iTRAQ-Based Identification of Proteins Related to Muscle Growth in the Pacific Abalone, Haliotis discus hannai
Abstract
:1. Introduction
2. Results
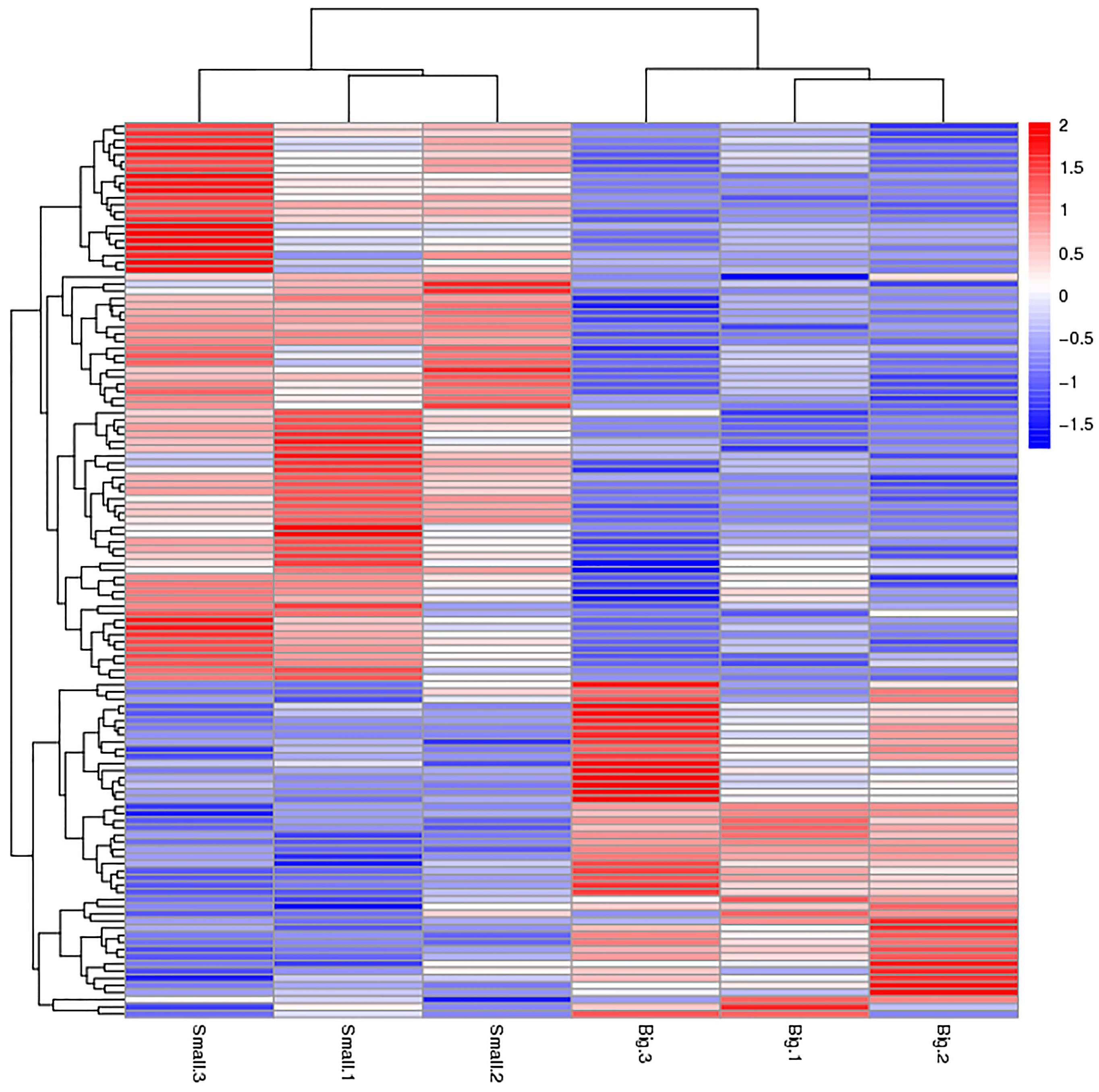
2.1. Protein Profiling
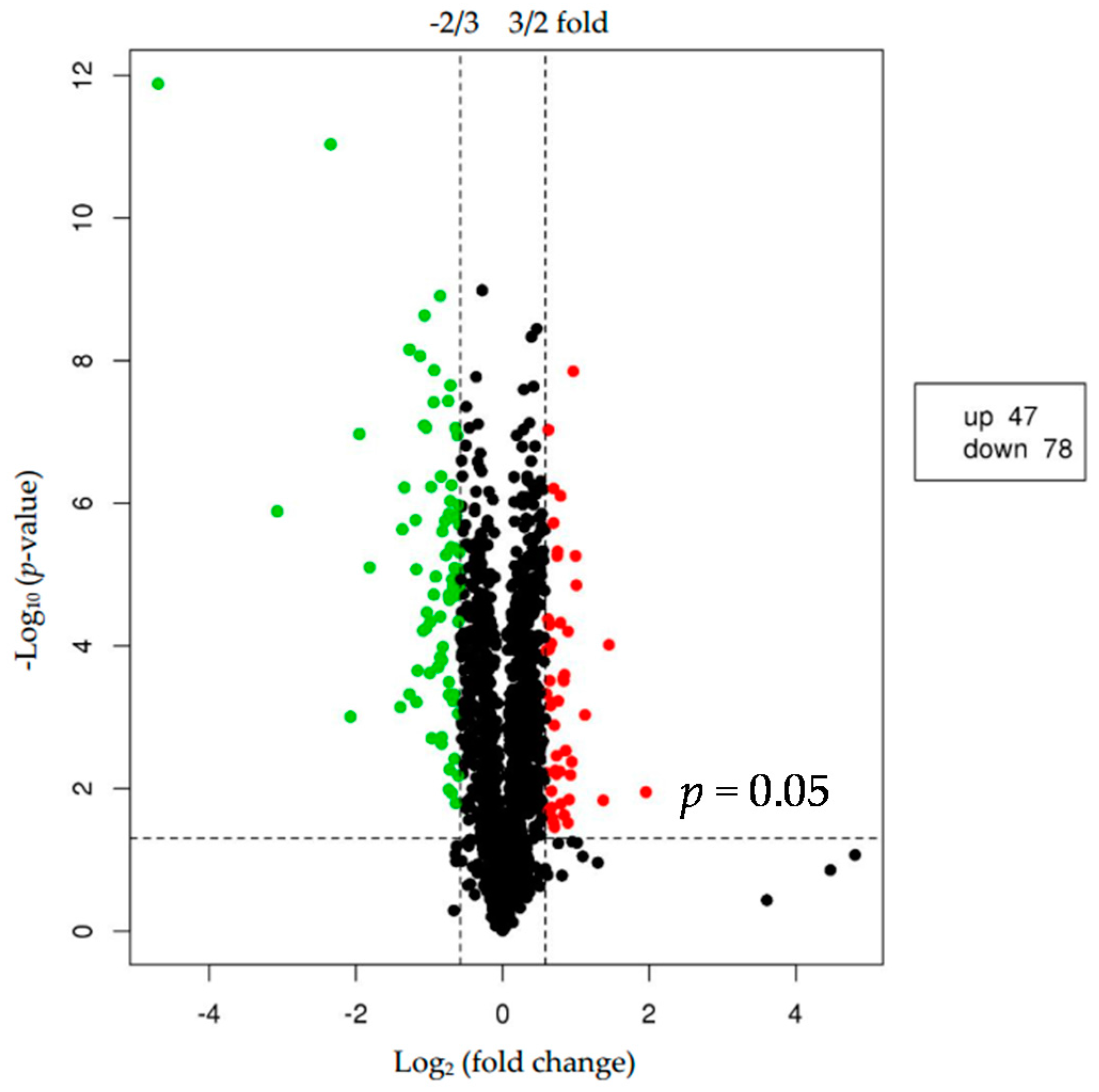
2.2. Differentially Expressed Proteins (DEPs)
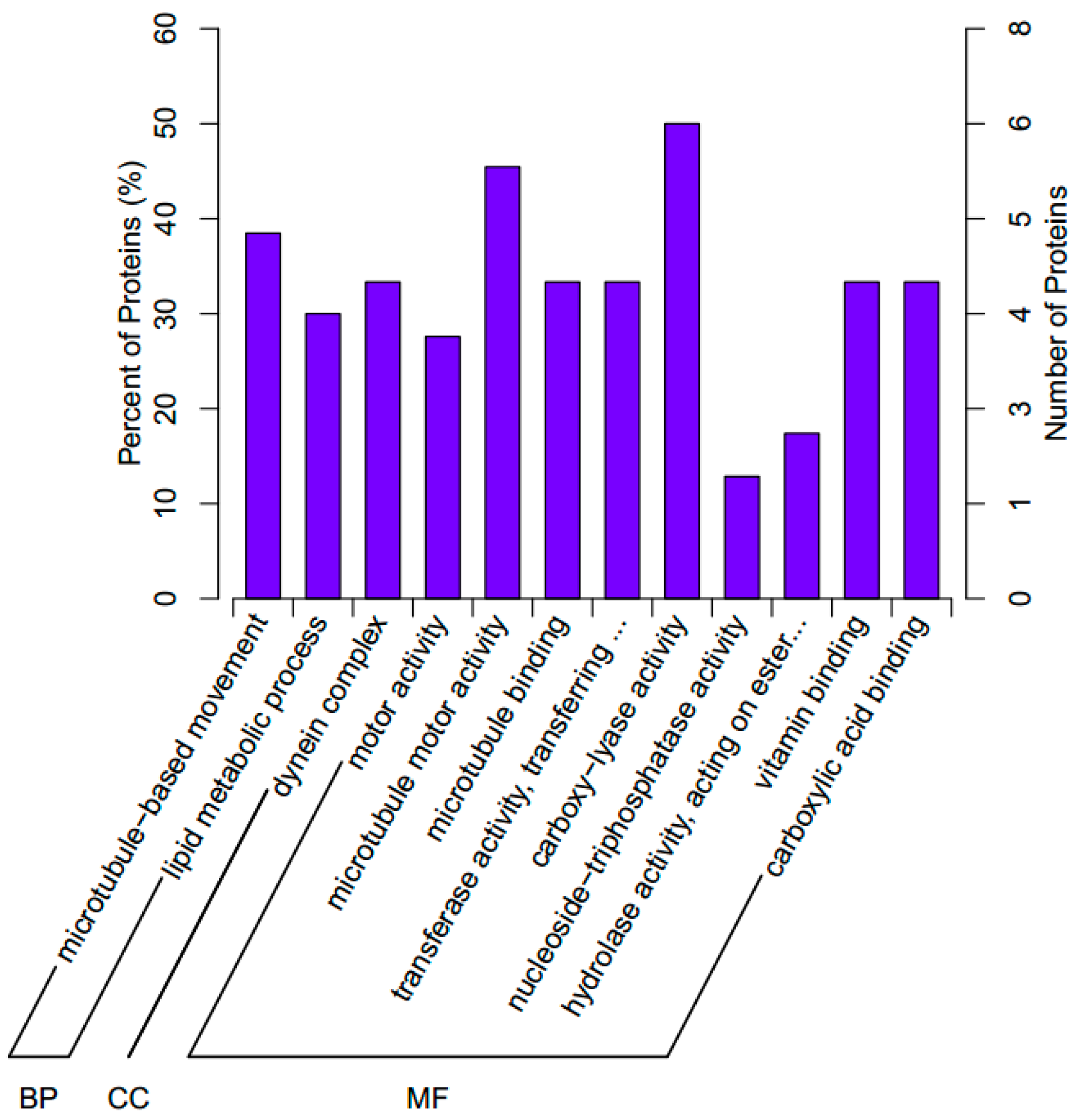
2.3. GO Functional Classification
2.4. KEGG Pathway Analysis
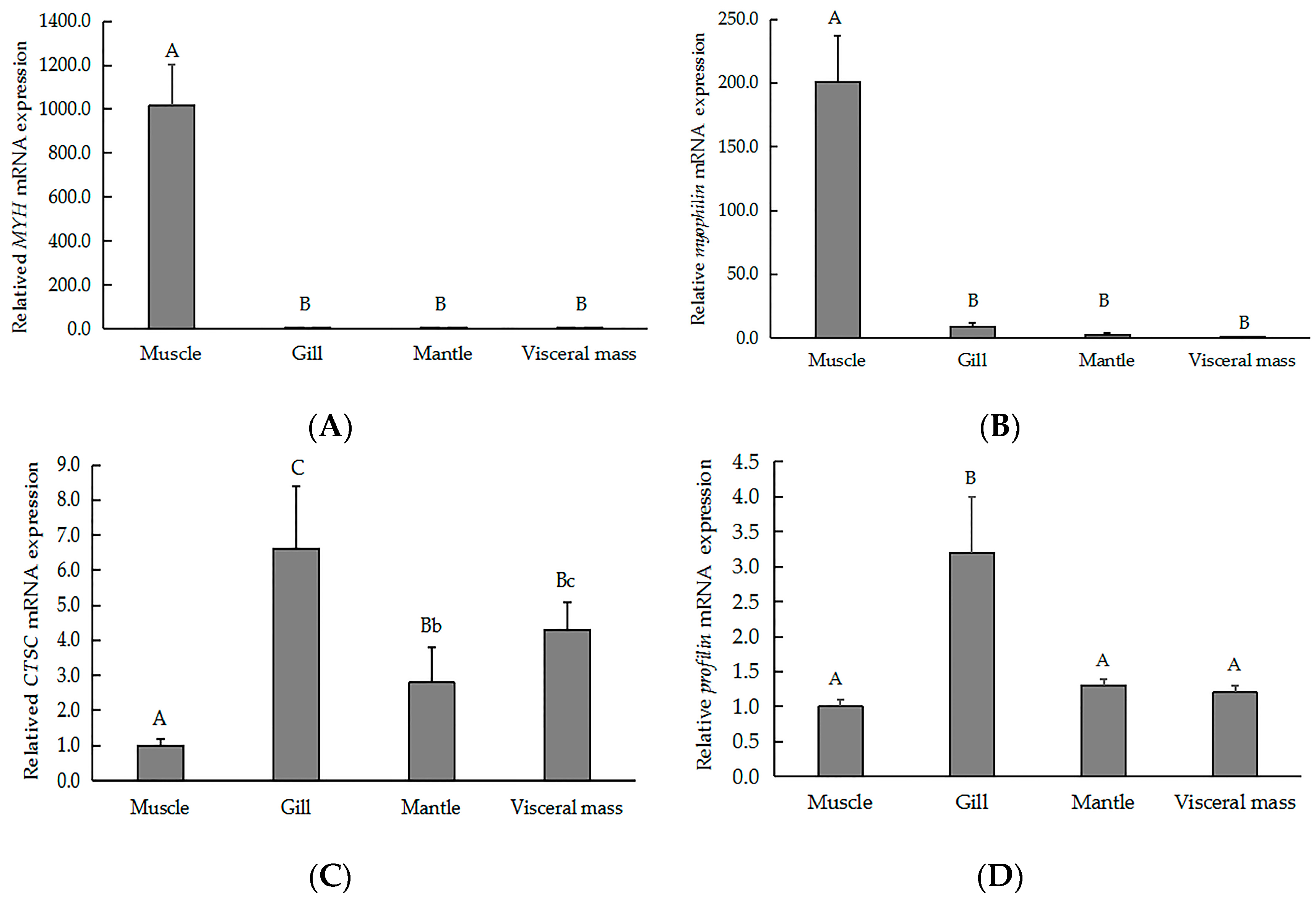
2.5. qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Experimental Tissue
4.2. Protein Preparation
4.3. iTRAQ Labeling of Peptides
4.4. HPLC Fractionation
4.5. LC-MS/MS Analysis
4.6. Data Analysis
4.7. qRT-PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, X.M.; Ford, S.E.; Zhang, F.S. Molluscan aquaculture in China. J. Shellfish Res. 1999, 18, 19–31. [Google Scholar]
- Luo, X.; Ke, C.H.; You, W.W.; Wang, D.X.; Chen, F. Molecular identification of interspecific hybrids between Haliotis discus hannai Ino and Haliotis gigantea Gmelin usingamplified fragment-length polymorphism and microsatellite markers. Aquacult. Res. 2010, 41, 1827–1834. [Google Scholar] [CrossRef]
- Chen, N.; Luo, X.; Gu, Y.T.; Han, G.D.; Dong, Y.W.; You, W.W.; Ke, C.H. Assessment of the thermal tolerance of abalone based on cardiac performance in Haliotis discus hannai, H. gigantea and their interspecific hybrid. Aquaculture 2016, 465, 258–264. [Google Scholar] [CrossRef]
- Guo, Y.F.; Zhao, W.W.; Gao, H.Q.; Wang, S.; Yu, P.M.; Yu, H.S.; Wang, D.; Wang, Q.; Wang, J.X.; Wang, Z.F.; et al. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2017. [Google Scholar]
- Naipil, C.C.; Muñoz, V.V.; Valdés, J.A.; Molina, A.; Escárate, C.G. RNA interference in Haliotis rufescens myostatin evidences upregulation of insulin signaling pathway. Agri Gene 2016, 1, 93–99. [Google Scholar] [CrossRef]
- Elliott, N.G. Genetic improvement programmes in abalone: What is the future? Aquac. Res. 2000, 31, 51–59. [Google Scholar] [CrossRef]
- Di, G.; Luo, X.; You, W.; Zhao, J.; Kong, X.; Ke, C. Proteomic analysis of muscle between hybrid abalone andparental lines Haliotis gigantea Reeve and Haliotis discus hannai Ino. Heredity 2015, 114, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Meng, B.; Chen, W.H.; Ge, X.M.; Liu, S.Q.; Yu, J. A proteomic study on postdiapaused embryonic development of brine shrimp (Artemia franciscana). Proteomics 2007, 7, 3580–3591. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, M.; Rodríguez-Piñeiro, A.M.; Oliveira, E.; Páez de la Cadena, M.; Rolán-Alvarez, E. Proteomic Comparison between Two Marine Snail Ecotypes Reveals Details about the Biochemistry of Adaptation. J. Proteome Res. 2008, 7, 4926–4934. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y.; Thiyagarajan, V.; Qian, P.Y.; Qiu, J.W. Protein expression during the embryonic development of a gastropod. Proteomics 2010, 10, 2701–2711. [Google Scholar] [CrossRef] [PubMed]
- Karp, N.A.; Huber, W.; Sadowski, P.G.; Charles, P.D.; Hester, S.V.; Lilley, K.S. Addressing Accuracy and Precision Issues in iTRAQ Quantitation. Mol. Cell. Proteom. 2010, 9, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ba, H.X.; Zhang, W.; Coates, D.; Li, C.Y. iTRAQ-Based Quantitative Proteomic Analysis of the Potentiated and Dormant Antler Stem Cells. Int. J. Mol. Sci. 2016, 17, 1778. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.; Unwin, R.D.; Evans, C.A.; Griffiths, S.; Carney, L.; Zhang, L.; Jaworska, E.; Lee, C.F.; Blinco, D.; Okoniewski, M.J.; et al. Eight-channel iTRAQ enables comparison of the activity of six leukemogenic tyrosine kinases. Mol. Cell. Proteom. 2008, 7, 853–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.S.; Liu, X.H.; Liu, L.X.; Lou, W.H.; Jin, D.Y.; Yang, P.Y.; Wang, X.L. iTRAQ-based quantitative proteomics reveals myoferlin as a novel prognostic predictor in pancreatic adenocarcinoma. J. Proteom. 2013, 91, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Malécot, M.; Marie, A.; Dao, S.P.; Edery, M. iTRAQ-based proteomic study of the effectsof microcystin-LR on medaka fish liver. Proteomics 2011, 11, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Marancik, D.P.; Fast, M.D.; Camus, A.C. Proteomic characterization of the acute-phase response of yellow stingrays Urobatis jamaicensis after injection with a Vibrio anguillarum-ordalii bacterin. Fish Shellfish Immunol. 2013, 34, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Lü, A.; Hu, X.C.; Wang, Y.; Shen, X.J.; Li, X.; Zhu, A.H.; Tian, J.; Ming, Q.L.; Feng, Z.J. iTRAQ analysis of gill proteins from the zebrafish (Danio rerio) infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2014, 36, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Mu, H.W.; Li, J.; Zhang, Y.H.; Xu, F.J.; Xiang, Z.M.; Qin, P.Y.; Qiu, J.W.; Yu, Z.N. Proteomic Basis of Stress Responses in the Gills of the Pacific Oyster Crassostrea gigas. J. Proteom. Res. 2015, 14, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Yu, X.; Gao, B.Q.; Li, J.; Liu, P. iTRAQ-based identification of differentially expressed proteins related to growth in the swimming crab, Portunus trituberculatus. Aquac. Res. 2016, 1–11. [Google Scholar] [CrossRef]
- Xu, D.X.; Sun, L.N.; Liu, S.L.; Zhang, L.B.; Yang, H.S. Understanding the Heat Shock Response in the Sea Cucumber Apostichopus japonicus, Using iTRAQ-Based Proteomics. Int. J. Mol. Sci. 2016, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.V.; Portilla, M.D.; Escárate, C.G. Characterization of the growth-related transcriptome in California red abalone (Haliotis rufescens) through RNA-Seq analysis. Mar. Genom. 2015, 24, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Kim, G.D.; Kim, J.M.; Lim, H.K. Differentially-Expressed Genes Associated with Faster Growth of the Pacific Abalone, Haliotis discus hannai. Int. J. Mol. Sci. 2015, 16, 27520–27534. [Google Scholar] [CrossRef] [PubMed]
- Hevrøy, E.M.; Jordal, A.E.O.; Hordvik, I.; Espe, M.; Hemre, G.I.; Olsvik, P.A. Myosin heavy chain mRNA expression correlates higher with muscle protein accretion than growth in Atlantic salmon, Salmo salar. Aquaculture 2006, 252, 453–461. [Google Scholar] [CrossRef]
- Gauvry, L.; Fauconneau, B. Cloning of a trout fast skeletal myosin heavy chain expressed both in embryo and adult muscles and in myotubes neoformed in vitro. Comp. Biochem. Physiol. 1996, 115, 183–190. [Google Scholar] [CrossRef]
- Young, R.B.; Hsieh, M.Y.; Hudson, J.R., Jr.; Richter, H.E.; Scott, M. Expression pattern and partial sequence analysis of a fetal bovine myosin heavy chain gene. J. Anim. Sci. 1994, 72, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Dong, J.J.; Sun, C.F.; Tian, Y.Y.; Ye, X. cDNA Cloning and Analyses of Two Myosin Heavy Chain Isoforms of Mandarin Fish (Siniperca chuatsi) Based on Transcriptome Sequencing. Prog. Fish. Sci. 2017, 38, 51–61. [Google Scholar] [CrossRef]
- Chen, T.L.; Kowalczyk, P.A.; Ho, G.; Chisholm, R.L. Targeted disruption of the Dictyostelium myosin essential light chain gene produces cells defective in cytokinesis and morphogenesis. J. Cell Sci. 1995, 108, 3207–3218. [Google Scholar] [PubMed]
- Greenfield, N.J.; Montelione, G.T.; Farid, R.S.; Hitchcock-DeGregori, S.E. The structure of the N-terminus of striated muscle α-tropomyosin in a chimeric peptide: Nuclear magnetic resonance structure and circular dichroism studies. Biochemistry 1998, 37, 7834–7843. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W.; Vandekerckhove, J. Stucture and function of actin. Ann. Rev. Biophys. 1992, 21, 49–76. [Google Scholar]
- Patwary, M.U.; Reith, M.; Kenchington, E.L. Isolation and charcterization of a cDNA encoding an actin gene from sea scallop (Placopecten magellanicus). J. Shellfish Res. 1996, 15, 265–270. [Google Scholar]
- Pollard, T.D.; Cooper, J.A. Actin, a Central Player in Cell Shape and Movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- DesGroseillers, L.; Auclair, D.; Wickham, L. Nucleotide sequence of an actin cDNA gene from Aplysia californica. Nucleic Acids Res. 1990, 18, 3654. [Google Scholar] [CrossRef] [PubMed]
- DesGroseillers, L.; Auclair, D.; Wickham, L.; Maalouf, M. A novel actin cDNA is expressed in the neurons of Aplysia californica. Biochim. Biophys. Acta 1994, 1217, 322–324. [Google Scholar] [CrossRef]
- Miyamoto, H.; Hamaguchi, M.; Okoshi, K. Analysis of genes expressed in the mantle of oyster Crassostreagigas. Fish Sci. 2002, 68, 651–658. [Google Scholar] [CrossRef]
- VanLoon, A.E.; Goedemans, H.J.; Daemen, A.J.J.M.; van de Kamp, A.J.; van den Biggelaar, J.A.M. Actin genes expressed during early development of Patella vulgata. Rouxs Arch. Dev. Biol. 1993, 202, 77–84. [Google Scholar] [CrossRef]
- Bryant, M.J.; Flint, H.J.; Sin, F.Y.T. Isolation, Characterization, and Expression Analysis of Three Actin Genes in the New Zealand Black-Footed Abalone, Haliotis iris. Mar. Biotechnol. 2006, 8, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Gunning, P.W.; Ghoshdastider, U.; Whitaker, S.; Popp, D.; Robinson, R.C. The evolution of compositionally and functionally distinct actin filaments. J. Cell Sci. 2015, 128, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Zhang, Y.Z. Progress in Profilin. Chin. J. Cell Biol. 2007, 29, 325–330. [Google Scholar]
- Pernier, J.; Shekhar, S.; Jegou, A.; Guichard, B.; Carlier, M.F. Profilin Interaction with Actin Filament Barbed End Controls Dynamic Instability, Capping, Branching, and Motility. Dev. Cell 2016, 36, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.; Carroll, R.T.; Burke, T.A.; Christensen, J.R.; Bestul, A.J.; Sees, J.A.; James, M.L.; Sirotkin, V.; Kovar, D.R. Profilin Regulates F-Actin Network Homeostasis by Favoring Formin over Arp2/3 Complex. Dev. Cell 2015, 32, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Rotty, J.D.; Wu, C.; Haynes, E.M.; Suarez, C.; Winkelman, J.D.; Johnson, H.E.; Haugh, J.M.; Kovar, D.R.; Bear, J.E. Profilin-1 Serves as a Gatekeeper for Actin Assembly by Arp2/3-Dependent and Independent Pathways. Dev. Cell 2015, 32, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Sohn, R.H.; Clermont, P.J. Profilin: At the crossroads of signal transduction and the actin cytoskeleton. BioEssays 1994, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Gasser, R.B.; Jones, M.K.; Lightowlers, M.W. Identification and characterization of myophilin, a muscle-specific antigen of Echinococcus granulosus. Mol. Biochem. Parasitol. 1995, 70, 139–148. [Google Scholar] [CrossRef]
- Martin, R.M.; Colebrook, A.L.; Gasser, R.B.; Lightowlers, M.W. Antibody responses of patients with cystic hydatid disease to recombinant myophilin of Echinococcus granulosus. Acta Trop. 1996, 61, 307–314. [Google Scholar] [CrossRef]
- Peng, H.L.; Song, K.; Huang, C.Y.; Ye, S.; Song, H.G.; Hu, W.; Han, Z.G.; McManus, D.P.; Zhao, G.P.; Zhang, Q.H. Expression, immunolocalization and serodiagnostic value of a myophilin-like protein from Schistosoma japonicum. Exp. Parasitol. 2008, 119, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sloane, B.F. Cathepsin B and cystatins: Evidence for a role in cancer progression. Semin. Cancer Biol. 1990, 1, 137–152. [Google Scholar] [PubMed]
- Tong, B.; Wan, B.; Wei, Z.; Wang, T.; Zhao, P.; Dou, Y.; Lv, Z.; Xia, Y.; Dai, Y. Role of cathepsin B in regulating migration and invasion of fibroblast-like synoviocytes into inflamed tissue from patients with rheumatoid arthritis. Clin. Exp. Immunol. 2014, 177, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.D.; Ham, B.; Mogridge, J.; Saftig, P.; Lin, S.; Kim, S.O. Cathepsin B-mediated autophagy flux facilitates the anthrax toxin receptor 2-mediated delivery of anthrax lethal factor into the cytoplasm. J. Biol. Chem. 2010, 285, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.E.; Ho, C.C.; Yang, S.F.; Lin, S.H.; Yeh, K.T.; Lin, C.W.; Chen, M.K. Cathepsin B Expression and the Correlation with Clinical Aspects of Oral Squamous Cell Carcinoma. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Vivar, C.; Potter, M.C.; van Praag, H. All about running: Synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr. Top. Behav. Neurosci. 2012, 15, 189–210. [Google Scholar] [CrossRef]
- Houseweart, M.K.; Pennacchio, L.A.; Vilaythong, A.; Peters, C.; Noebels, J.L.; Myers, R.M. Cathepsin B but not cathepsins L or S contributes to the pathogenesis of Unverricht-Lundborg progressive myoclonus epilepsy (EPM1). J. Neurobiol. 2003, 56, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Kominami, E.; Ishido, K.; Muno, D.; Sato, N. The primary structure and tissue distribution of cathepsin C. Biol. Chem. 1992, 373, 367–373. [Google Scholar] [CrossRef]
- Dolenc, I.; Turk, B.; Pungerc, G.; Ritonja, A.; Turk, V. Oligomeric structure and substrate induced inhibition of human cathepsin C. J. Biol. Chem. 1995, 270, 21626–21631. [Google Scholar] [CrossRef] [PubMed]
- Muno, D.; Ishidoh, K.; Ueno, T.; Kominami, E. Processing and transport of the precursor of cathepsin C during its transfer into lysosomes. Arch. Biochem. Biophys. 1993, 306, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wolters, P.J.; Raymond, W.W.; Blount, J.L.; Caughey, G.H. Regulated expression, processing, and secretion of dog mast cell dipeptidyl peptidase I. J. Biol. Chem. 1998, 273, 15514–15520. [Google Scholar] [CrossRef] [PubMed]
- Martínez, D.S.; Krzywinska, E.; Rathore, M.G.; Saumet, A.; Cornillon, A.; Lopez-Royuela, N.; Martínez-Lostao, L.; Ramirez-Labrada, A.; Lu, Z.Y.; Rossi, J.F.; et al. All-trans retinoic acid (ATRA) induces miR-23a expression, decreases CTSC expression and granzyme B activity leading to impaired NK cell cytotoxicity. Int. J. Biochem. Cell Biol. 2014, 49, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods 2001, 25, 402–428. [Google Scholar] [CrossRef] [PubMed]

| Group Name | Total Spectra | Spectra | Ratio Identified | Peptides | Proteins |
|---|---|---|---|---|---|
| All | 425,477 | 44,436 | 10.40% | 10,097 | 1904 |
| KEGG Pathway | Upregulated Proteins | Downregulated Proteins |
|---|---|---|
| Lysosome | putative inorganic phosphate cotransporter (Picot; Accession Number: O61369), actin (Accession Number: Q93129) | ganglioside GM2 activator (GM2A; Accession Number: Q8HXX6), cathepsin C (CTSC; Accession Number: A0A023PJH7), cathepsin B (CTSB; Accession Number: A1E295), palmitoyl-protein thioesterase 1 (PPT1; Accession Number: Q8HXW6), N-acetylglucosamine-6-sulfatase (GNS; Accession Number: Q8BFR4), α-N-acetylgalactosaminidase (Accession Number: Q90744) |
| Adherens junction | actin A1 (Accession Number: Q5BQE5), actin-2 (Accession Number: P26197), actin | kitasatospora griseola strain MF730-N6 RKJC_4 (Accession Number: A0A0D0PVQ3), β actin (ACTB; Accession Number: G8HY07), epidermal growth factor receptor (EGFR; Accession Number: P0CY46), glycerophosphodiester phosphodiesterase domain-containing protein 1 (GDPD1; Accession Number: Q8N9F7) |
| Bladder cancer | - | thymidine phosphorylase (Tymp; Accession Number: Q5FVR2), EGFR, GDPD1 |
| Apoptosis | actin A1, actin-2, actin, myophilin (Accession Number: Q24799) | CTSC, GDPD1, CTSB |
| Thyroid hormone signaling pathway | actin A1, actin-2, actin, solute carrier family 2 facilitated glucose transporter member 3 (SLC2A3; Accession Number: P47843) | ACTB, GDPD1 |
| Endometrial cancer | - | kitasatospora griseola strain MF730-N6 RKJC_4, EGFR, GDPD1 |
| Shigellosis | actin A1, actin-2, actin | profilin (Accession Number: F4XXT7), ACTB, GDPD1 |
| Regulation of actin cytoskeleton | actin A1, actin-2, actin | profilin, ACTB, EGFR, GDPD1, phosphatidylinositol 5-phosphate 4-kinase type-2 β (PIP4K2B; Accession Number: P78356), myosin regulatory light chain sqh (Accession Number: P40423) |
| Salmonella infection | actin A1, actin-2, actin | profilin, ACTB, GDPD1 |
| Viral myocarditis | actin A1, actin-2, actin, myosin heavy chain (MYH; Accession Number: P24733), MYH II (Accession Number: O96700) | ACTB |
| Hippo signaling pathway-fly | actin A1, actin-2, actin, protocadherin Fat 4 (FAT4; Accession Number: Q6V0I7) | ACTB |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; You, W.; Luo, X.; Ke, C. iTRAQ-Based Identification of Proteins Related to Muscle Growth in the Pacific Abalone, Haliotis discus hannai. Int. J. Mol. Sci. 2017, 18, 2237. https://doi.org/10.3390/ijms18112237
Huang J, You W, Luo X, Ke C. iTRAQ-Based Identification of Proteins Related to Muscle Growth in the Pacific Abalone, Haliotis discus hannai. International Journal of Molecular Sciences. 2017; 18(11):2237. https://doi.org/10.3390/ijms18112237
Chicago/Turabian StyleHuang, Jianfang, Weiwei You, Xuan Luo, and Caihuan Ke. 2017. "iTRAQ-Based Identification of Proteins Related to Muscle Growth in the Pacific Abalone, Haliotis discus hannai" International Journal of Molecular Sciences 18, no. 11: 2237. https://doi.org/10.3390/ijms18112237




