. Mirnas in β-cell development, identity, and disease. Front. Genet. 2016, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bendala, J.; Inverardi, L.; Ricordi, C. Regeneration of pancreatic β-cell mass for the treatment of diabetes. Expert Opin. Biol. Ther. 2012, 12, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Bonner-Weir, S.; Weir, G.C. New sources of pancreatic β-cells. Nat. Biotechnol. 2005, 23, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xia, Q.; Zhou, Q. How to make insulin-producing pancreatic β cells for diabetes treatment. Sci. China Life Sci. 2017, 60, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Montanucci, P.; Calafiore, R. Islet transplantation versus stem cells for the cell therapy of type 1 diabetes mellitus. Minerva Endocrinol. 2015, 40, 267–282. [Google Scholar] [PubMed]
- Domouky, A.M.; Hegab, A.S.; Al-Shahat, A.; Raafat, N. Mesenchymal stem cells and differentiated insulin producing cells are new horizons for pancreatic regeneration in type I diabetes mellitus. Int. J. Biochem. Cell Biol. 2017, 87, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Soria, B.; Roche, E.; Berna, G.; Leon-Quinto, T.; Reig, J.A.; Martin, F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 2000, 49, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.; Gambelli, F.; Gava, B.; Sasdelli, F.; Tellone, V.; Masini, M.; Marchetti, P.; Dotta, F.; Sorrentino, V. Generation and expansion of multipotent mesenchymal progenitor cells from cultured human pancreatic islets. Cell Death Differ. 2007, 14, 1860–1871. [Google Scholar] [CrossRef] [PubMed]
- Bonner-Weir, S.; Sharma, A. Pancreatic stem cells. J. Pathol. 2002, 197, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Scharfmann, R.; Xiao, X.; Heimberg, H.; Mallet, J.; Ravassard, P. B cells within single human islets originate from multiple progenitors. PLoS ONE 2008, 3, e3559. [Google Scholar] [CrossRef] [PubMed]
- Bollimpelli, V.S.; Dholaniya, P.S.; Kondapi, A.K. Topoisomerase iiβ and its role in different biological contexts. Arch. Biochem. Biophys. 2017, 633, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; D’Hoker, J.; Stange, G.; Bonne, S.; De Leu, N.; Xiao, X.; Van de Casteele, M.; Mellitzer, G.; Ling, Z.; Pipeleers, D.; et al. B cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008, 132, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Corritore, E.; Lee, Y.S.; Sokal, E.M.; Lysy, P.A. B-cell replacement sources for type 1 diabetes: A focus on pancreatic ductal cells. Ther. Adv. Endocrinol. Metab. 2016, 7, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Polonsky, K.S. Pdx1 and other factors that regulate pancreatic β-cell survival. Diabetes Obes. Metab. 2009, 11 (Suppl. S4), 30–37. [Google Scholar] [CrossRef] [PubMed]
- Pedica, F.; Beccari, S.; Pedron, S.; Montagna, L.; Piccoli, P.; Doglioni, C.; Chilosi, M. Pdx-1 (pancreatic/duodenal homeobox-1 protein 1). Pathologica 2014, 106, 315–321. [Google Scholar] [PubMed]
- Bonner-Weir, S.; Toschi, E.; Inada, A.; Reitz, P.; Fonseca, S.Y.; Aye, T.; Sharma, A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr. Diabetes 2004, 5 (Suppl. S2), 16–22. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.S. In vivo regeneration of insulin-producing β-cells. Adv. Exp. Med. Biol. 2010, 654, 627–640. [Google Scholar] [PubMed]
- Xia, B.; Zhan, X.R.; Yi, R.; Yang, B. Can pancreatic duct-derived progenitors be a source of islet regeneration? Biochem. Biophys. Res. Commun. 2009, 383, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Saleem, S.; Yee, S.P.; Hardikar, A.A.; Wang, R. C-kit and stem cell factor regulate panc-1 cell differentiation into insulin- and glucagon-producing cells. Lab. Investig. 2010, 90, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Wright, C.; Perfetti, R. Glucagon-like peptide 1 induces differentiation of islet duodenal homeobox-1-positive pancreatic ductal cells into insulin-secreting cells. Diabetes 2001, 50, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Druelle, N.; Avolio, F.; Napolitano, T.; Navarro-Sanz, S.; Silvano, S.; Collombat, P. B-cell replacement strategies: The increasing need for a “β-cell dogma”. Front. Genet. 2017, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Assouline-Thomas, B.; Ellis, D.; Petropavlovskaia, M.; Makhlin, J.; Ding, J.; Rosenberg, L. Islet neogenesis associated protein (INGAP) induces the differentiation of an adult human pancreatic ductal cell line into insulin-expressing cells through stepwise activation of key transcription factors for embryonic β cell development. Differentiation 2015, 90, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Roscioni, S.S.; Migliorini, A.; Gegg, M.; Lickert, H. Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nat. Rev. Endocrinol. 2016, 12, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Dassaye, R.; Naidoo, S.; Cerf, M.E. Transcription factor regulation of pancreatic organogenesis, differentiation and maturation. Islets 2016, 8, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Sekine, K. Fgf-fgfr signaling in vertebrate organogenesis. Cell. Mol. Biol. 1999, 45, 631–638. [Google Scholar] [PubMed]
- Ameri, J.; Stahlberg, A.; Pedersen, J.; Johansson, J.K.; Johannesson, M.M.; Artner, I.; Semb, H. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem. Cells 2010, 28, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.W.; Baeza, N.; Apelqvist, A.; Edlund, H. Attenuation of fgf signalling in mouse β-cells leads to diabetes. Nature 2000, 408, 864–868. [Google Scholar] [PubMed]
- Hardikar, A.A.; Marcus-Samuels, B.; Geras-Raaka, E.; Raaka, B.M.; Gershengorn, M.C. Human pancreatic precursor cells secrete FGF2 to stimulate clustering into hormone-expressing islet-like cell aggregates. Proc. Natl. Acad. Sci. USA 2003, 100, 7117–7122. [Google Scholar] [CrossRef] [PubMed]
- Handwerger, S.; Freemark, M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J. Pediatr. Endocrinol. Metab. 2000, 13, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Brelje, T.C.; Scharp, D.W.; Lacy, P.E.; Ogren, L.; Talamantes, F.; Robertson, M.; Friesen, H.G.; Sorenson, R.L. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: Implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 1993, 132, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Vasavada, R.C.; Garcia-Ocana, A.; Zawalich, W.S.; Sorenson, R.L.; Dann, P.; Syed, M.; Ogren, L.; Talamantes, F.; Stewart, A.F. Targeted expression of placental lactogen in the β cells of transgenic mice results in β cell proliferation, islet mass augmentation, and hypoglycemia. J. Biol. Chem. 2000, 275, 15399–15406. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.F.; De Angelis, F.; Bova, L.; Bartolini, B.; Bertuzzi, F.; Nano, R.; Capuani, B.; Lauro, R.; Federici, M.; Lauro, D.; et al. Human placental lactogen (hPL-a) activates signaling pathways linked to cell survival and improves insulin secretion in human pancreatic islets. Islets 2011, 3, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarang, S.; Viswanathan, C. Umbilical cord derived mesenchymal stem cells useful in insulin production—Another opportunity in cell therapy. Int. J. Stem. Cells 2016, 9, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, M.; Mansouri, K.; Hosseinkhani, S.; Yarani, R.; Yari, K.; Bakhtiari, M.; Mostafaie, A. Differentiation of human skin-derived precursor cells into functional islet-like insulin-producing cell clusters. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Marchand, M.; Schroeder, I.S.; Markossian, S.; Skoudy, A.; Negre, D.; Cosset, F.L.; Real, P.; Kaiser, C.; Wobus, A.M.; Savatier, P. Mouse es cells over-expressing the transcription factor neurod1 show increased differentiation towards endocrine lineages and insulin-expressing cells. Int. J. Dev. Biol. 2009, 53, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Masini, M.; Marselli, L.; Himpe, E.; Martino, L.; Bugliani, M.; Suleiman, M.; Boggi, U.; Filipponi, F.; Occhipinti, M.; Bouwens, L.; et al. Co-localization of acinar markers and insulin in pancreatic cells of subjects with type 2 diabetes. PLoS ONE 2017, 12, e0179398. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt-Velin, N.; Lepore, M.G.; Cartoni, C.; Beermann, F.; Pedrazzini, T. Fgf-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J. Clin. Investig. 2005, 115, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Toyofuku, Y.; Lynn, F.C.; Chak, E.; Uchida, T.; Mizukami, H.; Fujitani, Y.; Kawamori, R.; Miyatsuka, T.; Kosaka, Y.; et al. Serotonin regulates pancreatic β cell mass during pregnancy. Nat. Med. 2010, 16, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Goyvaerts, L.; Schraenen, A.; Schuit, F. Serotonin competence of mouse β cells during pregnancy. Diabetologia 2016, 59, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Khadra, A.; Schnell, S. Development, growth and maintenance of β-cell mass: Models are also part of the story. Mol. Asp. Med. 2015, 42, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Bonner-Weir, S.; Inada, A.; Yatoh, S.; Li, W.C.; Aye, T.; Toschi, E.; Sharma, A. Transdifferentiation of pancreatic ductal cells to endocrine β-cells. Biochem. Soc. Trans. 2008, 36, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, S.; Uno, S.; Iwahashi, H.; Fujita, Y.; Yoshikawa, A.; Kozawa, J.; Okita, K.; Takiuchi, D.; Eguchi, H.; Nagano, H.; et al. Predominance of β-cell neogenesis rather than replication in humans with an impaired glucose tolerance and newly diagnosed diabetes. J. Clin. Endocrinol. Metab. 2013, 98, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Perfetti, R. Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur. J. Endocrinol. 2002, 146, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Melloul, D.; Marshak, S.; Cerasi, E. Regulation of insulin gene transcription. Diabetologia 2002, 45, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Inada, A.; Nienaber, C.; Katsuta, H.; Fujitani, Y.; Levine, J.; Morita, R.; Sharma, A.; Bonner-Weir, S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. USA 2008, 105, 19915–19919. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Sussel, L.; Conners, J.; Scheel, D.; Kalamaras, J.; Dela Cruz, F.; Schwitzgebel, V.; Hayes-Jordan, A.; German, M. Homeobox gene NKX6.1 lies downstream of NKX2.2 in the major pathway of β-cell formation in the pancreas. Development 2000, 127, 5533–5540. [Google Scholar] [PubMed]
- Hori, Y.; Rulifson, I.C.; Tsai, B.C.; Heit, J.J.; Cahoy, J.D.; Kim, S.K. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 16105–16110. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Tyrberg, B.; Itkin-Ansari, P.; Lakey, J.R.; Geron, I.; Monosov, E.Z.; Barcova, M.; Mercola, M.; Levine, F. B-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat. Med. 2006, 12, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Levine, F.; Itkin-Ansari, P. B-cell regeneration: Neogenesis, replication or both? J. Mol. Med. 2008, 86, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Kim, S.Y.; Lee, S.; Shin, Y.J.; Min, B.H.; Bendayan, M.; Park, I.S. Clusterin induces differentiation of pancreatic duct cells into insulin-secreting cells. Diabetologia 2006, 49, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Bottino, R.; Criscimanna, A.; Casu, A.; He, J.; Van der Windt, D.J.; Rudert, W.A.; Giordano, C.; Trucco, M. Recovery of endogenous β-cell function in nonhuman primates after chemical diabetes induction and islet transplantation. Diabetes 2009, 58, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Houbracken, I.; de Waele, E.; Lardon, J.; Ling, Z.; Heimberg, H.; Rooman, I.; Bouwens, L. Lineage tracing evidence for transdifferentiation of acinar to duct cells and plasticity of human pancreas. Gastroenterology 2011, 141, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Martin-Pagola, A.; Sisino, G.; Allende, G.; Dominguez-Bendala, J.; Gianani, R.; Reijonen, H.; Nepom, G.T.; Ricordi, C.; Ruiz, P.; Sageshima, J.; et al. Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia 2008, 51, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Seino, S. Current status of regeneration of pancreatic β-cells. J. Diabetes Investig. 2013, 4, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, R.; Katsuta, H.; Akashi, T.; Yatoh, S.; Weir, G.C.; Sharma, A.; Bonner-Weir, S. Differentiation of copas-sorted non-endocrine pancreatic cells into insulin-positive cells in the mouse. Diabetologia 2009, 52, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, F.; Zaldumbide, A.; Loomans, C.J.; van Rossenberg, E.; Engelse, M.; de Koning, E.J.; Hoeben, R.C. Isolated human islets contain a distinct population of mesenchymal stem cells. Islets 2010, 2, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Lowman, H.B.; Cunningham, B.C.; Wells, J.A. Mutational analysis and protein engineering of receptor-binding determinants in human placental lactogen. J. Biol. Chem. 1991, 266, 10982–10988. [Google Scholar] [PubMed]
- Marchetti, P.; Bugliani, M.; Lupi, R.; Marselli, L.; Masini, M.; Boggi, U.; Filipponi, F.; Weir, G.C.; Eizirik, D.L.; Cnop, M. The endoplasmic reticulum in pancreatic β cells of type 2 diabetes patients. Diabetologia 2007, 50, 2486–2494. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donadel, G.; Pastore, D.; Della-Morte, D.; Capuani, B.; Lombardo, M.F.; Pacifici, F.; Bugliani, M.; Grieco, F.A.; Marchetti, P.; Lauro, D. FGF-2b and h-PL Transform Duct and Non-Endocrine Human Pancreatic Cells into Endocrine Insulin Secreting Cells by Modulating Differentiating Genes. Int. J. Mol. Sci. 2017, 18, 2234. https://doi.org/10.3390/ijms18112234
Donadel G, Pastore D, Della-Morte D, Capuani B, Lombardo MF, Pacifici F, Bugliani M, Grieco FA, Marchetti P, Lauro D. FGF-2b and h-PL Transform Duct and Non-Endocrine Human Pancreatic Cells into Endocrine Insulin Secreting Cells by Modulating Differentiating Genes. International Journal of Molecular Sciences. 2017; 18(11):2234. https://doi.org/10.3390/ijms18112234
Chicago/Turabian StyleDonadel, Giulia, Donatella Pastore, David Della-Morte, Barbara Capuani, Marco F. Lombardo, Francesca Pacifici, Marco Bugliani, Fabio A. Grieco, Piero Marchetti, and Davide Lauro. 2017. "FGF-2b and h-PL Transform Duct and Non-Endocrine Human Pancreatic Cells into Endocrine Insulin Secreting Cells by Modulating Differentiating Genes" International Journal of Molecular Sciences 18, no. 11: 2234. https://doi.org/10.3390/ijms18112234