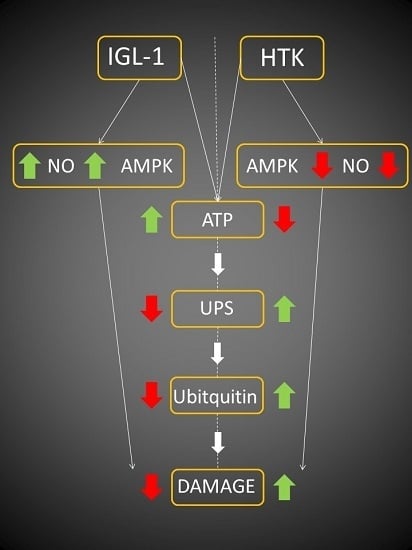
The Relevance of the UPS in Fatty Liver Graft Preservation: A New Approach for IGL-1 and HTK Solutions
Abstract
:1. Introduction
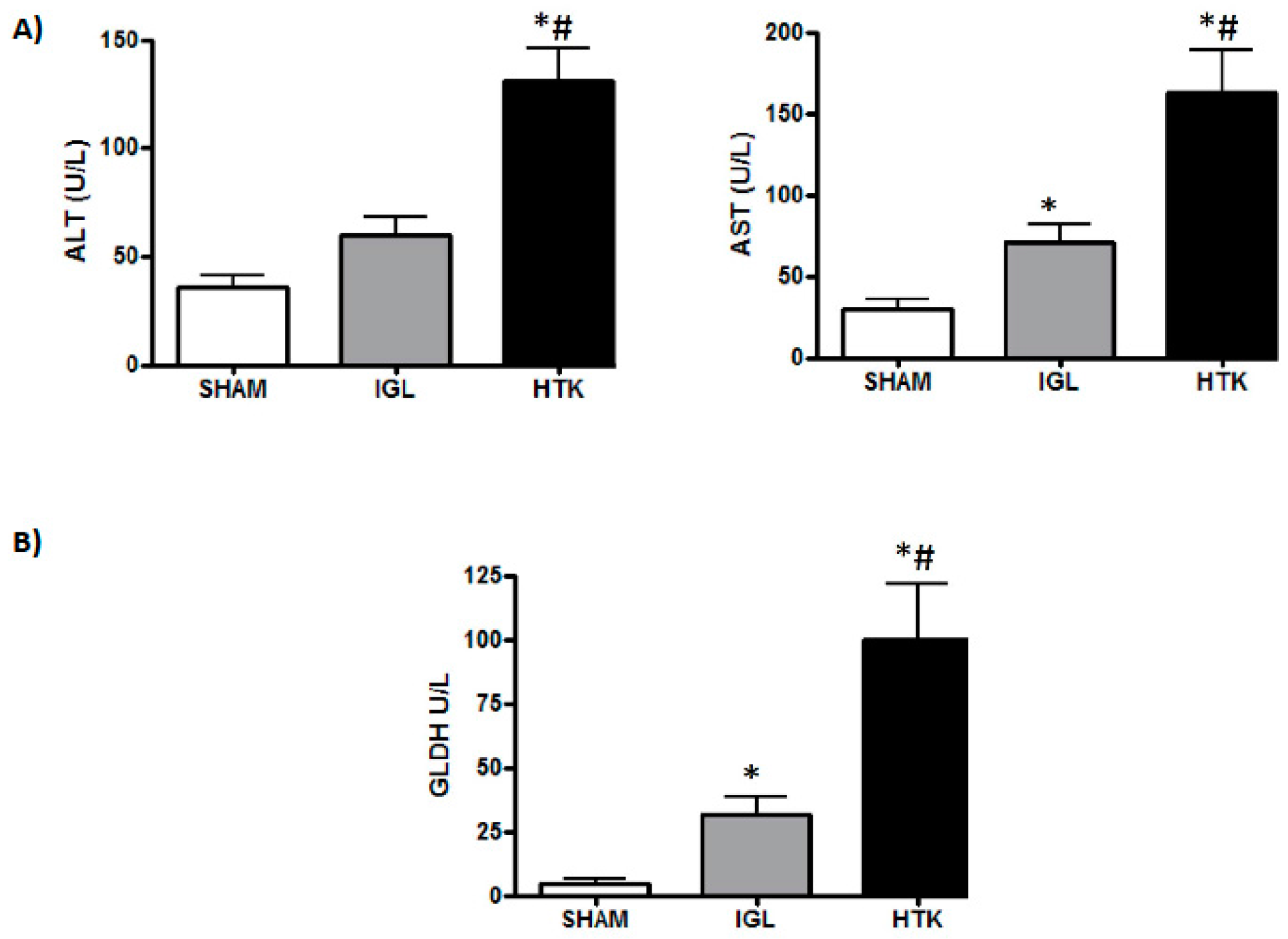
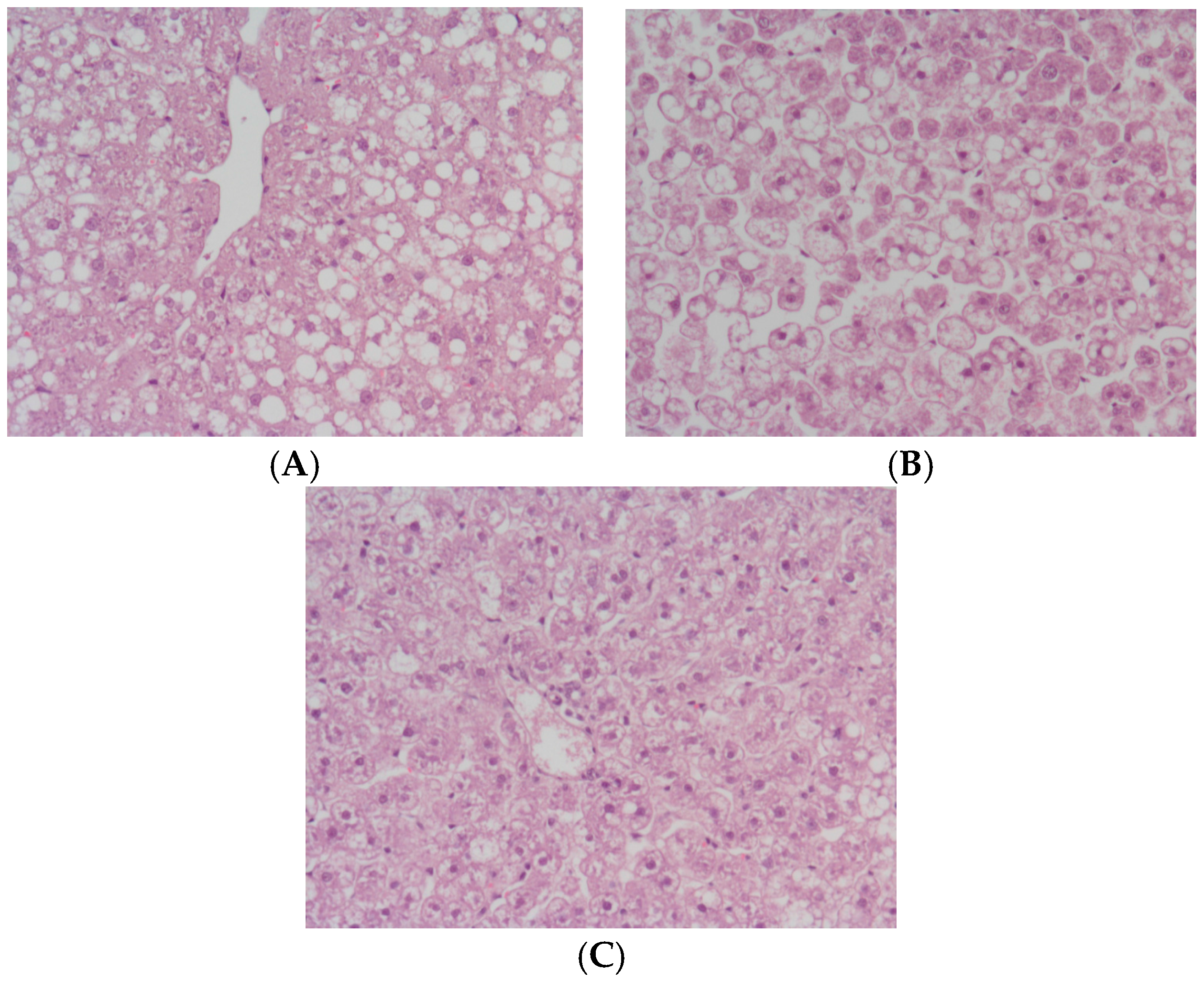
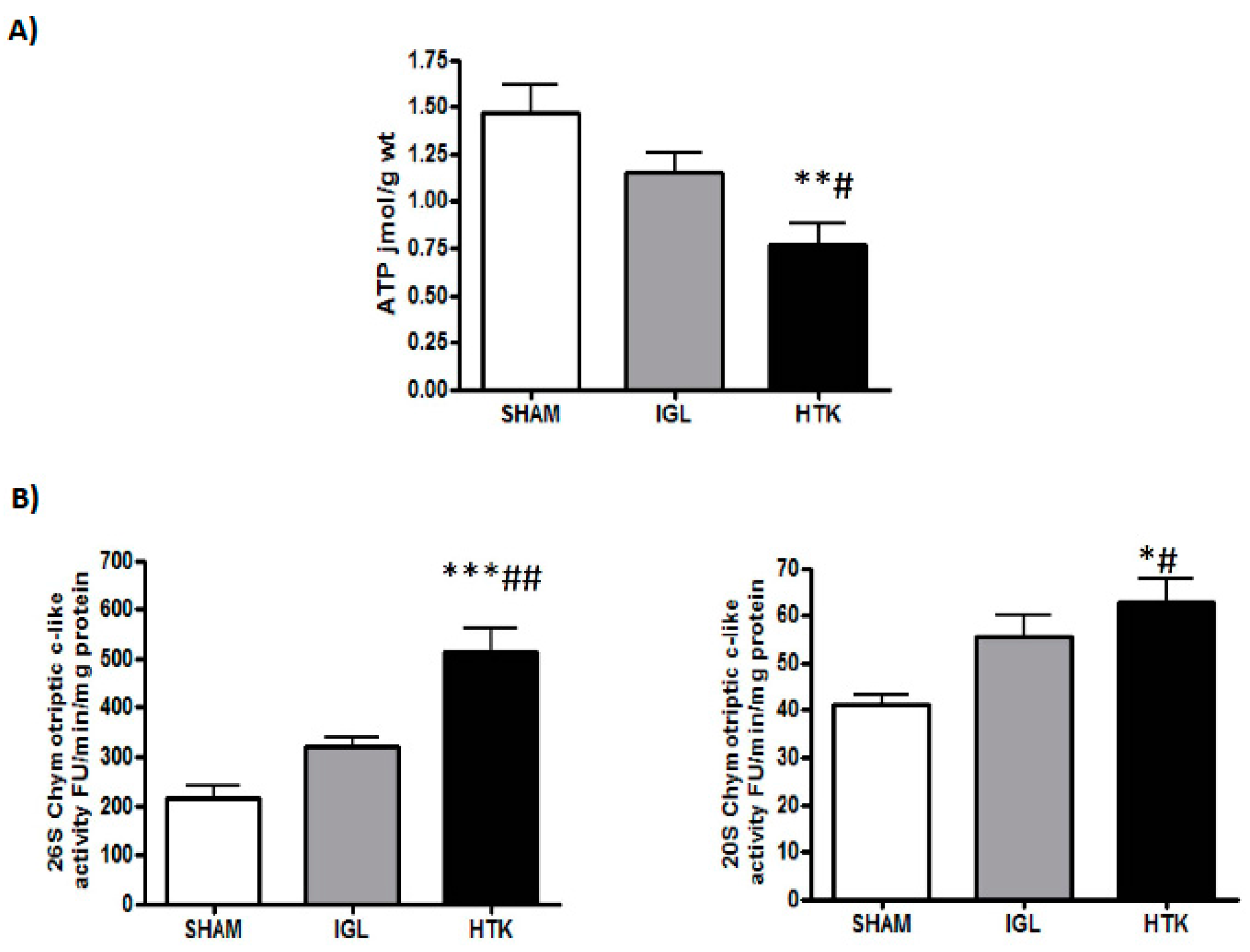
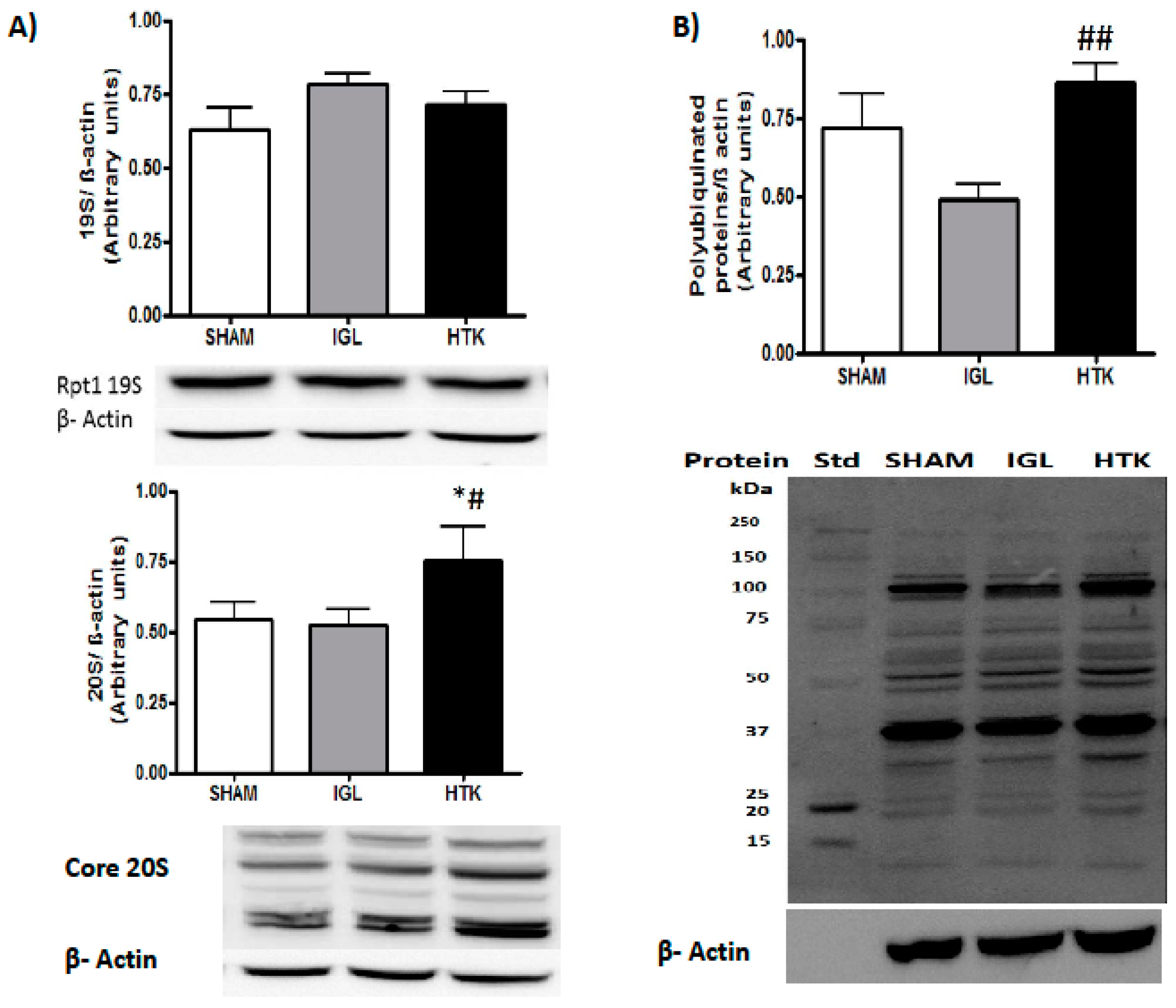
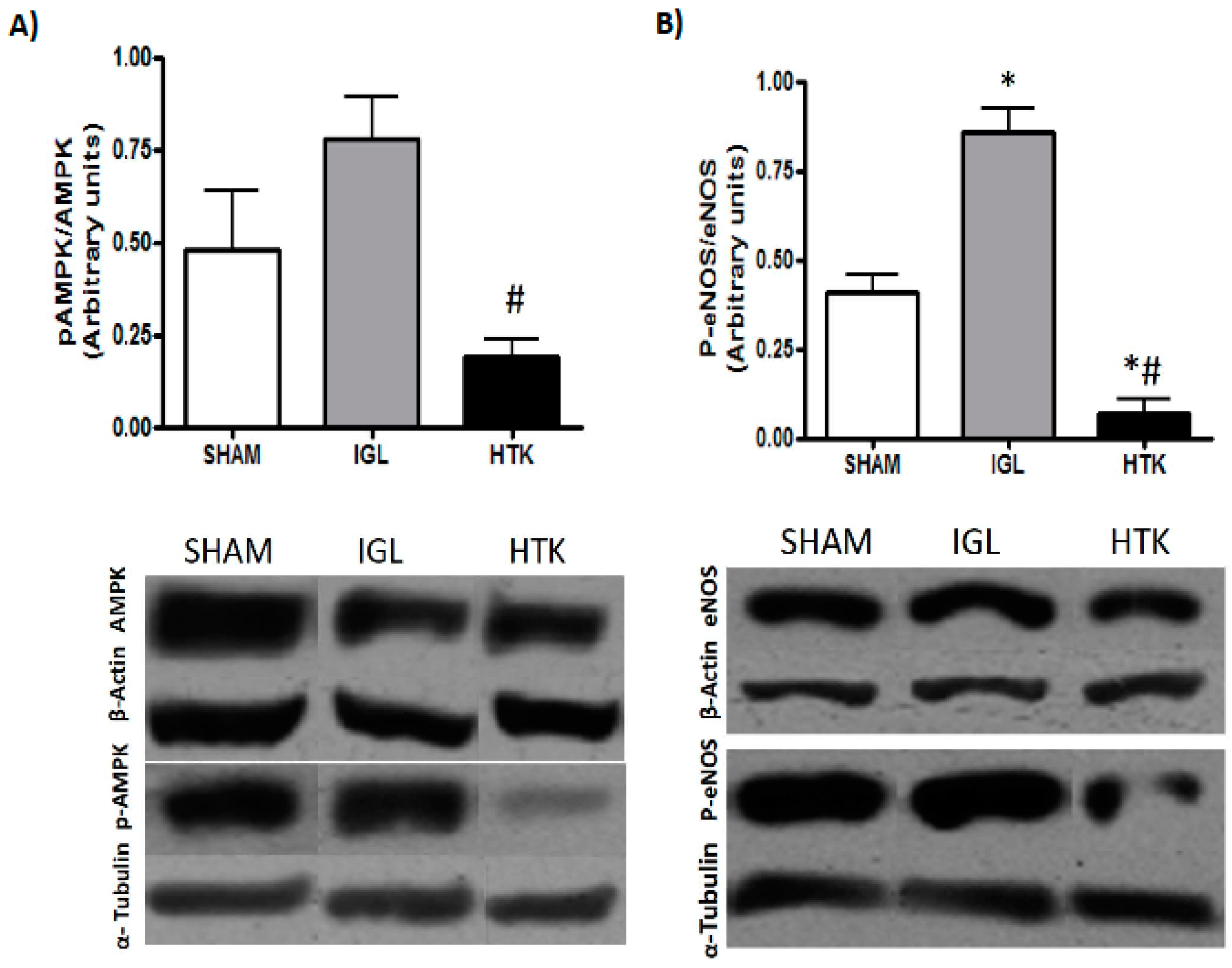
2. Results
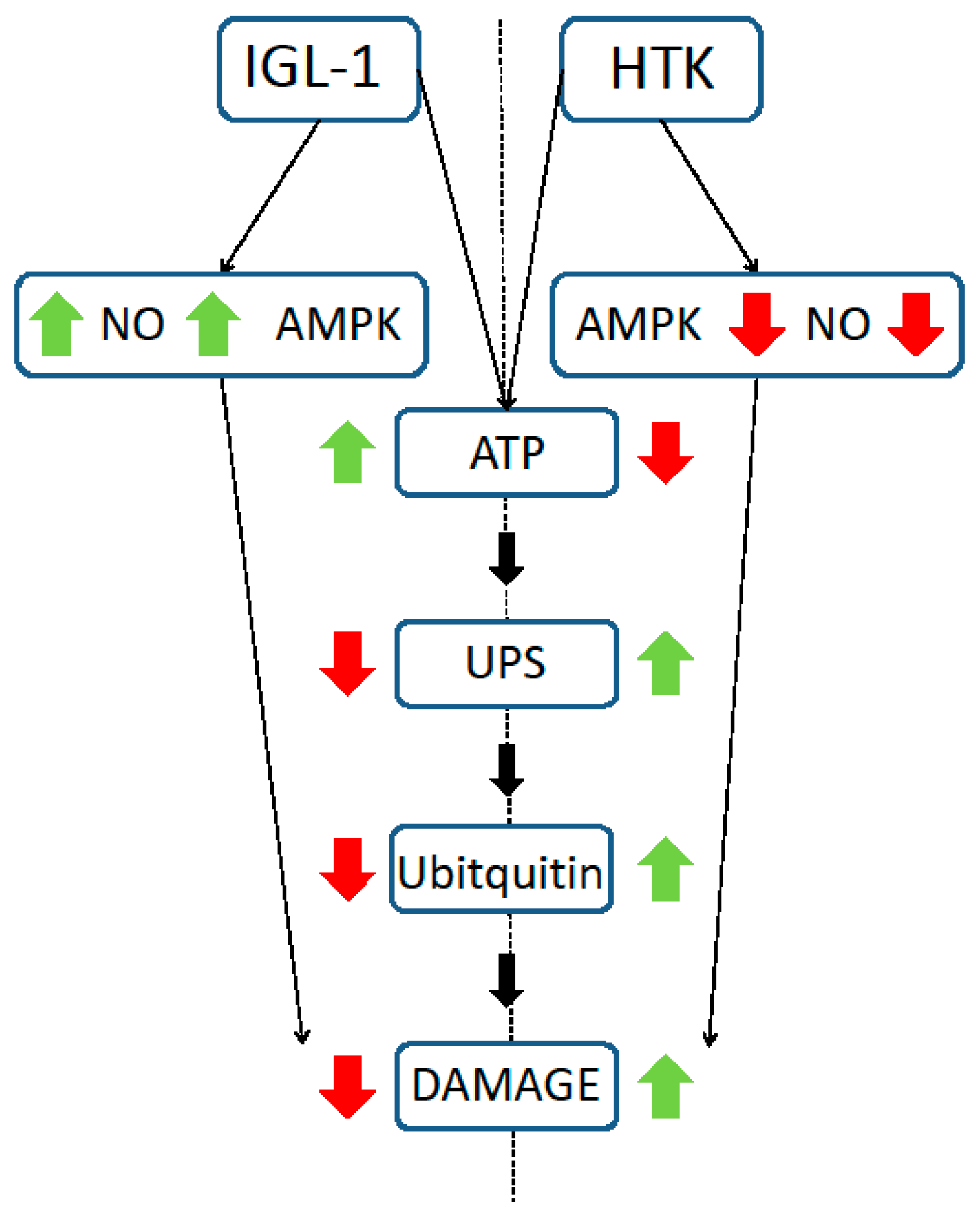
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Experimental Groups
- Group 1 (SHAM; n = 6): Animals underwent transverse laparotomy and received silk ligatures in the right suprarenal vein, diaphragmatic vein, and hepatic artery.
- Group 2 (IGL-1; n = 6): After organ recovery, the livers were flushed with 40 mL of IGL-1 solution and stored in IGL-1 preservation solution for 24 h at 4 °C.
- Group 3 (HTK; n = 6): After organ recovery, the livers were flushed with 100 mL of HTK solution (2.5 times more than IGL-1) and stored in HTK solution for 24 h at 4 °C.
4.3. Biochemical Determinations
4.3.1. Proteasome Chymotryptic-Like Activity Assay
4.3.2. Transaminase Assay
4.4. Histology
4.5. Glutamate Dehydrogenase Activity
4.6. Energy Metabolism (ATP Breakdown)
4.7. Western-Blotting Analysis
4.8. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goldberg, A.L. Protein degradation and protection against misfolded or damaged proteins. Nature 2003, 426, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.H.; Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef] [PubMed]
- Drews, O.; Taegtmeyer, H. Targeting the ubiquitin-proteasome system in heart disease: The basis for new therapeutic strategies. Antioxid. Redox Signal. 2014, 21, 2322–2343. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A. The ubiquitin-proteasome proteolytic pathway. Cell 1994, 79, 13–21. [Google Scholar] [CrossRef]
- Padrissa-Altes, S.; Zaouali, M.A.; Bartrons, R.; Rosello-Catafau, J. Ubiquitin-proteasome system inhibitors and AMPK regulation in hepatic cold ischaemia and reperfusion injury: Possible mechanisms. Clin. Sci. 2012, 123, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeschke, H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G15–G26. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ramirez, A.; Mosbah, I.B.; Ramalho, F.; Rosello-Catafau, J.; Peralta, C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006, 79, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Hawley, S.A.; Scott, J.W. AMP-activated protein kinase--development of the energy sensor concept. J. Physiol. 2006, 574, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. The AMP-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology 2003, 144, 5179–5183. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Boncompagni, E.; Reiter, R.J.; Bejaoui, M.; Freitas, I.; Pantazi, E.; Folch-Puy, E.; Abdennebi, H.B.; Garcia-Gil, F.A.; Rosello-Catafau, J. AMPK involvement in endoplasmic reticulum stress and autophagy modulation after fatty liver graft preservation: A role for melatonin and trimetazidine cocktail. J. Pineal Res. 2013, 55, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Abdennebi, H.B.; Padrissaaltes, S.; Mahfoudhboussaid, A.; Rosellocatafau, J. Pharmacological strategies against cold ischemia reperfusion injury. Expert Opin. Pharmacother. 2010, 11, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Mangus, R.S.; Tector, A.J.; Fridell, J.A.; Kazimi, M.; Hollinger, E.; Vianna, R.M. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in intestinal and multivisceral transplantation. Transplantation 2008, 86, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Meine, M.H.; Leipnitz, I.; Zanotelli, M.L.; Schlindwein, E.S.; Kiss, G.; Martini, J.; de Medeiros Fleck, A., Jr.; Mucenic, M.; de Mello Brandao, A.; Marroni, C.A.; et al. Comparison Between IGL-1 and HTK Preservation Solutions in Deceased Donor Liver Transplantation. Transplant. Proc. 2015, 47, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.A.; Geng, Q.; Romero, J.; Picken, M.M.; Gamelli, R.L.; Majetschak, M. Prolongation of myocardial viability by proteasome inhibition during hypothermic organ preservation. Biochem. Biophys. Res. Commun. 2010, 401, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Romero, J.; Saini, V.; Baker, T.A.; Picken, M.M.; Gamelli, R.L.; Majetschak, M. A subset of 26S proteasomes is activated at critically low ATP concentrations and contributes to myocardial injury during cold ischemia. Biochem. Biophys. Res. Commun. 2009, 390, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Bardag-Gorce, F.; Carbonell, T.; Oliva, J.; Pantazi, E.; Bejaoui, M.; Abdennebi, H.B.; Rimola, A.; Rosellocatafau, J. Proteasome inhibitors protect the steatotic and non-steatotic liver graft against cold ischemia reperfusion injury. Exp. Mol. Pathol. 2013, 94, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Padrissa-Altes, S.; Zaouali, M.A.; Boncompagni, E.; Bonaccorsi-Riani, E.; Carbonell, T.; Bardag-Gorce, F.; Oliva, J.; French, S.W.; Bartrons, R.; Rosellocatafau, J. The use of a reversible proteasome inhibitor in a model of Reduced-Size Orthotopic Liver transplantation in rats. Exp. Mol. Pathol. 2012, 93, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ben Mosbah, I.; Rosellocatafau, J.; Franco-Gou, R.; Abdennebi, H.B.; Saidane, D.; Ramella-Virieux, S.; Boillot, O.; Peralta, C. Preservation of steatotic livers in IGL-1 solution. Liver Transplant. 2006, 12, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, F.S.; Fernandez-Monteiro, I.; Rosellocatafau, J.; Peralta, C. Hepatic microcirculatory failure. Acta Cir. Bras. 2006, 21, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Selzner, M.; Clavien, P.A. Fatty liver in liver transplantation and surgery. Semin. Liver Dis. 2001, 21, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Armon, T.; Ganoth, D.; Hershko, A. Assembly of the 26 S complex that degrades proteins ligated to ubiquitin is accompanied by the formation of ATPase activity. J. Biol. Chem. 1990, 265, 20723–20726. [Google Scholar] [PubMed]
- Babbitt, S.E.; Kiss, A.; Deffenbaugh, A.E.; Chang, Y.H.; Bailly, E.; Erdjument-Bromage, H.; Tempst, P.; Buranda, T.; Sklar, L.A.; Baumler, J.; et al. ATP hydrolysis-dependent disassembly of the 26S proteasome is part of the catalytic cycle. Cell 2005, 121, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Li, X.; Thompson, D.; Wooding, K.; Chang, T.L.; Tang, Z.; Yu, H.; Thomas, P.J.; DeMartino, G.N. ATP binding and ATP hydrolysis play distinct roles in the function of 26S proteasome. Mol. Cell 2006, 24, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Majetschak, M. Regulation of the proteasome by ATP: Implications for ischemic myocardial injury and donor heart preservation. Am. J. Physiol. 2013, 305, H267–H278. [Google Scholar] [CrossRef] [PubMed]
- Majetschak, M.; Patel, M.B.; Sorell, L.T.; Liotta, C.; Li, S.; Pham, S.M. Cardiac proteasome dysfunction during cold ischemic storage and reperfusion in a murine heart transplantation model. Biochem. Biophys. Res. Commun. 2008, 365, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Zaouali, M.A.; Folch-Puy, E.; Pantazi, E.; Bardag-Gorce, F.; Carbonell, T.; Oliva, J.; Rimola, A.; Abdennebi, H.B.; Rosellocatafau, J. Bortezomib enhances fatty liver preservation in Institut George Lopez-1 solution through adenosine monophosphate activated protein kinase and Akt/mTOR pathways. J. Pharm. Pharmacol. 2014, 66, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Abdennebi, H.B.; Zaouali, M.A.; Alfany-Fernandez, I.; Tabka, D.; Rosellocatafau, J. How to protect liver graft with nitric oxide. World J. Gastroenterol. 2011, 17, 2879–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco-Chaumel, E.; Rosellocatafau, J.; Bartrons, R.; Franco-Gou, R.; Xaus, C.; Casillas, A.; Gelpi, E.; Rodes, J.; Peralta, C. Adenosine monophosphate-activated protein kinase and nitric oxide in rat steatotic liver transplantation. J. Hepatol. 2005, 43, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Panisello-Rosello, A.; Lopez, A.; Benitez, C.C.; Folch-Puy, E.; Garcia-Gil, A.; Carbonell, T.; Adam, R.; Rosellocatafau, J. Relevance of proteolysis and proteasome activation in fatty liver graft preservation: An Institut Georges Lopez-1 vs. University of Wisconsin appraisal. World J. Gastroenterol. 2017, 23, 4211–4221. [Google Scholar] [CrossRef] [PubMed]
| Electrolyte (m·mol/L) | IGL-1 | HTK | Colloids (g/L) | IGL-1 | HTK |
|---|---|---|---|---|---|
| K+ | 25 | 9 | Polyethylene glycol-35 | 1 | |
| Na+ | 125 | 15 | |||
| Mg2+ | 5 | 4 | |||
| Cl− | 50 | ||||
| SO42− | 5 | ||||
| Buffers (m·mol/L) | Antioxydants (m·mol/L) | ||||
| Diphosphate | 25 | Glutathione | 3 | ||
| Histidine | 180 | ||||
| Histidine-HO | 18 | Allopurinol | 1 | ||
| Tryptophan | 2 | ||||
| Impermeants (m·mol/L ) | Precursors (m·mol/L ) | ||||
| Raffinose | 30 | Adenosine | 5 | ||
| Lactobionic acid | 100 | Ketoglutarate | 1 | ||
| Mannitol | 30 | pH | 7.4 | 7.2 | |
| Osmolarity | 290 | 310 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panisello-Roselló, A.; Verde, E.; Amine Zaouali, M.; Flores, M.; Alva, N.; Lopez, A.; Folch-Puy, E.; Carbonell, T.; Hotter, G.; Adam, R.; et al. The Relevance of the UPS in Fatty Liver Graft Preservation: A New Approach for IGL-1 and HTK Solutions. Int. J. Mol. Sci. 2017, 18, 2287. https://doi.org/10.3390/ijms18112287
Panisello-Roselló A, Verde E, Amine Zaouali M, Flores M, Alva N, Lopez A, Folch-Puy E, Carbonell T, Hotter G, Adam R, et al. The Relevance of the UPS in Fatty Liver Graft Preservation: A New Approach for IGL-1 and HTK Solutions. International Journal of Molecular Sciences. 2017; 18(11):2287. https://doi.org/10.3390/ijms18112287
Chicago/Turabian StylePanisello-Roselló, Arnau, Eva Verde, Mohamed Amine Zaouali, Marta Flores, Norma Alva, Alexandre Lopez, Emma Folch-Puy, Teresa Carbonell, Georgina Hotter, René Adam, and et al. 2017. "The Relevance of the UPS in Fatty Liver Graft Preservation: A New Approach for IGL-1 and HTK Solutions" International Journal of Molecular Sciences 18, no. 11: 2287. https://doi.org/10.3390/ijms18112287