Low Temperature Extends the Lifespan of Bursaphelenchus xylophilus through the cGMP Pathway
Abstract
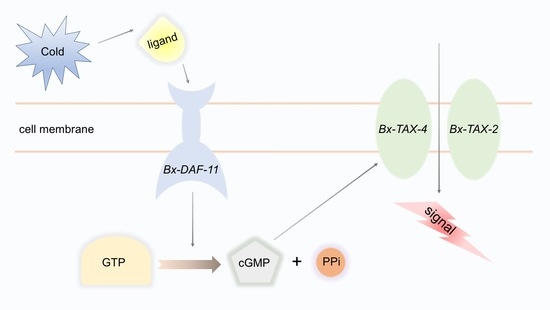
:1. Introduction
2. Results
2.1. Cloning of the Three cGMP Genes and Alignment of Deduced Amino Acids
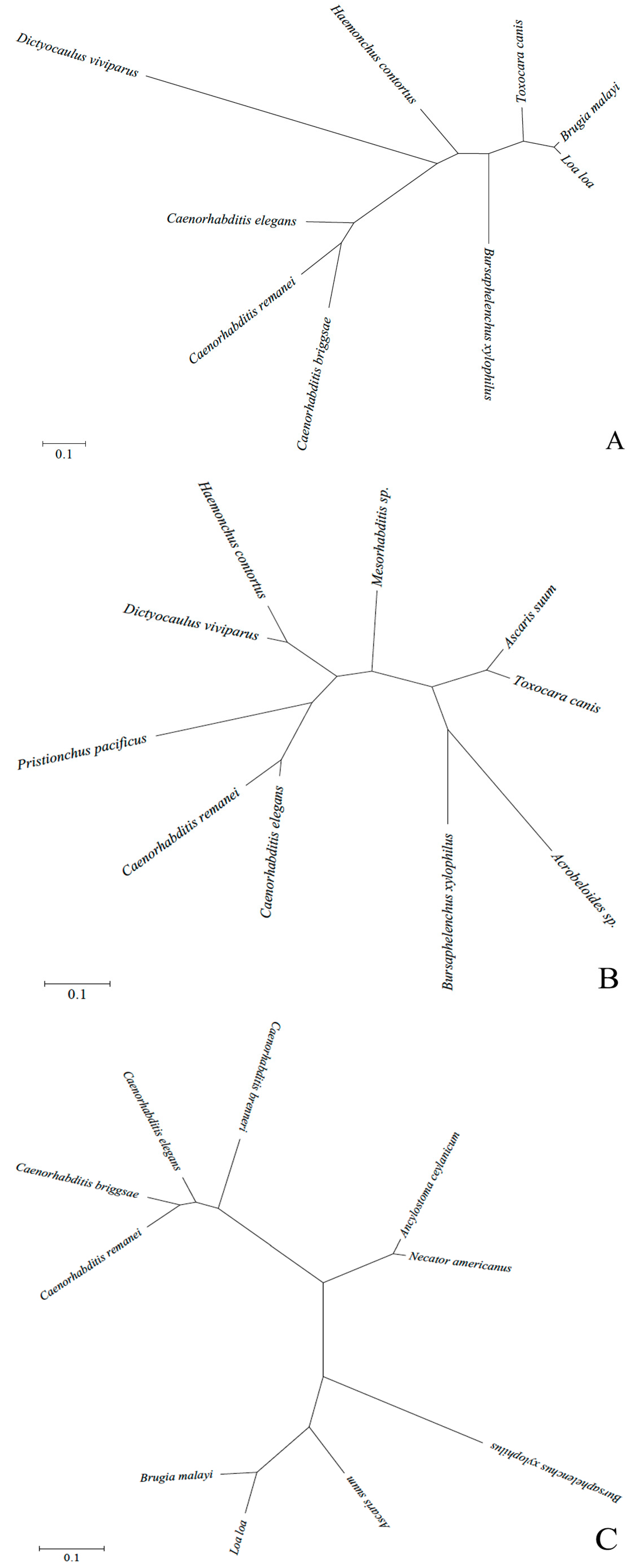
2.2. Bioinformatics Analysis of Deduced Proteins
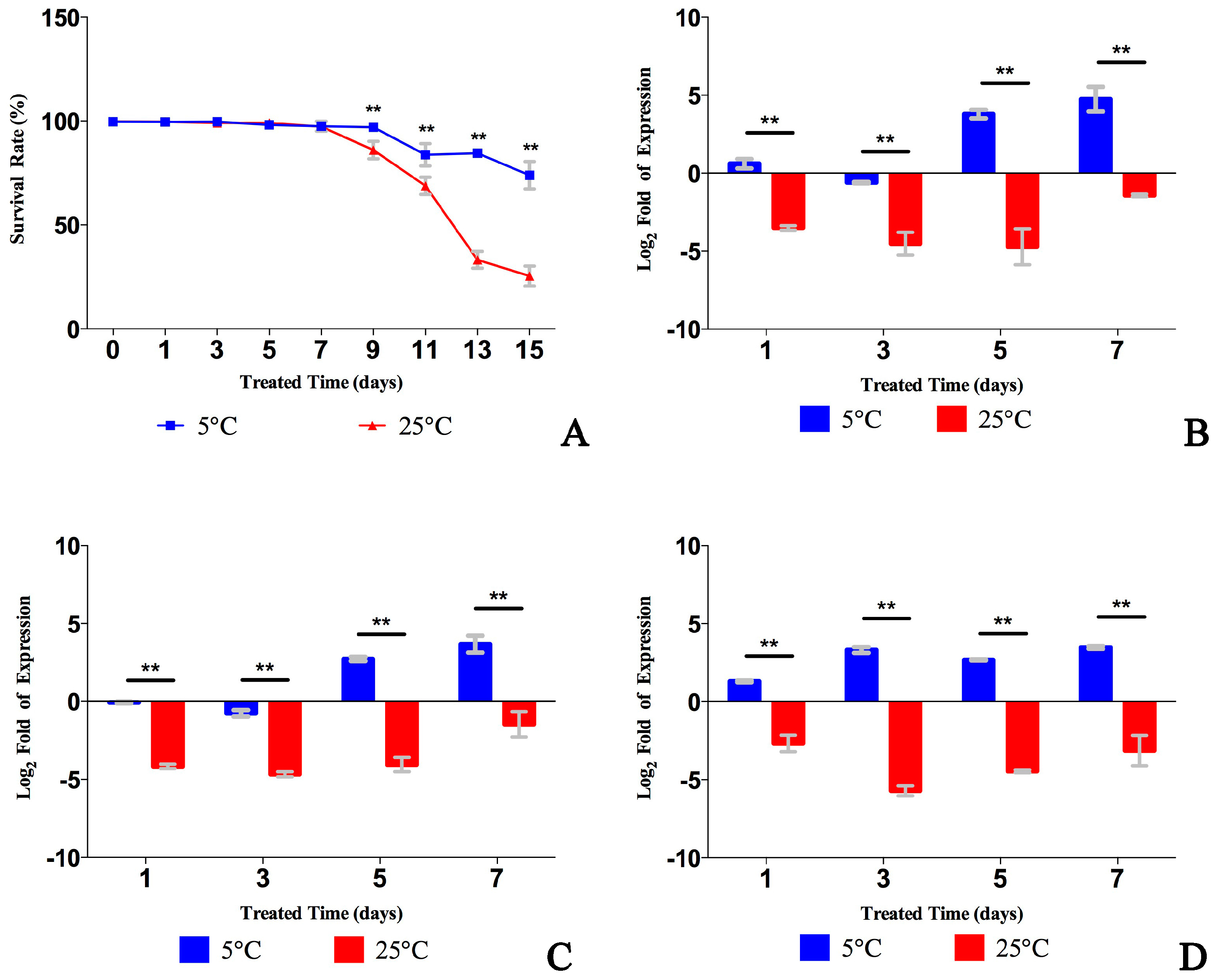
2.3. Analysis of Transcript Abundance at Low Temperature
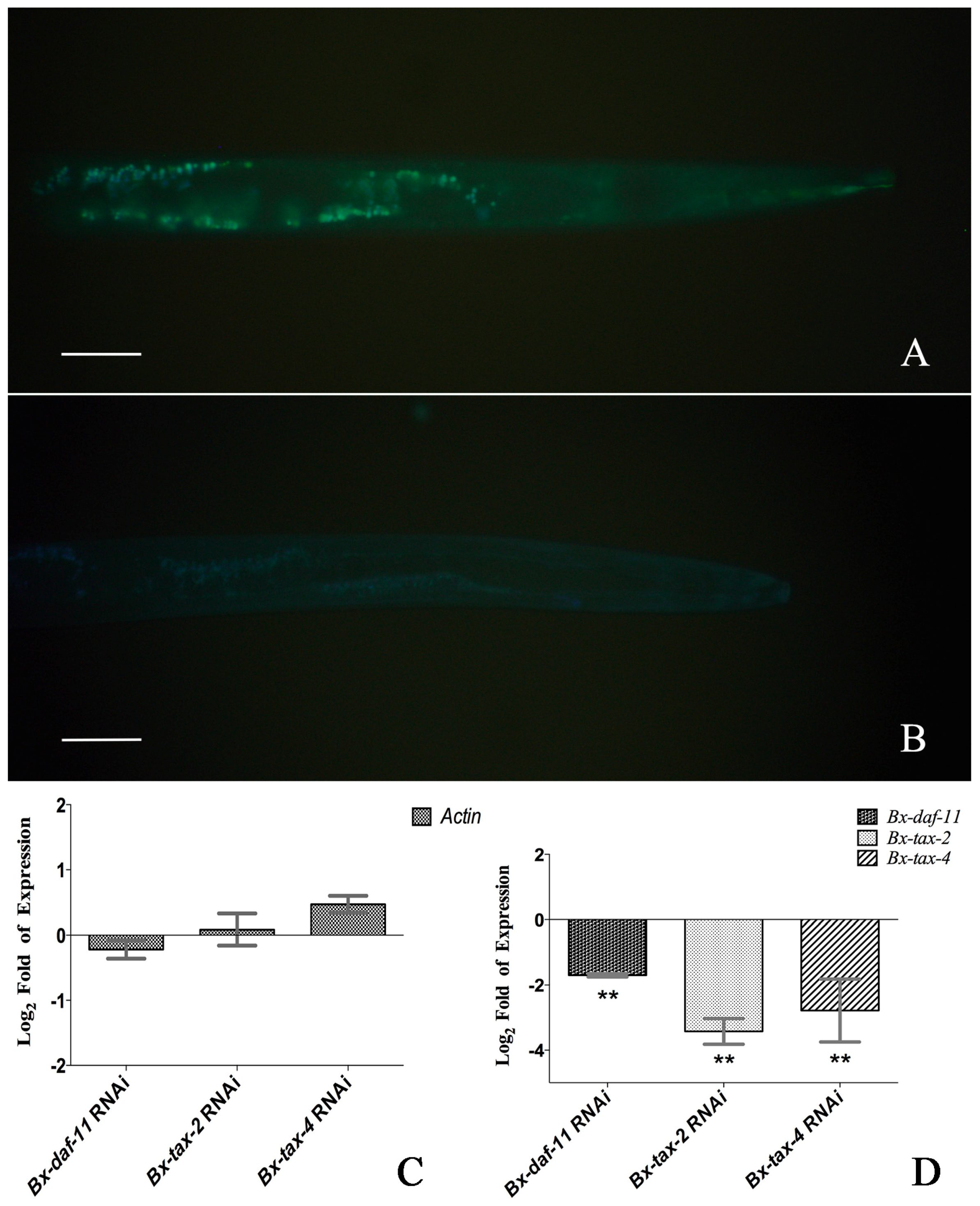
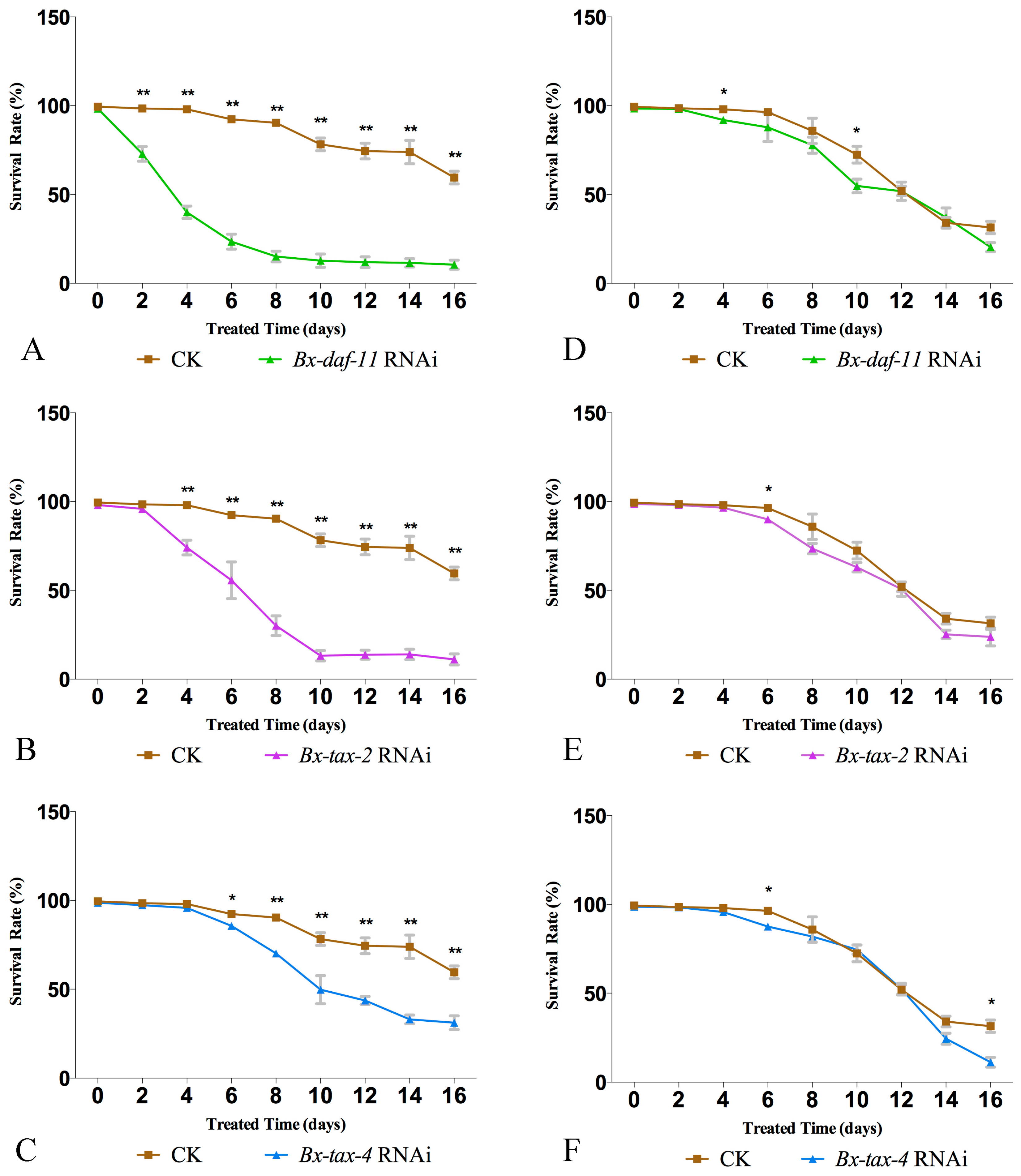
2.4. RNAi of Bx-DAF-11, Bx-TAX-2, and Bx-TAX-4
3. Discussion
4. Materials and Methods
4.1. Nematode Culture and Extraction
4.2. RNA Extraction and Cloning of Bx-daf-11, Bx-tax-2, and Bx-tax-4
4.3. Cold Treatment and Survival Rate Counting
4.4. Bioinformatic Analysis
4.5. Analysis of Transcript Abundance
4.6. RNAi of Bx-daf-11, Bx-tax-2, and Bx-tax-4
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PWN | pine wood nematode |
| RNAi | RNA interference |
| cGMP | cyclic guanosine monophosphate |
| CDS | coding sequence |
| SD | standard deviation |
| qPCR | quantitative real-time PCR |
| dsRNA | double-stranded RNA |
| FITC | fluorescein isothiocyanate |
| J2 | second-stage propagative juveniles |
| DL3 | specialized third-stage dauer larva |
| DL4 | specialized forth-stage dauer larva |
References
- Richardson, D.M.; Pysek, P.; Elton, C.S. The Ecology of Invasions by Animals and Plants; The University of Chicago Press: Chicago, IL, USA, 2007; pp. 659–666. [Google Scholar]
- Bartell, S.M.; Nair, S.K. Establishment risks for invasive species. Risk Anal. 2004, 24, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Panov, V.E.; Krylov, P.I.; Riccardi, N. Role of diapause in dispersal and invasion success by aquatic invertebrates. J. Limnol. 2004, 63, 56–69. [Google Scholar] [CrossRef]
- Lounibos, L.P.; Escher, R.L.; Lourenço-De-Oliveira, R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 2003, 96, 512–518. [Google Scholar] [CrossRef]
- Diaz, R.; Overholt, W.A.; Dan, H.; Samayoa, A.C. Diapause Induction in Gratiana boliviana (Coleoptera: Chrysomelidae), a biological control agent of tropical soda apple in Florida. Ann. Entomol. Soc. Am. 2011, 104, 1319–1326. [Google Scholar] [CrossRef]
- Mota, M.M.; Futai, K.; Vieira, P. Pine wilt disease and The pinewood nematode, Bursaphelenchus Xylophilus. Integr. Manag. Plant Pests Dis. 2009, 4, 253–274. [Google Scholar]
- Zamora, P.; Rodriguez, V.; Renedo, F.; Sanz, A.V.; Dominguez, J.C.; Perez-Escolar, G.; Miranda, J.; Alvarez, B.; Gonzalez-Casas, A.; Mayor, E.; et al. First report of Bursaphelenchus xylophilus causing pine wilt disease on Pinus radiata in Spain. Plant Dis. 2015, 99, 1449. [Google Scholar] [CrossRef]
- Dwinell, L.D. First report of pinewood nematode (Bursaphelenchus xylophilus) in Mexico. Plant Dis. 1993. [Google Scholar] [CrossRef]
- Wu, H.Y.; Tan, Q.Q.; Jiang, S.X. First report of pine wilt disease caused by Bursaphelenchus xylophilus on Pinta thunbergii in the Inland City of Zibo, Shandong, China. Plant Dis. 2013, 97, 1126. [Google Scholar] [CrossRef]
- Gruffudd, H.R.; Jenkins, T.A.R.; Evans, H.F. Using an evapo-transpiration model (ETpN) to predict the risk and expression of symptoms of pine wilt disease (PWD) across Europe. Biol. Invasions 2016, 18, 1–18. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, W.; Liang, J.; Yan, D.; Jia, X.; Zhang, X. Potential suitability assessment of Bursaphelenchus xylophilus in China. For. Res. 2005, 18, 460–464. [Google Scholar]
- Zhao, L.L.; Wei, W.; Kulhavy, D.L.; Zhang, X.Y.; Sun, J.H. Low temperature induces two growth-arrested stages and change of secondary metabolites in Bursaphelenchus xylophilus. Nematology 2007, 9, 663–670. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, B.; Dong, Y.; Gong, J.; Xu, T.; Liu, J.; Xu, X.Z. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 2013, 152, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, A.; Okumura, M.; Kimata, T.; Tanizawa, Y.; Takano, R.; Kimura, K.D.; Inada, H.; Matsumoto, K.; Mori, I. Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science 2008, 320, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Beverly, M.; Anbil, S.; Sengupta, P. Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in Caenorhabditis elegans. J. Neurosci. 2011, 31, 11718–11727. [Google Scholar] [CrossRef] [PubMed]
- Garrity, P.A.; Goodman, M.B.; Samuel, A.D.; Sengupta, P. Running hot and cold: Behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Gene. Dev. 2010, 24, 2365–2382. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Ujisawa, T.; Sonoda, S.; Kuhara, A. Light and pheromone-sensing neurons regulates cold habituation through insulin signalling in Caenorhabditis elegans. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Sugi, T.; Nishida, Y.; Mori, I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nat. Neurosci. 2011, 14, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Birnby, D.A.; Link, E.M.; Vowels, J.J.; Tian, H.; Colacurcio, P.L.; Thomas, J.H. A transmembrane guanylyl cyclase (DAF-11) and Hsp90 (DAF-21) regulate a common set of chemosensory behaviors in Caenorhabditis elegans. Genetics 2000, 155, 85–104. [Google Scholar] [PubMed]
- Mori, I.; Ohshima, Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 1995, 376, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Noriyuki, O.; Atsushi, K.; Fumiya, N.; Yoshifumi, O.; Ikue, M. Bidirectional regulation of thermotaxis by glutamate transmissions in Caenorhabditis elegans. EMBO J. 2011, 30, 1376–1388. [Google Scholar]
- Kikuchi, T.; Cotton, J.A.; Dalzell, J.J.; Hasegawa, K.; Kanzaki, N.; McVeigh, P.; Takanashi, T.; Tsai, I.J.; Assefa, S.A.; Cock, P.J.A.; et al. Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog. 2011, 7, e1002219. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Z.; Li, D.; Chen, Q. Identification and Characterization of a Bursaphelenchus xylophilus (Aphelenchida: Aphelenchoididae) thermotolerance-related gene: Bx-HSP90. Int. J. Mol. Sci. 2012, 13, 8819–8833. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, D.; Chen, Q.; Ma, L. Genome-wide survey and characterization of the small heat shock protein gene family in Bursaphelenchus xylophilus. Gene 2015, 579, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.L.; Peng, D.L.; Huang, W.K.; Peng, H.A.; Long, H.B.; Wang, G.F. Cloning and sequence analysis of three new heat shock protein 90 genes (hsp90) from Bursaphelenchus xylophilus, B. mucronatus and B. doui. J. Agric. Biotechnol. 2011, 19, 916–923. [Google Scholar]
- Mamiya, Y. The life history of the pine wood nematode, Bursaphelenchus lignicolus. Jpn. J. Nematol. 1975, 5, 16–25. [Google Scholar]
- Mamiya, Y. Pathology of the Pine Wilt Disease Caused by Bursaphelenchus xylophilus. Ann. Rev. Phytopathol. 1983, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, D.; Wang, F.; Zhang, R.; Ling, Y. Trehalose metabolism genes of Aphelenchoides besseyi (Nematoda: Aphelenchoididae) in hypertonic osmotic pressure survival. Biol. Open 2017, 6, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Vanholme, B.; Haegeman, A. Four transthyretin-like genes of the migratory plant-parasitic nematode Radopholus similis: Members of an extensive nematode-specific family. Gene 2007, 402, 9–19. [Google Scholar] [CrossRef] [PubMed]
| Name of Primer | Sequence (5′–3′) | Reference |
|---|---|---|
| Bx-daf-11-F | GATGCGATCCAGGTTTCTAC | This study |
| Bx-daf-11-R | TAATGTTACCGTTCCTCCGA | This study |
| Bx-tax-2-F | TCTCCCAATACTCCACAAGT | This study |
| Bx-tax-2-R | TTGGGTAACTGAGCAGAACT | This study |
| Bx-tax-4-F | TGTCACCAACATGAATGGAC | This study |
| Bx-tax-4-R | GGTGATGACTTCATCGTCTG | This study |
| q-Bx-daf-11-F | TGTTGGGACAATTGGTCAGG | This study |
| q-Bx-daf-11-R | TCACATTGTCATGGATTAACTGC | This study |
| q-Bx-tax-2-F | TGTGGACAATCAGTCGGAGA | This study |
| q-Bx-tax-2-F | CCAGGCCATGTAACTTTTGC | This study |
| q-Bx-tax-4-F | AACTCACACAGGGTTTCTGG | This study |
| q-Bx-tax-4-R | ACGTAGTCATGTAATCCAATGGAAG | This study |
| 28S-F | TACGATCGGTGTTCGTTGC | Qiaoli Chen et al. [28] |
| 28S-R | CTCACATCGTCGACATCCAA | Qiaoli Chen et al. [28] |
| i-Bx-daf-11-F | GCTAATACGACTCACTATAGGGATGCGCGTTCATGGGATTTTTG | This study |
| i-Bx-daf-11-R | AGTAATACGACTCACTATAGGGATCGCATGTCCCTTTTGTCGAAC | This study |
| i-Bx-tax-2-F | GCTAATACGACTCACTATAGGGATTCACTTAAGCGCGAGAGATG | This study |
| i-Bx-tax-2-R | AGTAATACGACTCACTATAGGGATCAAGGCGTTGTAGAGAAAGGC | This study |
| i-Bx-tax-4-F | GCTAATACGACTCACTATAGGGATGAGCAAGGGCTTCTGGTTAG | This study |
| i-Bx-tax-4-R | AGTAATACGACTCACTATAGGGATCCATCGGGGAGTGACTGTTTG | This study |
| q-Actin-F | GAAAGAGGGCCGGAAGAG | Jacob J et al. [29] |
| q-Actin-R | AGATCGTCCGCGACATAAAG | Jacob J et al. [29] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Ma, L.; Wang, F.; Wang, B.; Hao, X.; Xu, J.; Ma, Y. Low Temperature Extends the Lifespan of Bursaphelenchus xylophilus through the cGMP Pathway. Int. J. Mol. Sci. 2017, 18, 2320. https://doi.org/10.3390/ijms18112320
Wang B, Ma L, Wang F, Wang B, Hao X, Xu J, Ma Y. Low Temperature Extends the Lifespan of Bursaphelenchus xylophilus through the cGMP Pathway. International Journal of Molecular Sciences. 2017; 18(11):2320. https://doi.org/10.3390/ijms18112320
Chicago/Turabian StyleWang, Bowen, Ling Ma, Feng Wang, Buyong Wang, Xin Hao, Jiayao Xu, and Yan Ma. 2017. "Low Temperature Extends the Lifespan of Bursaphelenchus xylophilus through the cGMP Pathway" International Journal of Molecular Sciences 18, no. 11: 2320. https://doi.org/10.3390/ijms18112320