Deep Eutectic Solvents (DESs) for the Isolation of Willow Lignin (Salix matsudana cv. Zhuliu)
Abstract
:1. Introduction
2. Results and Discussion
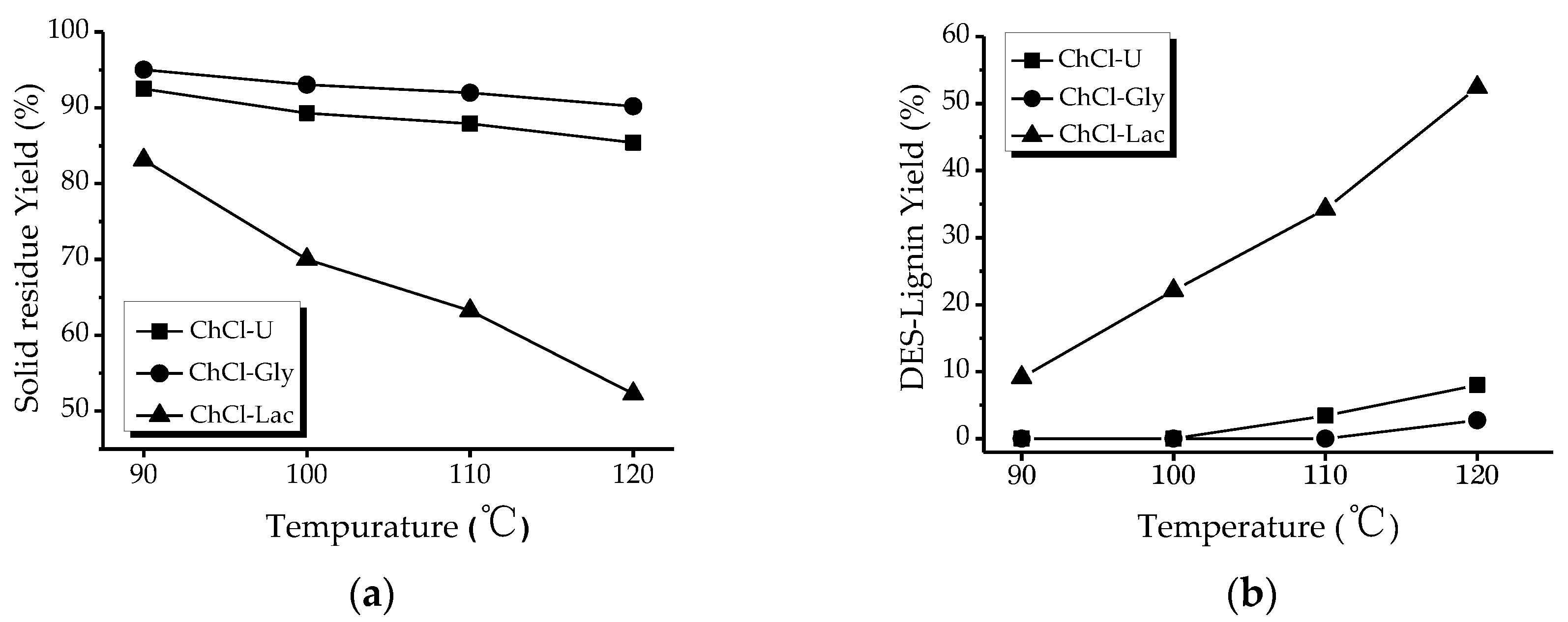
2.1. Effect of Different Deep Eutectic Solvents (DESs) and Temperture on the Extraction of Lignin from Willow
2.2. Effect of Different Mole Ratio of Choline Chloride (ChCl) to Lactic Acid (Lac) on the Extraction of Lignin from Willow
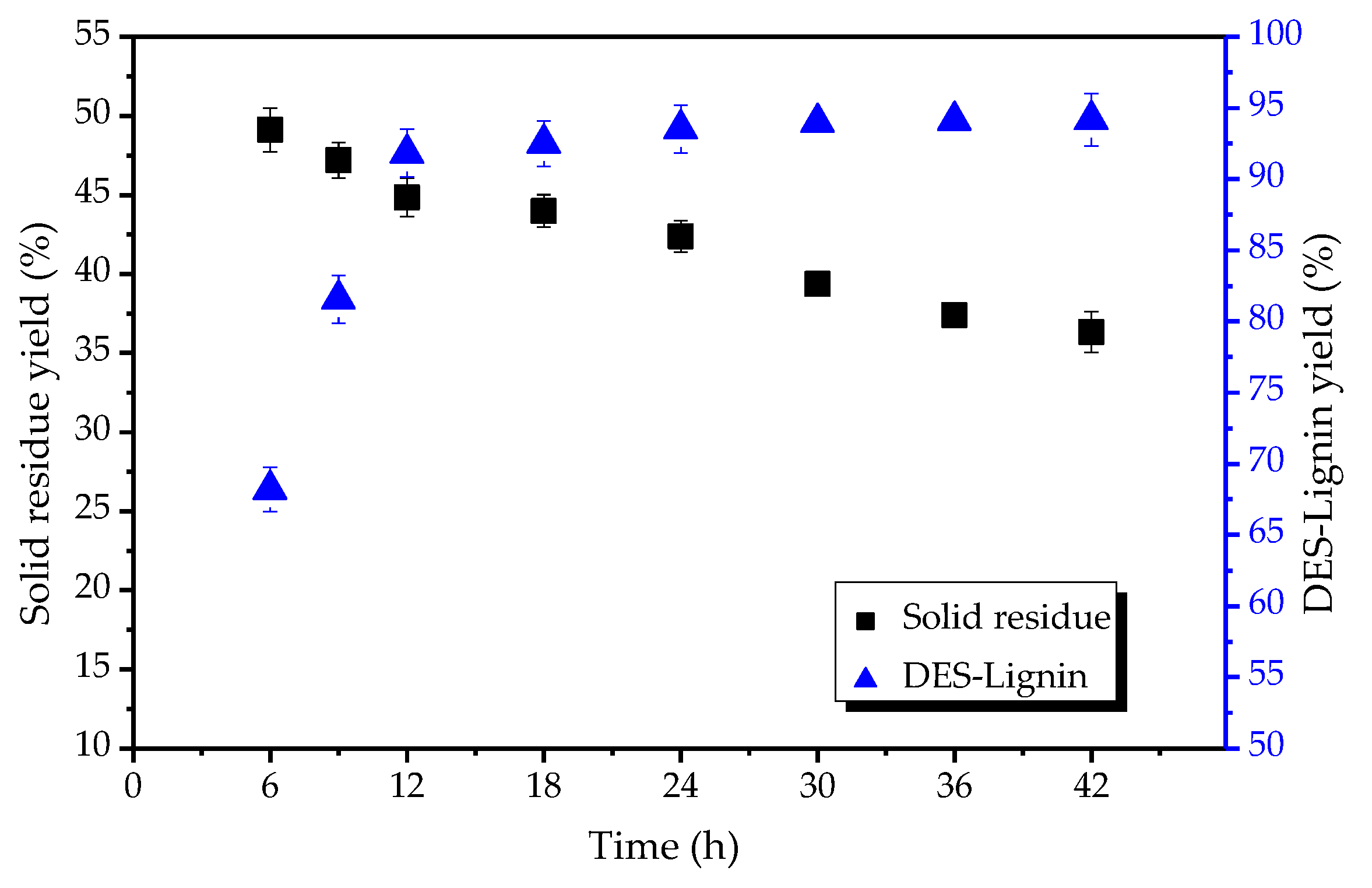
2.3. Effect of the DESs Treatment Time on Extraction of Lignin from Willow
2.4. Chemical Composition Analysis of Solid Residue and Dissolved Carbohydrates in the Mixtures
2.5. Lignin Purity Analysis
2.6. Structure Characterization of DES-Lignin
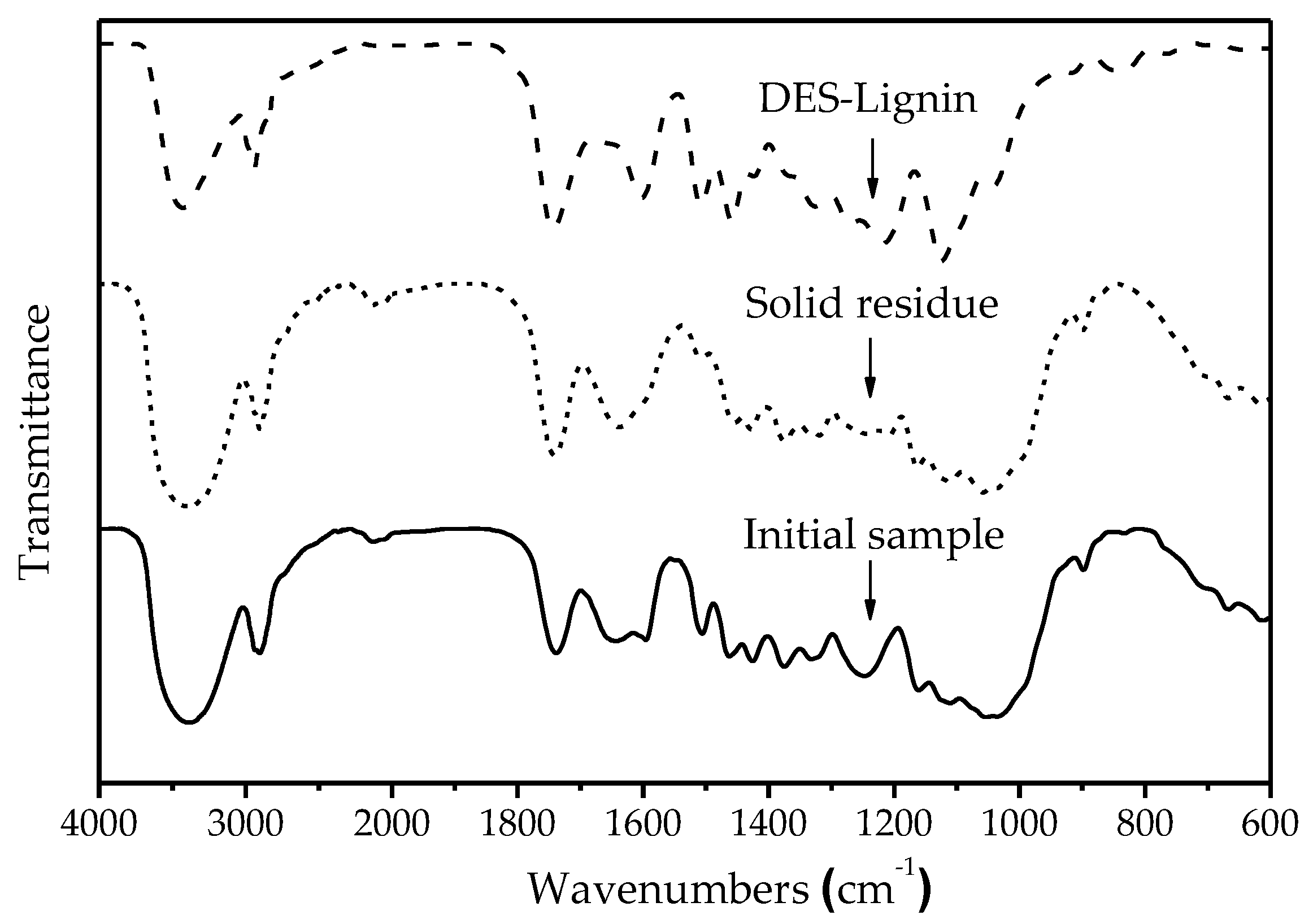
2.6.1. Fourier Transform Infrared Spectroscopy (FT-IR)
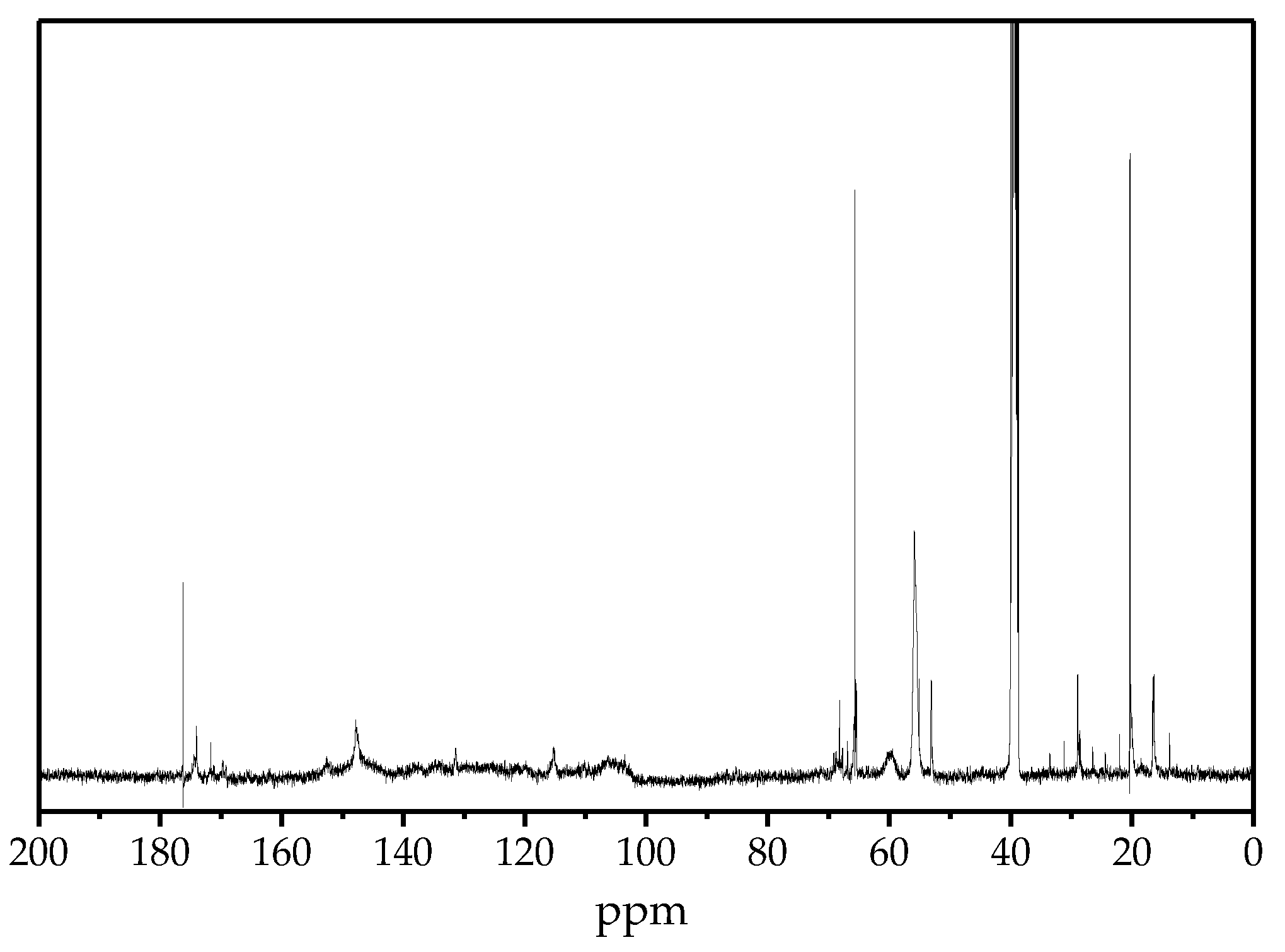
2.6.2. 13C-NMR
2.6.3. 31P-NMR
3. Materials and Methods
3.1. Materials
3.2. DESs Preparation
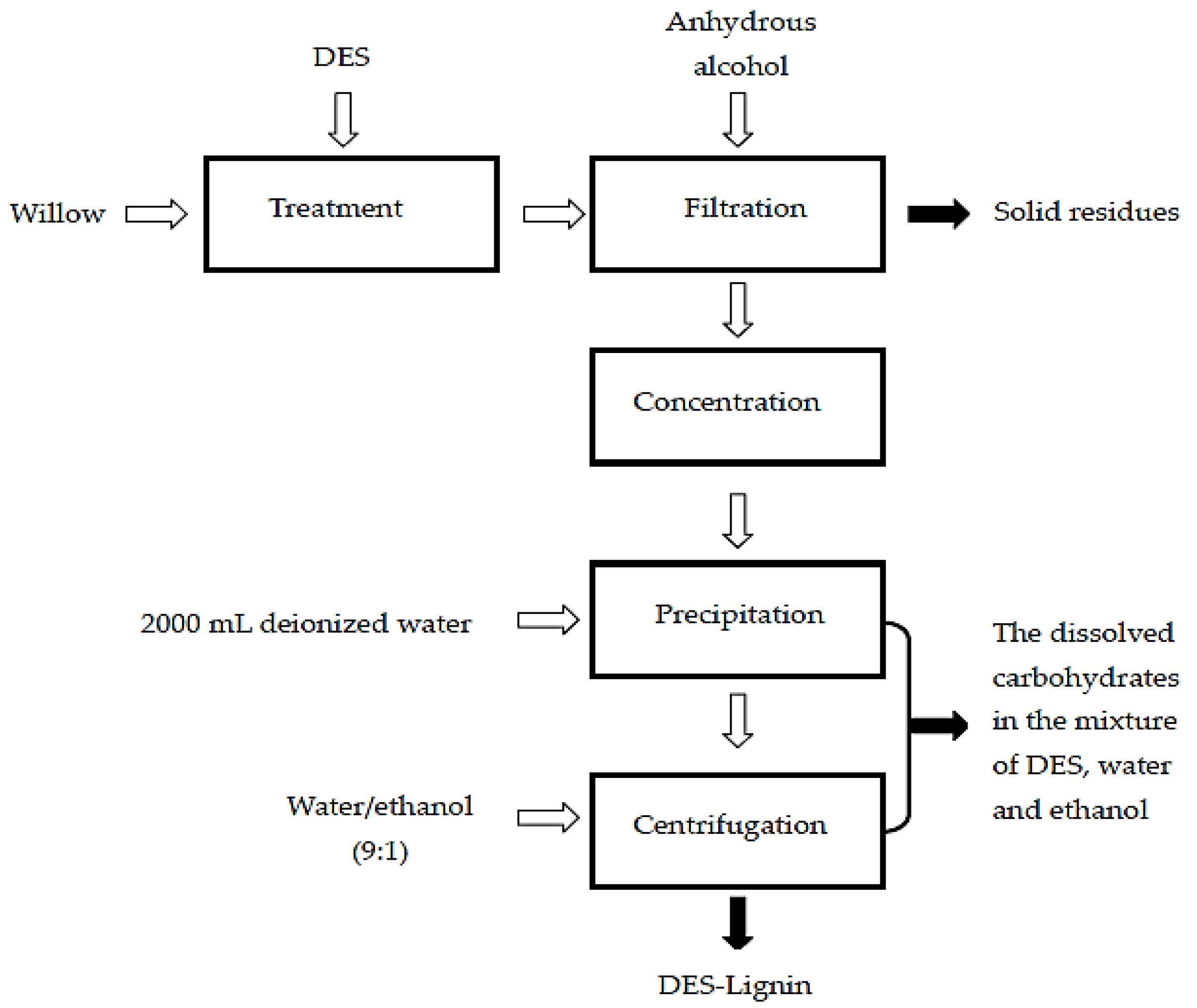
3.3. DESs Treatment of Biomass (Willow)
3.4. Chemical Composition of Solid Residue, DES-Lignin, and the Dissolved Carbohydrates in the Mixtures
3.5. Structure Characterization of the DES-Lignin
3.5.1. FT-IR Spectroscopy
3.5.2. 13C-NMR
3.5.3. 31P-NMR
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, J.S.; Saddler, J.; Binod, P. Pretreatment of biomass. Bioresour. Technol. 2016, 199, 1. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Zheng, J.; Rehmann, L. Extrusion pretreatment of lignocellulosic biomass: A review. Int. J. Mol. Sci. 2014, 15, 18967–18984. [Google Scholar] [CrossRef] [PubMed]
- Guo, T. The Analysis of Willow’s Chemical Components and Pulping Properties. Master’s Thesis, Qilu University of Technology, Jinan, China, 10 June 2016. [Google Scholar]
- Guo, T.; Liu, Y.; Liu, Y.; Yang, G.-H.; Chen, J.-C.; Lucia, L.A. Chemical elucidation of structurally diverse willow lignins. Bioresources 2016, 11. [Google Scholar] [CrossRef]
- Zhang, Q.-G.; Hu, J.J.; Lee, D.-J. Pretreatment of biomass using ionic liquids: Research updates. Renew. Energy 2017, 111, 77–84. [Google Scholar] [CrossRef]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-N.; Li, C.-P.; Yin, J.-M.; Li, S.-M.; Jia, Y.-P.; Bao, M. Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride. J. Mol. Liq. 2017, 236, 338–343. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Natesan, J.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kumaraguru, S.; Pavulraj, R.; Vijayakumar, J.; Mohan, S. Electrodeposition of cobalt/silver multilayers from deep eutectic solvent and their giant magnetoresistance. J. Alloys Compd. 2017, 693, 1143–1149. [Google Scholar] [CrossRef]
- Bryant, S.J.; Atkin, R.; Warr, G.G. Effect of deep eutectic solvent nanostructure on phospholipid bilayer phases. Langmuir 2017, 33, 6878–6884. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Gunny, A.A.; Arbain, D.; Mohamed Daud, M.Z.; Jamal, P. Synergistic action of deep eutectic solvents and cellulases for lignocellulosic biomass hydrolysis. Mater. Res. Innov. 2014, 18, 65–67. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.U.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Feofilova, E.; Mysyakina, I. Lignin: Chemical structure, biodegradation, and practical application (a review). Appl. Biochem. Microbiol. 2016, 52, 573–581. [Google Scholar] [CrossRef]
- Pang, K.; Hou, Y.-C.; Wu, W.-Z.; Guo, W.-J.; Wei, P.-G.; Marsh, K.N. Efficient separation of phenols from oils via forming deep eutectic solvents. Green Chem. 2012, 14, 2398–2401. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic parameters for solvents of interest in green chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Yu, H.; Hu, J.; Chang, J. Selective separation of wood components based on hansen’s theory of solubility. Ind. Eng. Chem. Res. 2011, 50, 7513–7519. [Google Scholar] [CrossRef]
- Wei, L.; Fan, Y.-J. Progress of deep eutectic solvents and their applications. Chemistry 2011, 74, 333–339. [Google Scholar] [CrossRef]
- Li, J.-B.; Dong, H.-L.; Xiu, H.-J.; Zhang, M.-Y.; Reddy, K.S.; Zhang, X.-F.; Ji, Y. Extraction, separation and refining of microcrystalline cellulose from wheat straw using various pretreatments. Int. J. Agric. Biol. Eng. 2016, 2, 137–145. [Google Scholar] [CrossRef]
- Saeed, A.; Jahan, M.S.; Li, H.; Liu, Z.; Ni, Y.; Heiningen, A.V. Mass balances of components dissolved in the pre-hydrolysis liquor of kraft-based dissolving pulp production process from canadian hardwoods. Biomass Bioenergy 2012, 39, 14–19. [Google Scholar] [CrossRef]
- Li, L.-F.; Hu, Y.-C. Application of ionic liquids in lignin processing. Chem. Ind. For. Prod. 2015, 35, 163–170. [Google Scholar] [CrossRef]
- Sun, Y.-C.; Xu, J.-K.; Xu, F.; Sun, R.-C. Efficient separation and physico-chemical characterization of lignin from eucalyptus using ionic liquid–organic solvent and alkaline ethanol solvent. Ind. Crops Prod. 2013, 47, 277–285. [Google Scholar] [CrossRef]
- Constant, S.; Basset, C.; Dumas, C.; di Renzo, F.; Robitzer, M.; Barakat, A.; Quignard, F. Reactive organosolv lignin extraction from wheat straw: Influence of lewis acid catalysts on structural and chemical properties of lignins. Ind. Crops Prod. 2015, 65, 180–189. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FT-IR and PY-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Li, L.-Q.; Zheng, M.-Y.; Wang, X.; Ma, H.-L.; Wang, K.-J. Effect of alkali pretreatment on cellulosic structural changes of corn stover. Environ. Sci. Technol. 2012, 35, 27–31. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; Dam, J.E.G.V. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Juan, D.-R.; Rafael, S.; Eduardo, E.; Davide, S.; Pierluigi, M.; Alessandro, P.; Alejandro, R. Isolation and characterization of gramineae and fabaceae soda lignins. Int. J Mol. Sci. 2017, 18, 327. [Google Scholar] [CrossRef]
- Jahan, M.S.; Mun, S.P. Characteristics of dioxane lignins isolated at different ages of nalita wood (trema orientalis). J. Wood Chem. Technol. 2007, 27, 83–98. [Google Scholar] [CrossRef]
- Yang, G.; Jahan, M. Structural characterization of pre-hydrolysis liquor lignin and its comparison with other technical lignins. Curr. Org. Chem. 2013, 17, 1589–1595. [Google Scholar] [CrossRef]
- Wikberg, H.; Maunu, S. Characterisation of thermally modified hard- and softwoods by 13C CPMAS NMR. Carbohydr. Polym. 2004, 58, 461–466. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Yue, Y.-Y.; Fu, Y.; Chang, J. Solubility selectivity for lignin of eucalyptus in deep eutectic solvents. Fine Chem. 2016, 33, 1287–1294. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Sun, R.-C. Structural elucidation of whole lignin from eucalyptus based on preswelling and enzymatic hydrolysis. Green Chem. 2015, 17, 1589–1596. [Google Scholar] [CrossRef]
- Jiang, W.-K.; Lyu, G.-J.; Wu, S.-B.; Lucia, L.-A.; Yang, G.-H.; Liu, Y. Supercritical water-induced lignin decomposition reactions: A structural and quantitative study. BioResources 2016, 11, 5660–5675. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report for National Renewable Energy Laboratory: Golden, CO, USA, 2010. [Google Scholar]
- Shen, J.; Fatehi, P.; Soleimani, P.; Ni, Y. Recovery of lignocelluloses from pre-hydrolysis liquor in the lime kiln of kraft-based dissolving pulp production process by adsorption to lime mud. Bioresour. Technol. 2011, 102, 10035–10039. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.-P.; Wu, C.-J.; Zhao, C.-S.; Yu, D.-M. Effect of boric acid addition on the prehydrolysis of whangee. BioResources 2016, 11, 9628–9637. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Wang, X.-J.; Fu, Y.-J.; Yuan, Z.-W.; Qin, M.-H. Saccharide separation from wood prehydrolysis liquor: Comparison ofselectivity toward non-saccharide compounds with separate techniques. RSC Adv. 2015, 5, 28925–28931. [Google Scholar] [CrossRef]


| Component | Arabinose (%) | Galactose (%) | Glucose (%) | Xylose (%) | Mannose (%) | Lignin (%) |
|---|---|---|---|---|---|---|
| Solid residue | - | - | 82.07 | 10.77 | - | 6.20 |
| Dissolved in the mixtures | 70.69 | 72.00 | 14.13 | 73.52 | 81.3 | 91.8 * |
| Component | Amount (%) |
|---|---|
| Acid insoluble lignin (AIL) | 92.57 ± 0.7 |
| Acid soluble lignin (ASL) | 1.89 ± 0.1 |
| Glucose | 0.21 ± 0.05 |
| Xylose | 0.15 ± 0.03 |
| Arabinose | - |
| Galactose | - |
| Mannose | - |
| Ash | 0.51 ± 0.1 |
| Lignin purity * | 94.46 ± 0.8 |
| Wavenumbers (cm−1) | Assignment (Bond) | DES-Lignin | Solid Residue | Initial Sample |
|---|---|---|---|---|
| 3410 | O–H stretching vibration | 3410 | 3410 | 3410 |
| 2924 | C–H stretching vibration in methyl, methylene | 2924 | 2924 | 2924 |
| 1710 | C=O stretching vibration | 1710 | 1710 | 1710 |
| 1600, 1510 | Aromatic ring skeleton vibration | 1600, 1510 | - | 1600, 1510 |
| 1460 | C–H deformation vibration in –CH2– | 1460 | 1460 | 1460 |
| 1426 | C–H bending vibration in –CH2– of cellulose | - | 1426 | 1426 |
| 1373 | C–H bending vibration of aliphatic compounds | - | 1373 | 1373 |
| 1325, 1220 | C–O stretching vibration of syringyl units | 1325, 1220 | 1325 | 1325, 1220 |
| 1270 | C–O stretching vibration of guaiacyl units | 1270 | - | 1270 |
| 1164 | C–O–C symmetrical stretching vibration in carbohydrate | - | 1164 | 1164 |
| 1120/835 | C–H stretching vibration of syringyl units | 1120/835 | - | 1120/835 |
| 1056 | C=O stretching vibration in carbohydrate | - | 1056 | 1056 |
| 1035 | C–H bending vibration of guaiacyl units | 1035 | 1035 | 1035 |
| Chemical Shift (ppm) | Assignment |
|---|---|
| 171–174 | Phenolic OH |
| 169–171 | Aliphatic OH |
| 150.3–153.0 | CAr in etherified syringyl units |
| 146.2–149.0 | CAr in non-etherified guaiacyl units |
| 131.0–132.1 | CAr in non-etherified syringyl units |
| 114.2–116.0 | CAr in p-coumaric acid ester |
| 103–108 | CAr in syringyl units |
| 67–71 | Cα in β-O-4 |
| 57–61 | Cγ in β-O-4 |
| 54–57 | CH3O |
| 52–54 | Cβ in β-β or β-5 |
| Chemical Shift (ppm) | Assignment | Content (mmol·g−1) |
|---|---|---|
| 133.8–135.0 | Carboxyl | 0.182 |
| 137.0–138.4 | p-phenol hydroxyl (H) | 0.110 |
| 138.4–140.2 | Guaiacyl phenol hydroxyl (G) | 0.285 |
| 142.4–143.7 | Syringyl phenol hydroxyl (S) | 0.520 |
| - | G:H:S | 2.59:1:4.73 |
| 140.2–142.4, 143.7–144.4 | Condensation phenol hydroxyl | 0.539 |
| 145.2–150.1 | Aliphatic hydroxyl | 0.606 |
| - | Total phenol hydroxyl | 0.915 |
| - | Total hydroxyl | 2.06 |
| Composition | Willow (wt %) |
|---|---|
| Arabinose | 0.58 |
| Galactose | 0.75 |
| Glucose | 49.13 |
| Xylose | 17.74 |
| Mannose | 1.24 |
| Total lignin | 21.63 |
| Acid insoluble lignin | 18.63 |
| Acid soluble lignin | 3.00 |
| Benzene-alcohol extractive | 3.10 |
| Ash | 0.91 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Lyu, G.; Liu, Y.; Lou, R.; Lucia, L.A.; Yang, G.; Chen, J.; Saeed, H.A.M. Deep Eutectic Solvents (DESs) for the Isolation of Willow Lignin (Salix matsudana cv. Zhuliu). Int. J. Mol. Sci. 2017, 18, 2266. https://doi.org/10.3390/ijms18112266
Li T, Lyu G, Liu Y, Lou R, Lucia LA, Yang G, Chen J, Saeed HAM. Deep Eutectic Solvents (DESs) for the Isolation of Willow Lignin (Salix matsudana cv. Zhuliu). International Journal of Molecular Sciences. 2017; 18(11):2266. https://doi.org/10.3390/ijms18112266
Chicago/Turabian StyleLi, Tengfei, Gaojin Lyu, Yu Liu, Rui Lou, Lucian A. Lucia, Guihua Yang, Jiachuan Chen, and Haroon A. M. Saeed. 2017. "Deep Eutectic Solvents (DESs) for the Isolation of Willow Lignin (Salix matsudana cv. Zhuliu)" International Journal of Molecular Sciences 18, no. 11: 2266. https://doi.org/10.3390/ijms18112266








