Effects of Late Gestational Fetal Exposure to Dexamethasone Administration on the Postnatal Hypothalamus-Pituitary-Adrenal Axis Response to Hypoglycemia in Pigs
Abstract
:1. Introduction
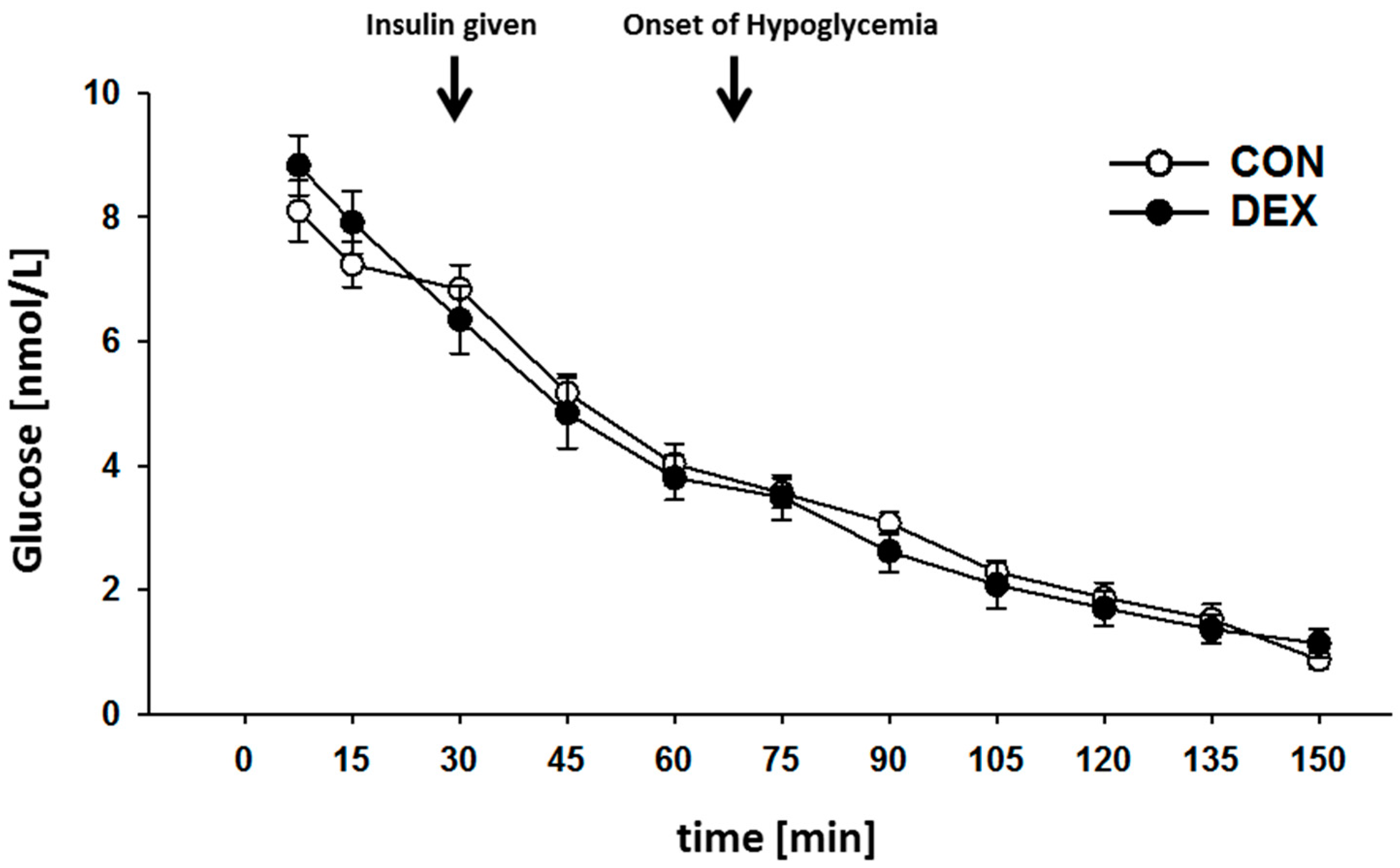
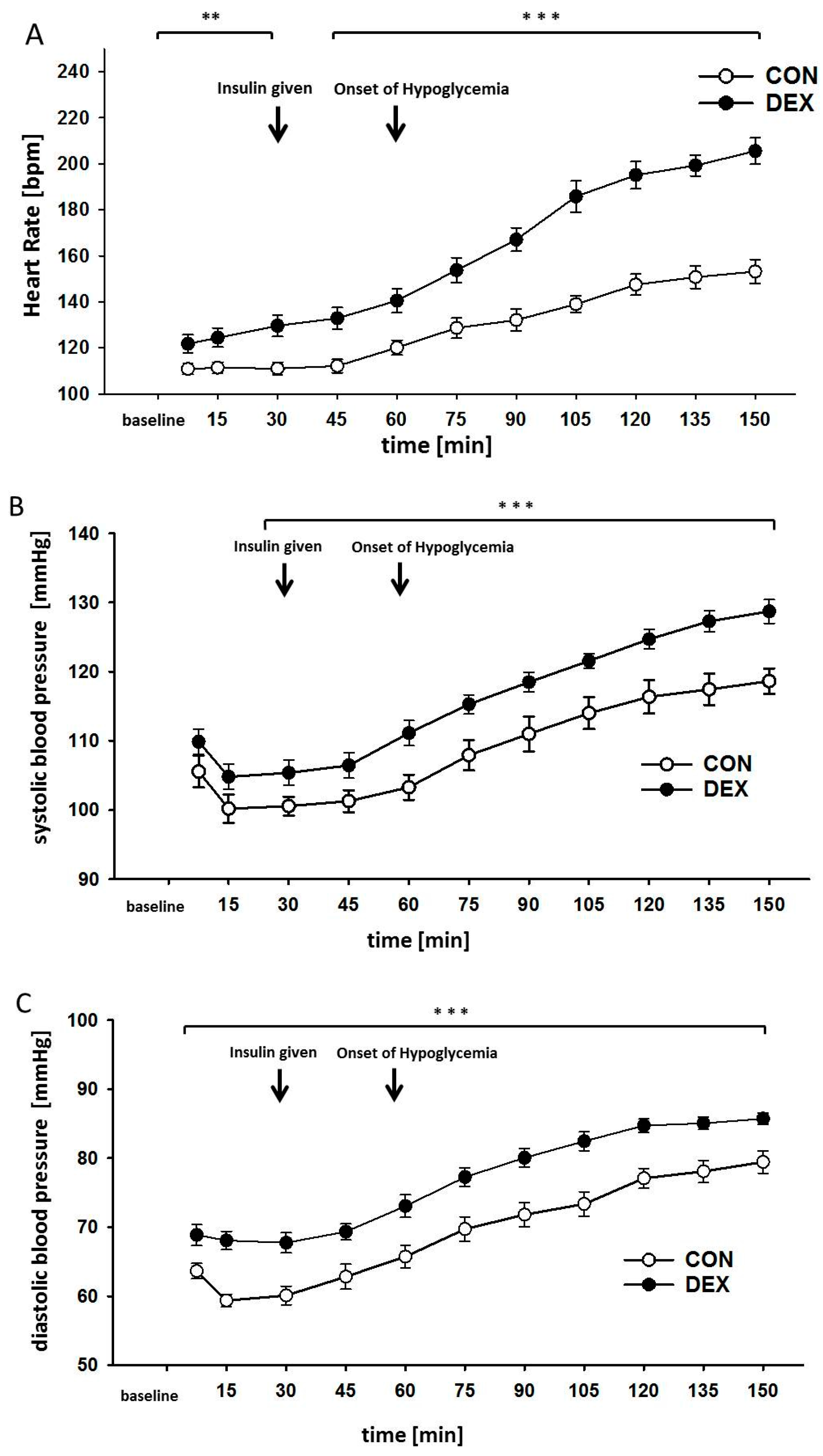
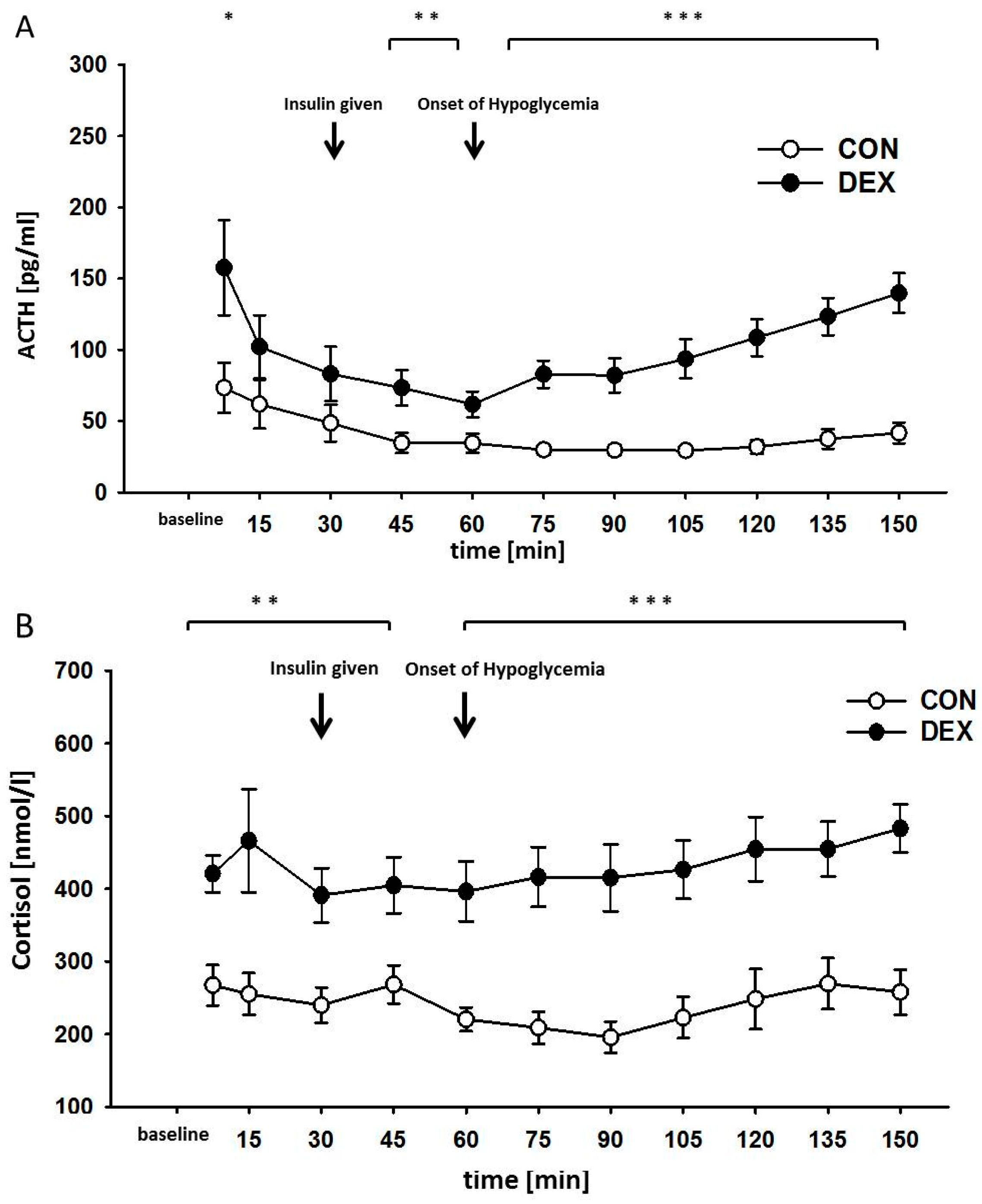
2. Results
3. Discussion
4. Methods
4.1. Breeding and Experimental Groups
4.2. Surgical Procedures
4.3. Hypoglycemic Clamp
4.4. Measurement of ACTH
4.5. Measurement of Cortisol
4.6. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACTH | Adenocorticotropic hormone |
| CON | Control group |
| DEX | Dexamethasone-treated group |
| EDTA | Ethylenediamine tetraacetic acid |
| GCs | Synthetic glucocorticoids |
| HPAA | Hypothalamic-pituitary-adrenocortical axis |
References
- Meyer, C.; Dostou, J.M.; Gerich, J.E. Role of the human kidney in glucose counterregulation. Diabetes 1999, 48, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Noll, G.; Wenzel, R.R.; Schneider, M.; Oesch, V.; Binggeli, C.; Shaw, S.; Weidmann, P.; Luscher, T.F. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive parents. Circulation 1996, 93, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.W.; Straznicky, N.E.; Lambert, E.A.; Dixon, J.B.; Schlaich, M.P. Sympathetic nervous activation in obesity and the metabolic syndrome-Causes, consequences and therapeutic implications. Pharmacol. Therapeut. 2010, 126, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Ueno, Y. Autonomic Nervous System and the Liver. Hepatol. Res. 2017, 47, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Collazo, P.; Ferno, J.; Gonzalez, F.; Dieguez, C.; Leis, R.; Nogueiras, R.; Lopez, M. Hypothalamic-autonomic control of energy homeostasis. Endocrine 2015, 50, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Sloboda, D.M.; Moss, T.J.; Li, S.; Doherty, D.; Nitsos, I.; Challis, J.R.; Newnham, J.P. Prenatal betamethasone exposure results in pituitary-adrenal hyporesponsiveness in adult sheep. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E61–E70. [Google Scholar] [CrossRef] [PubMed]
- Jellyman, J.K.; Allen, V.L.; Forhead, A.J.; Holdstock, N.B.; Fowden, A.L. Hypothalamic-pituitary-adrenal axis function in pony foals after neonatal ACTH-induced glucocorticoid overexposure. Equine Vet. J. Suppl. 2012, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Jellyman, J.K.; Gardner, D.S.; McGarrigle, H.H.; Fowden, A.L.; Giussani, D.A. Pituitary-adrenal responses to acute hypoxemia during and after maternal dexamethasone treatment in sheep. Pediatr. Res. 2004, 56, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Kvetnansky, R.; Pacak, K.; Fukuhara, K.; Viskupic, E.; Hiremagalur, B.; Nankova, B.; Goldstein, D.S.; Sabban, E.L.; Kopin, I.J. Sympathoadrenal system in stress—Interaction with the hypothalamic-pituitary-adrenocortical system. Stress 1995, 771, 131–158. [Google Scholar]
- Cersosimo, E.; Garlick, P.; Ferretti, J. Renal glucose production during insulin-induced hypoglycemia in humans. Diabetes 1999, 48, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, E.; Molina, P.E.; Abumrad, N.N. Renal glucose production during insulin-induced hypoglycemia. Diabetes 1997, 46, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Triplitt, C.L. Understanding the kidneys’ role in blood glucose regulation. Am. J. Manag. Care 2012, 18, S11–S16. [Google Scholar] [PubMed]
- Bischoff, S.J.; Schmidt, M.; Lehmann, T.; Schwab, M.; Matziolis, G.; Saemann, A.; Schiffner, R. Renal glucose release during hypoglycemia is partly controlled by sympathetic nerves—A study in pigs with unilateral surgically denervated kidneys. Physiol. Rep. 2015, 3, e12603. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Seckl, J. Glucocorticoids, prenatal stress and the programming of disease. Horm. Behav. 2011, 59, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Dunn, E.; Kostaki, A.; Andrews, M.H.; Matthews, S.G. Fetal programming of hypothalamo-pituitary-adrenal function: Prenatal stress and glucocorticoids. J. Physiol. Lond. 2006, 572, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Petropoulos, S.; Matthews, S.G. Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res. Rev. 2008, 57, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Newnham, J.P.; Jobe, A.H. Should we be prescribing repeated courses of antenatal corticosteroids? Semin. Fetal Neonatal Med. 2009, 14, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, E.; Brunelli, R.; Anceschi, M.M. Review and meta-analysis: Benefits and risks of multiple courses of antenatal corticosteroids. J. Matern.-Fetal Neonatal Med. 2010, 23, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, O.M.; Kari, M.A.; Hallman, M. Repeated antenatal corticosteroid treatment: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2011, 90, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Wapner, R.J.; Sorokin, Y.; Mele, L.; Johnson, F.; Dudley, D.J.; Spong, C.Y.; Peaceman, A.M.; Leveno, K.J.; Malone, F.; Caritis, S.N.; et al. Long-term outcomes after repeat doses of antenatal corticosteroids. N. Engl. J. Med. 2007, 357, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Crowther, C.A.; Harding, J.E.; Middleton, P.F.; Andersen, C.C.; Ashwood, P.; Robinson, J.S.; Grp, A.S.S. Australasian randomised trial to evaluate the role of maternal intramuscular dexamethasone versus betamethasone prior to preterm birth to increase survival free of childhood neurosensory disability (A*STEROID): Study protocol. BMC Pregnancy Childbirth 2013, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Waffarn, F.; Davis, E.P. Effects of antenatal corticosteroids on the hypothalamic-pituitary-adrenocortical axis of the fetus and newborn: Experimental findings and clinical considerations. Am. J. Obstet. Gynecol. 2012, 207, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P.; Kino, T. Glucocorticoid Signaling in the Cell Expanding Clinical Implications to Complex Human Behavioral and Somatic Disorders. Ann. N. Y. Acad. Sci. 2009, 1179, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Chrousos, G.P.; Gold, P.W. The Concepts of Stress and Stress System Disorders—Overview of Physical and Behavioral Homeostasis. J. Am. Med. Assoc. 1992, 267, 1244–1252. [Google Scholar] [CrossRef]
- Adams, H.A.; Russ, W.; Kling, D.; Moosdorf, R.; Hempelmann, G. Plasma-Catecholamines, Circulatory Parameters, and Endocrine Stress Response during Carotid Endarterectomy. Anaesthesist 1988, 37, 224–230. [Google Scholar] [PubMed]
- Adams, H.A.; Russ, W.; Leisin, M.; Borner, U.; Gips, H.; Hempelmann, G. Inhalation Anesthetic Agents and Endocrine Stress Response during Surgery—A Comparison of Halothane, Enflurane, and Isoflurane. Anaesthesist 1987, 36, 159–165. [Google Scholar] [PubMed]
- Schricker, T.; Lattermann, R. Perioperative catabolism. Can. J. Anesth. 2015, 62, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Katagiri, H. Avenues of communication between the brain and tissues/organs involved in energy homeostasis. Endocr. J. 2007, 54, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Minson, C.T. Obesity and adipokines: Effects on sympathetic overactivity. J. Physiol. Lond. 2012, 590, 1787–1801. [Google Scholar] [CrossRef] [PubMed]
- Fibiger, W.; Singer, G.; Miller, A.J.; Armstrong, S.; Datar, M. Cortisol and Catecholamines Changes as Functions of Time-of-Day and Self-Reported Mood. Neurosci. Biobehav. Rew. 1984, 8, 523–530. [Google Scholar] [CrossRef]
- Klemcke, H.G.; Nienaber, J.A.; Hahn, G.L. Plasma Adrenocorticotropic Hormone and Cortisol in Pigs—Effects of Time of Day on Basal and Stressor-Altered Concentrations. Proc. Soc. Exp. Biol. Med. 1989, 190, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.C.; Christensen, R.; Clewell, W.H.; Dalton, M.E.; Davidson, E.C.; Escobedo, M.B.; Gjerdingen, D.K.; Goddardfinegold, J.; Goldenberg, R.K.; Grimes, D.A.; et al. Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. J. Am. Med. Assoc. 1995, 273, 413–418. [Google Scholar] [CrossRef]
- Rotenberg, S.; McGrath, J.J. Inter-relation between autonomic and HPA axis activity in children and adolescents. Biol. Psychol. 2016, 117, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Casati, D.; Frasch, M.G. Analysis of fetal heart rate variability in frequency domain: Methodical considerations. Exp. Physiol. 2014, 99, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gold, N.; Frasch, M.G.; Huang, H.; Thiriet, M.; Wang, X. Mathematical Model of Cardiovascular and Metabolic Responses to Umbilical Cord Occlusions in Fetal Sheep. Bull. Math. Biol. 2015, 77, 2264–2293. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Meunier-Salaun, M.C.; Brulaud, F.; Monnier, M.; Mormede, P. Assessment of hypothalamic-pituitary-adrenal axis and sympathetic nervous system activity in pregnant sows through the measurement of glucocorticoids and catecholamines in urine. J. Anim. Sci. 2000, 78, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals; Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2011; Volume 8. [Google Scholar]
- Schleussner, E. The prevention, diagnosis and treatment of premature labor. Deutsch. Arztebl. Int. 2013, 110, 227–235. [Google Scholar]
- Shepherd, E.; Salam, R.A.; Middleton, P.; Makrides, M.; McIntyre, S.; Badawi, N.; Crowther, C.A. Antenatal and intrapartum interventions for preventing cerebral palsy: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2017, 8, CD012077. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.F.; Kemp, M.W.; Kannan, P.S.; Kramer, B.W.; Newnham, J.P.; Kallapur, S.G.; Jobe, A.H. Antenatal dexamethasone vs. betamethasone dosing for lung maturation in fetal sheep. Pediatr. Res. 2017, 81, 496–503. [Google Scholar] [CrossRef] [PubMed]
| Timepoint Heading | Control Group (CON) (kg) | Dexamethasone-Treated Group (DEX) (kg) |
|---|---|---|
| Birth | 1.6 ± 0.02 | 1.3 ± 0.01 * |
| 28 days of age | 8.0 ± 0.1 | 6.8 ± 0.3 * |
| 67 days of age | 28.6 ± 0.2 | 24.1 ± 0.3 * |
| Variables | Controls (CON) | Dexamethasone-Treated (DEX) |
|---|---|---|
| Total number of pregnant sows (n) | 6 | 6 |
| Weight (kg) | 310.7 ± 48.5 | 316 ± 38.9 |
| Age (years) | 3.1 ± 0.4 | 3.1 ± 0.4 |
| Litter size per sow (n) | 13.6 ± 0.7 | 13.2 ± 0.8 |
| Total offspring number (n) | 80 | 78 |
| gender (nmale/nfemale) | 40/40 | 36/42 |
| Total number of offspring used (n) | 12 | 18 |
| offspring weight (kg) | 28.5 ± 4.1 | 24.2 ± 2.7 |
| Age (days) | 74.2 ± 2.7 | 73.5 ± 2.3 |
| Piglet gender (nmale/nfemale) | 6/6 | 9/9 |
| Body temperature at baseline (°C) | 37.6 ± 0.5 | 37.5 ± 0.4 |
| Body temperature during hypoglycemia (°C) | 35.3 ± 0.6 | 35.8 ± 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiffner, R.; Rodríguez-González, G.L.; Rakers, F.; Nistor, M.; Nathanielsz, P.W.; Daneva, T.; Schwab, M.; Lehmann, T.; Schmidt, M. Effects of Late Gestational Fetal Exposure to Dexamethasone Administration on the Postnatal Hypothalamus-Pituitary-Adrenal Axis Response to Hypoglycemia in Pigs. Int. J. Mol. Sci. 2017, 18, 2241. https://doi.org/10.3390/ijms18112241
Schiffner R, Rodríguez-González GL, Rakers F, Nistor M, Nathanielsz PW, Daneva T, Schwab M, Lehmann T, Schmidt M. Effects of Late Gestational Fetal Exposure to Dexamethasone Administration on the Postnatal Hypothalamus-Pituitary-Adrenal Axis Response to Hypoglycemia in Pigs. International Journal of Molecular Sciences. 2017; 18(11):2241. https://doi.org/10.3390/ijms18112241
Chicago/Turabian StyleSchiffner, René, Guadalupe L. Rodríguez-González, Florian Rakers, Marius Nistor, Peter W. Nathanielsz, Teodora Daneva, Matthias Schwab, Thomas Lehmann, and Martin Schmidt. 2017. "Effects of Late Gestational Fetal Exposure to Dexamethasone Administration on the Postnatal Hypothalamus-Pituitary-Adrenal Axis Response to Hypoglycemia in Pigs" International Journal of Molecular Sciences 18, no. 11: 2241. https://doi.org/10.3390/ijms18112241





