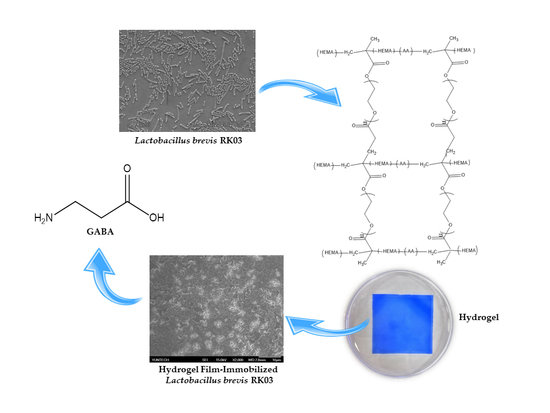
Hydrogel Film-Immobilized Lactobacillus brevis RK03 for γ-Aminobutyric Acid Production
Abstract
:1. Introduction
2. Results
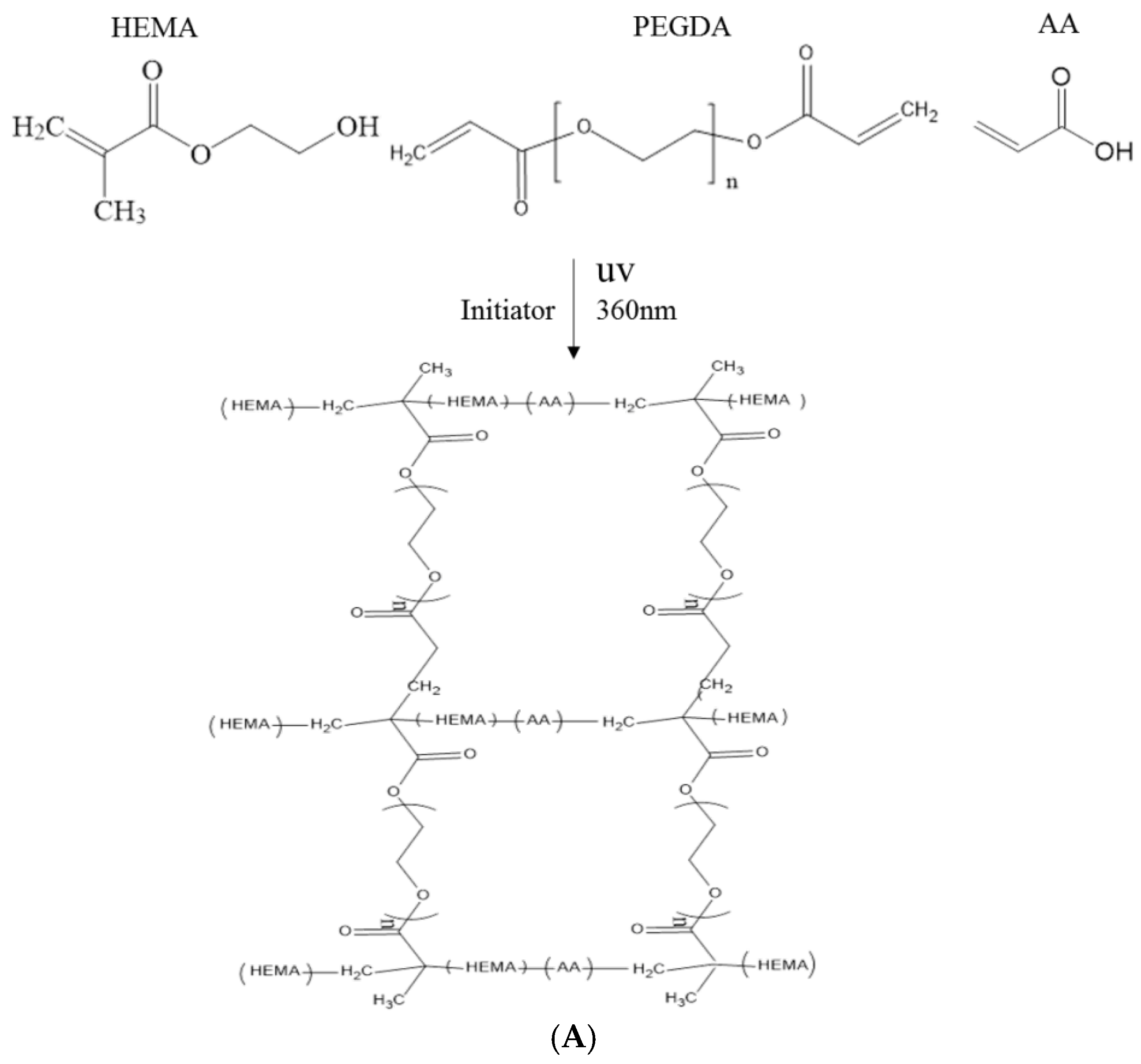
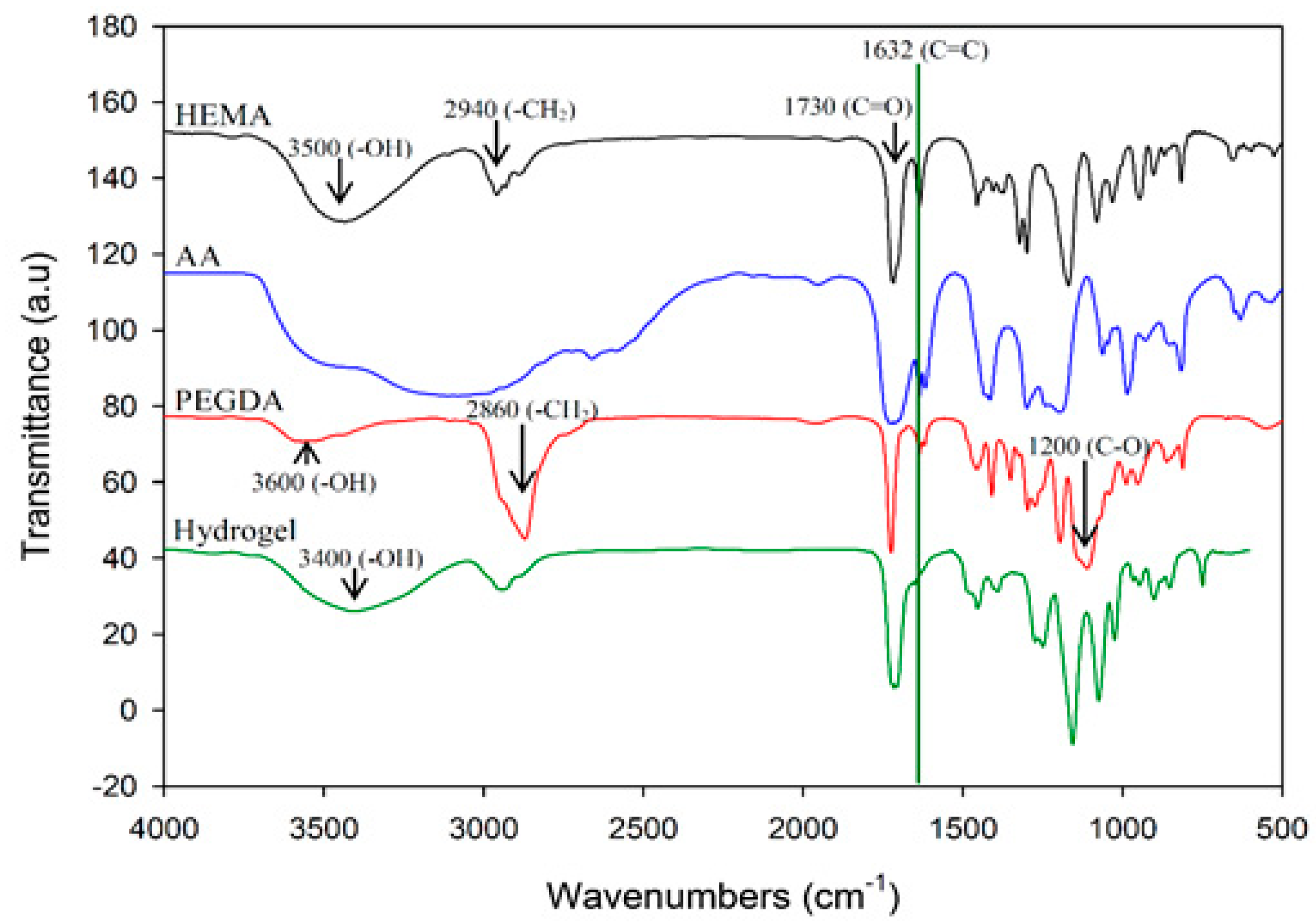
2.1. Synthesis and Characterization of Polyacrylic Hydrogel Films
2.2. GABA Production by Immobilized Cells on Polyacrylic Hydrogel Films
2.3. GABA Production by Immobilized Cells on Polyacrylic Hydrogel Films Using Semi-Continuous Fermentation
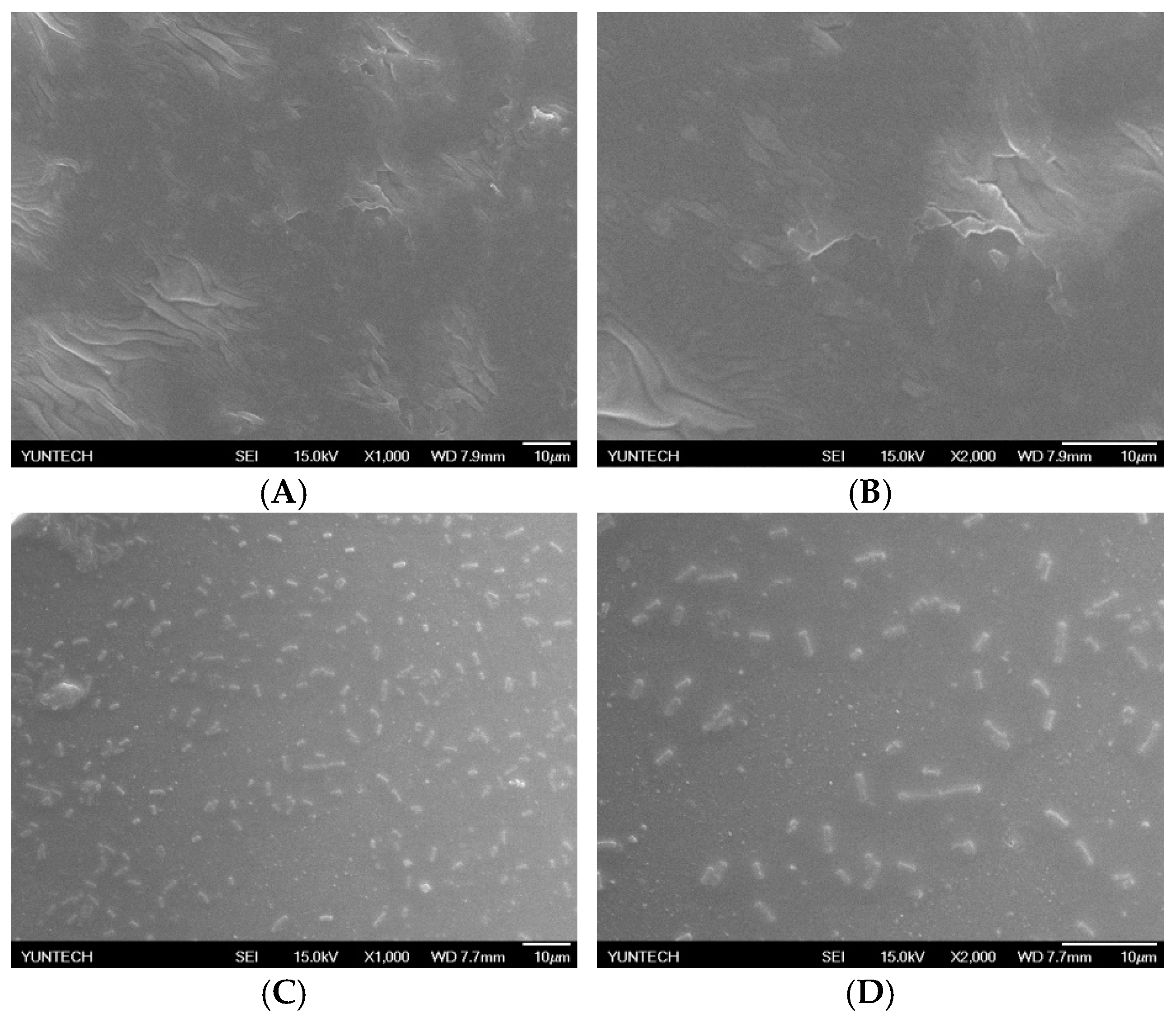
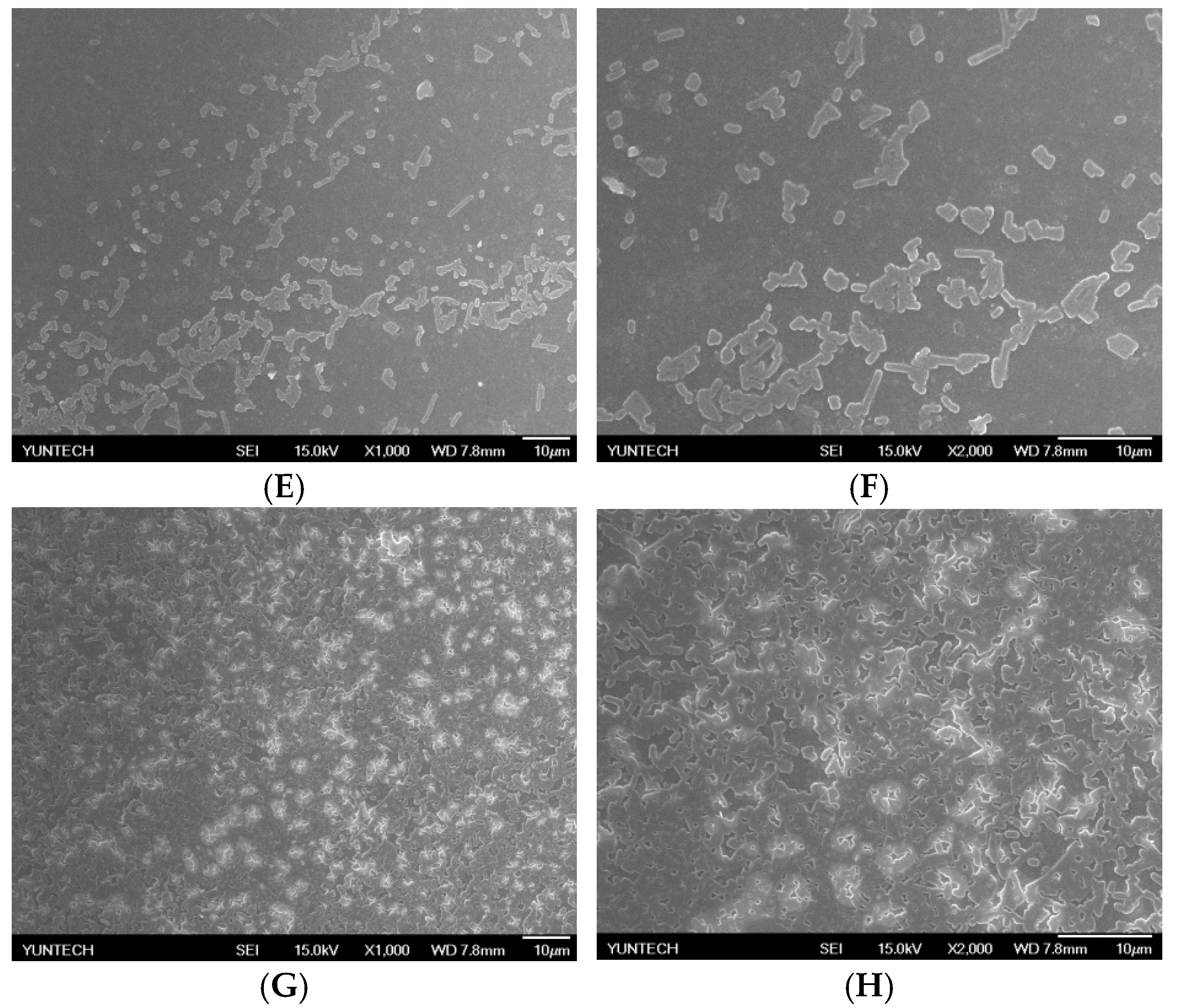
2.4. Immobilization of L. brevis RK03 onto Polyacrylic Hydrogel Films
3. Discussion
4. Materials and Methods
4.1. Isolation and Identification of GABA-Producing LAB
4.2. Scanning Electron Microscopy of Lactobacillus brevis RK03
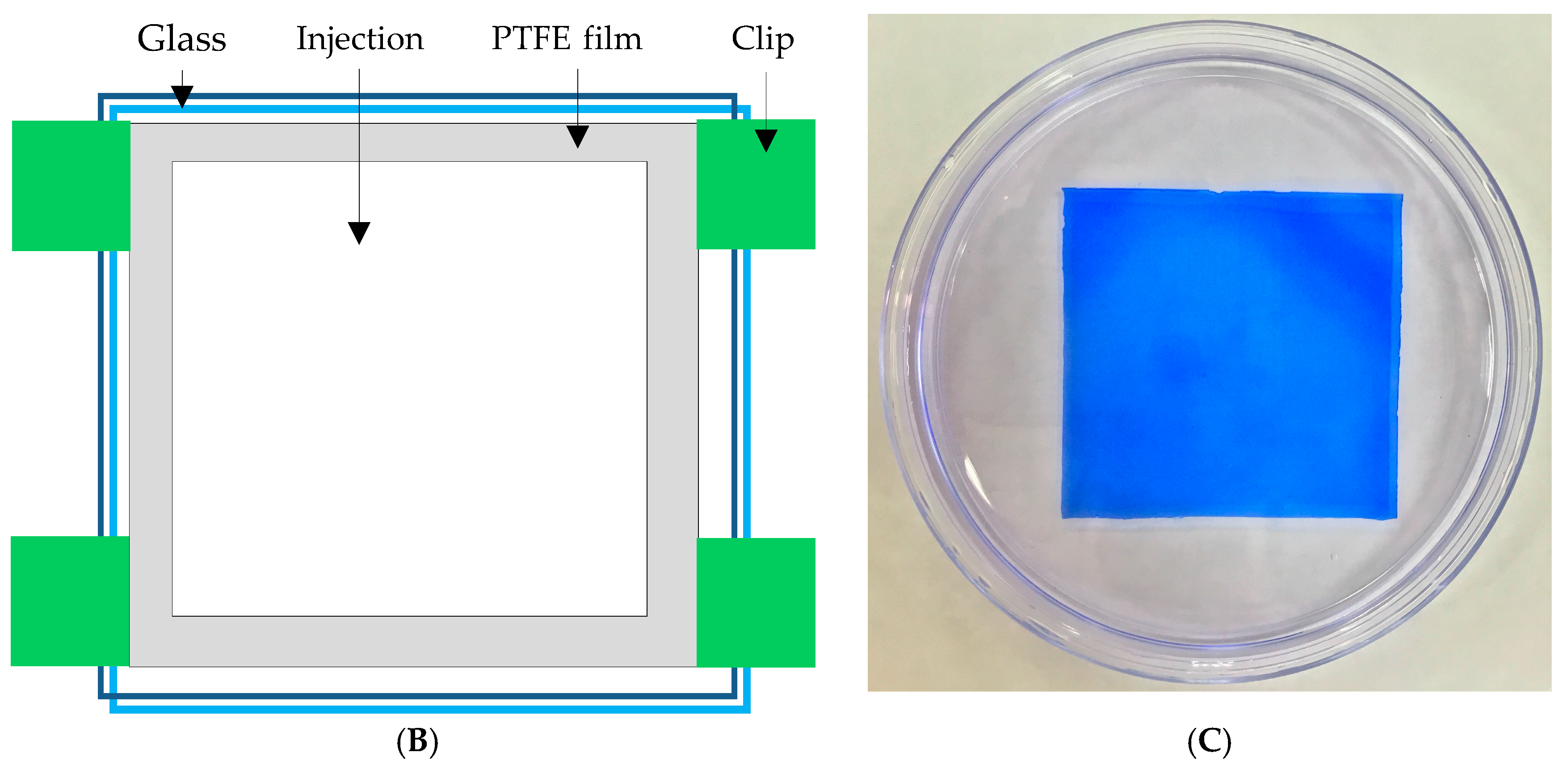
4.3. Preparation of Polyacrylic Hydrogel Films for Cell Immobilization
4.4. Tensile Strength and Water Content Measurement
4.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.6. GABA and MSG Analysis
4.7. GABA and MSG Analysis in Semi-Continuous Conditions
4.8. Measurement of GABA Content with High-Performance Liquid Chromatography (HPLC)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chung, H.J.; Park, T.G. Self-assembled and nanostructured hydrogels for drug delivery and tissue engineering. Nano Today 2009, 4, 429–437. [Google Scholar] [CrossRef]
- Stanton, H.C. Mode of action of gamma-aminobutyric acid on the cardiovascular system. Arch. Int. Pharmacodyn. Ther. 1963, 143, 195–204. [Google Scholar] [PubMed]
- Lee, S.; Ahn, J.; Kim, Y.-G.; Jung, J.-K.; Lee, H.; Lee, E.G. Gamma-aminobutyric acid production using immobilized glutamate decarboxylase followed by downstream processing with cation exchange chromatography. Int. J. Mol. Sci. 2013, 14, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Ahn, J.; Lee, J.; Lee, H.; Kim, C.; Jung, J.-K.; Lee, H.; Lee, E.G. Expression, immobilization and enzymatic properties of glutamate decarboxylase fused to a cellulose-binding domain. Int. J. Mol. Sci. 2011, 13, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Seki, T.; Ariga, T. The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2004, 68, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.R.; Chang, J.Y.; Chang, H.C. Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J. Microbiol. Biotechnol. 2007, 17, 104–109. [Google Scholar] [PubMed]
- Karlgard, C.; Wong, N.; Jones, L.; Moresoli, C. In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. Int. J. Pharm. 2003, 257, 141–151. [Google Scholar] [CrossRef]
- Bae, Y.H.; Okano, T.; Kim, S.W. “On–Off” thermocontrol of solute transport. I. Temperature dependence of swelling of N-isopropylacrylamide networks modified with hydrophobic components in water. Pharm. Res. 1991, 8, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Liaw, W.-C.; Chen, C.-S.; Chang, W.-S.; Chen, K.-P. Xylitol production from rice straw hemicellulose hydrolyzate by polyacrylic hydrogel thin films with immobilized Candida subtropicalis WF79. J. Biosci. Bioeng. 2008, 105, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Narayan, V.S.; Nair, P. Metabolism, enzymology and possible roles of 4-aminobutyrate in higher plants. Phytochemistry 1990, 29, 367–375. [Google Scholar] [CrossRef]
- Seo, M.-J.; Nam, Y.-D.; Lee, S.-Y.; Park, S.-L.; Yi, S.-H.; Lim, S.-I. Expression and characterization of a glutamate decarboxylase from Lactobacillus brevis 877G producing γ-aminobutyric acid. Biosci. Biotechnol. Biochem. 2013, 77, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Steffan, T.; Renukappa-Gutke, T.; Höfner, G.; Wanner, K.T. Design, synthesis and SAR studies of GABA uptake inhibitors derived from 2-substituted pyrrolidine-2-yl-acetic acids. Bioorgan. Med. Chem. 2015, 23, 1284–1306. [Google Scholar] [CrossRef] [PubMed]
- Govindpani, K.; Guzmán, B.C.-F.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Towards a Better understanding of GABAergic remodeling in Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 1813. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Lin, H.-T.V.; Tsai, G.J. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem. 2013, 48, 559–568. [Google Scholar] [CrossRef]
- Kiriyama, A.; Honbo, A.; Nishimura, A.; Shibata, N.; Iga, K. Pharmacokinetic-pharmacodynamic analyses of antihypertensive drugs, nifedipine and propranolol, in spontaneously hypertensive rats to investigate characteristics of effect and side effects. Regul. Toxicol. Pharm. 2016, 76, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Taneera, J.; Jin, Z.; Jin, Y.; Muhammed, S.J.; Zhang, E.; Lang, S.; Salehi, A.; Korsgren, O.; Renström, E.; Groop, L. γ-Aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes. Diabetologia 2012, 55, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Dhakal, R.; Bajpai, V.K.; Baek, K.-H. Production of GABA (γ-aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Ho, K.-J.; Hsieh, Y.-J.; Wang, L.-T.; Mau, J.-L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. Lwt-Food Sci. Technol. 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Song, N.E.; Baik, S.H. Identification and characterization of high GABA and low biogenic amine producing indigenous yeasts isolated from Korean traditional fermented Bokbunja (Rubus coreanus Miquel) wine. J. Biotechnol. 2014, 185, S83. [Google Scholar] [CrossRef]
- Binh, T.T.T.; Ju, W.-T.; Jung, W.-J.; Park, R.-D. Optimization of γ-amino butyric acid production in a newly isolated Lactobacillus brevis. Biotechnol. Lett. 2014, 36, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, V.; Quiberoni, A.; Reinheimer, J.; Suárez, V. Functional properties of Lactobacillus plantarum strains: A study in vitro of heat stress influence. Food Microbiol. 2016, 54, 154–161. [Google Scholar] [CrossRef]
- Franciosi, E.; Carafa, I.; Nardin, T.; Schiavon, S.; Poznanski, E.; Cavazza, A.; Larcher, R.; Tuohy, K.M. Biodiversity and γ-aminobutyric acid production by lactic acid bacteria isolated from traditional alpine raw cow’s milk cheeses. BioMed Res. Int. 2015, 2015, 625740. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Ferranti, P.; Smacchi, E.; Goffredi, F.; Addeo, F. Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks started by Lactobacillus delbrueckiisubsp. bulgaricus SS1 and Lactococcus lactissubsp. cremoris FT4. Appl. Environ. Microb. 2000, 66, 3898–3904. [Google Scholar] [CrossRef]
- Hiraga, K.; Ueno, Y.; Sukontasing, S.; Tanasupawat, S.; Oda, K. Lactobacillus senmaizukei sp. nov., isolated from Japanese pickle. Int. J. Syst. Evol. Microbiol. 2008, 58, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Komatsuzaki, N.; Shima, J.; Kawamoto, S.; Momose, H.; Kimura, T. Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 2005, 22, 497–504. [Google Scholar] [CrossRef]
- Li, H.; Qiu, T.; Gao, D.; Cao, Y. Medium optimization for production of gamma-aminobutyric acid by Lactobacillus brevis NCL912. Amino Acids 2010, 38, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, T.; Huang, G.; Cao, Y. Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb. Cell Fact. 2010, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Kimoto, H.; Someya, Y.; Furukawa, S.; Suzuki, I. Production of γ-aminobutyric acid by cheese starters during cheese ripening. J. Dairy Sci. 1998, 81, 1486–1491. [Google Scholar] [CrossRef]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sanchart, C.; Rattanaporn, O.; Haltrich, D.; Phukpattaranont, P.; Maneerat, S. Technological and safety properties of newly isolated GABA-producing Lactobacillus futsaii strains. J. Appl. Microbiol. 2016, 121, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.M.; Brown, L.; de Giori, G.S.; Hebert, E.M. Optimization of batch culture conditions for GABA production by Lactobacillus brevis CRL 1942, isolated from quinoa sourdough. LWT Food Sci. Technol. 2016, 67, 22–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, L.; Gao, Q.; Yu, S.M.; Li, L.; Gao, N.F. The two-step biotransformation of monosodium glutamate to GABA by Lactobacillus brevis growing and resting cells. Appl. Microbiol. Biot. 2012, 94, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-S.; Liu, M.-K.; Wu, C.-H.; Yen, Y.-T.; Lin, Y.-C. Calcium alginate microcapsule generation on a microfluidic system fabricated using the optical disk process. J. Micromech. Microeng. 2007, 17, 1428–1434. [Google Scholar] [CrossRef]
- Qureshi, N.; Lai, L.; Blaschek, H. Scale-up of a high productivity continuous biofilm reactor to produce butanol by adsorbed cells of Clostridium beijerinckii. Food Bioprod. Process. 2004, 82, 164–173. [Google Scholar] [CrossRef]
- Yao, W.; Wu, X.; Zhu, J.; Sun, B.; Miller, C. In vitro enzymatic conversion of γ-aminobutyric acid immobilization of glutamate decarboxylase with bacterial cellulose membrane (BCM) and non-linear model establishment. Enzyme Microb. Technol. 2013, 52, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mei, L.-H.; Wu, H.; Lin, D.-Q. Biosynthesis of γ-aminobutyric acid (GABA) using immobilized whole cells of Lactobacillus brevis. World J. Microb. Biot. 2007, 23, 865–871. [Google Scholar] [CrossRef]
- Al Mardini, H.; Al Jumaili, B.; Record, C.; Burke, D. Effect of protein and lactulose on the production of gamma-aminobutyric acid by faecal Escherichia coli. Gut 1991, 32, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
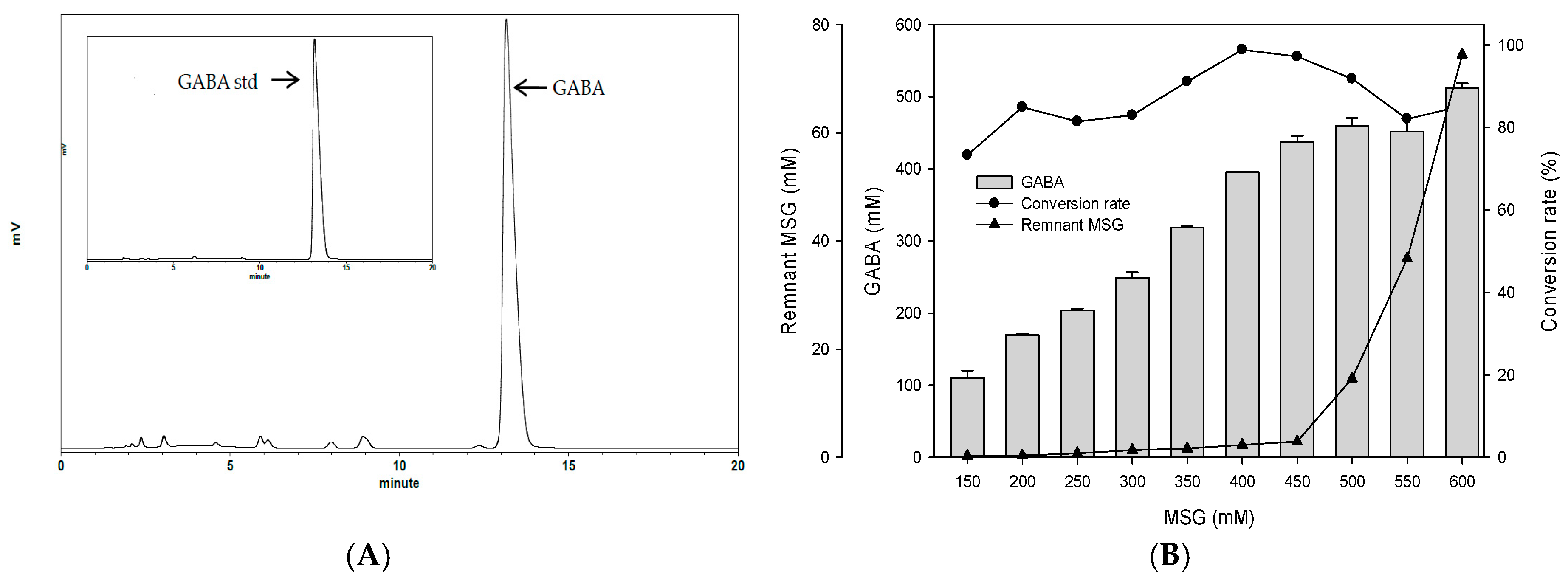
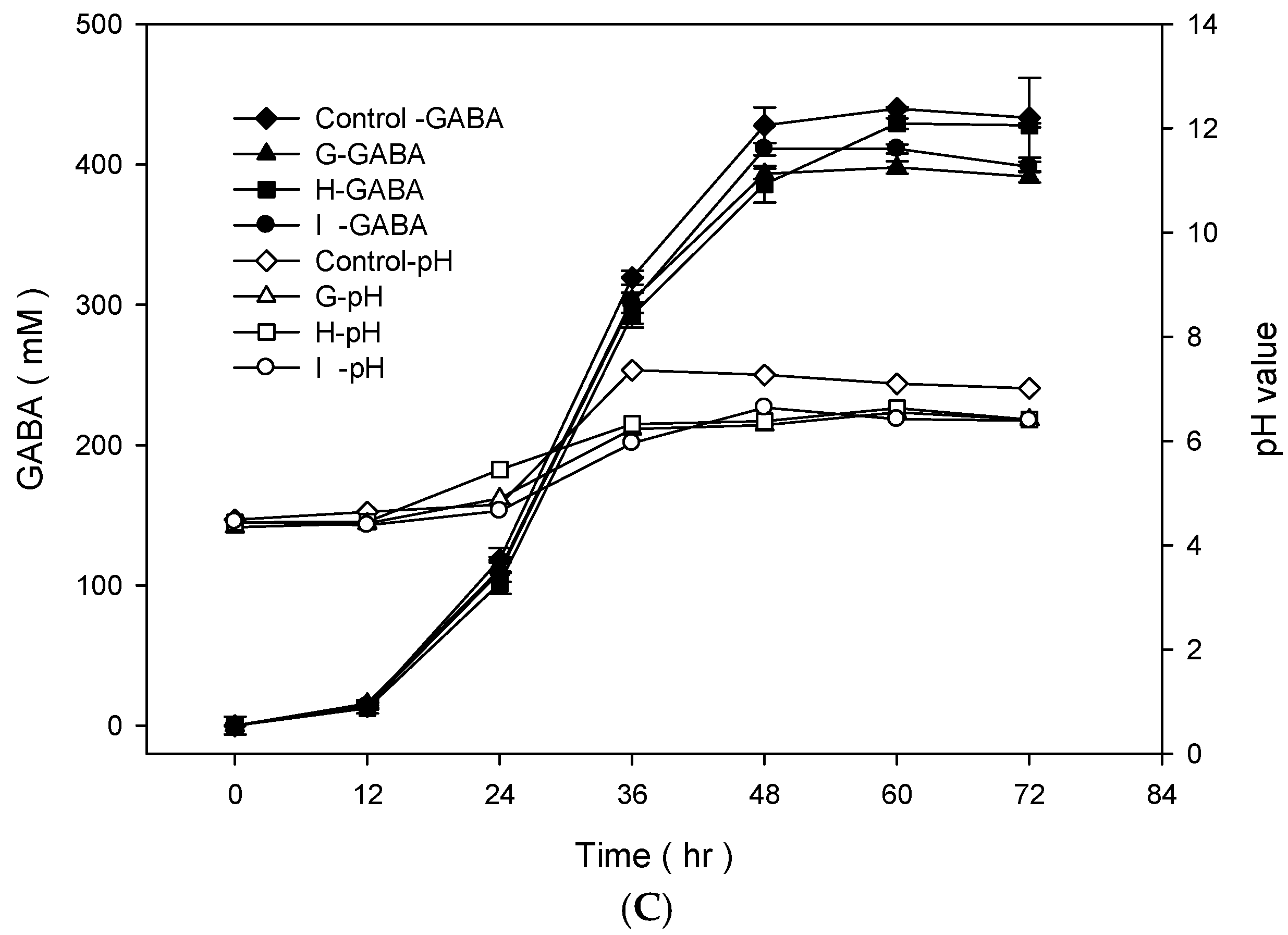
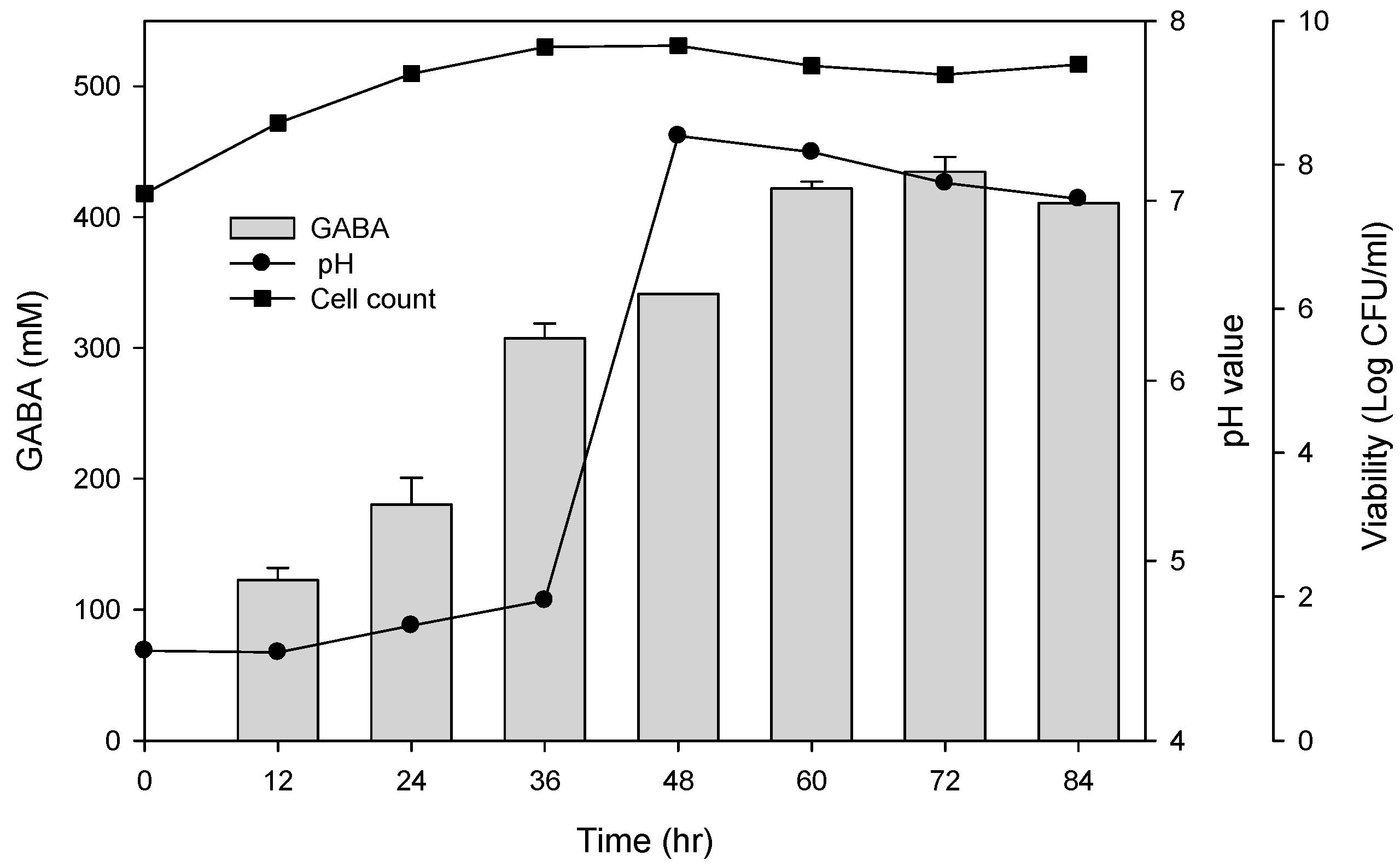
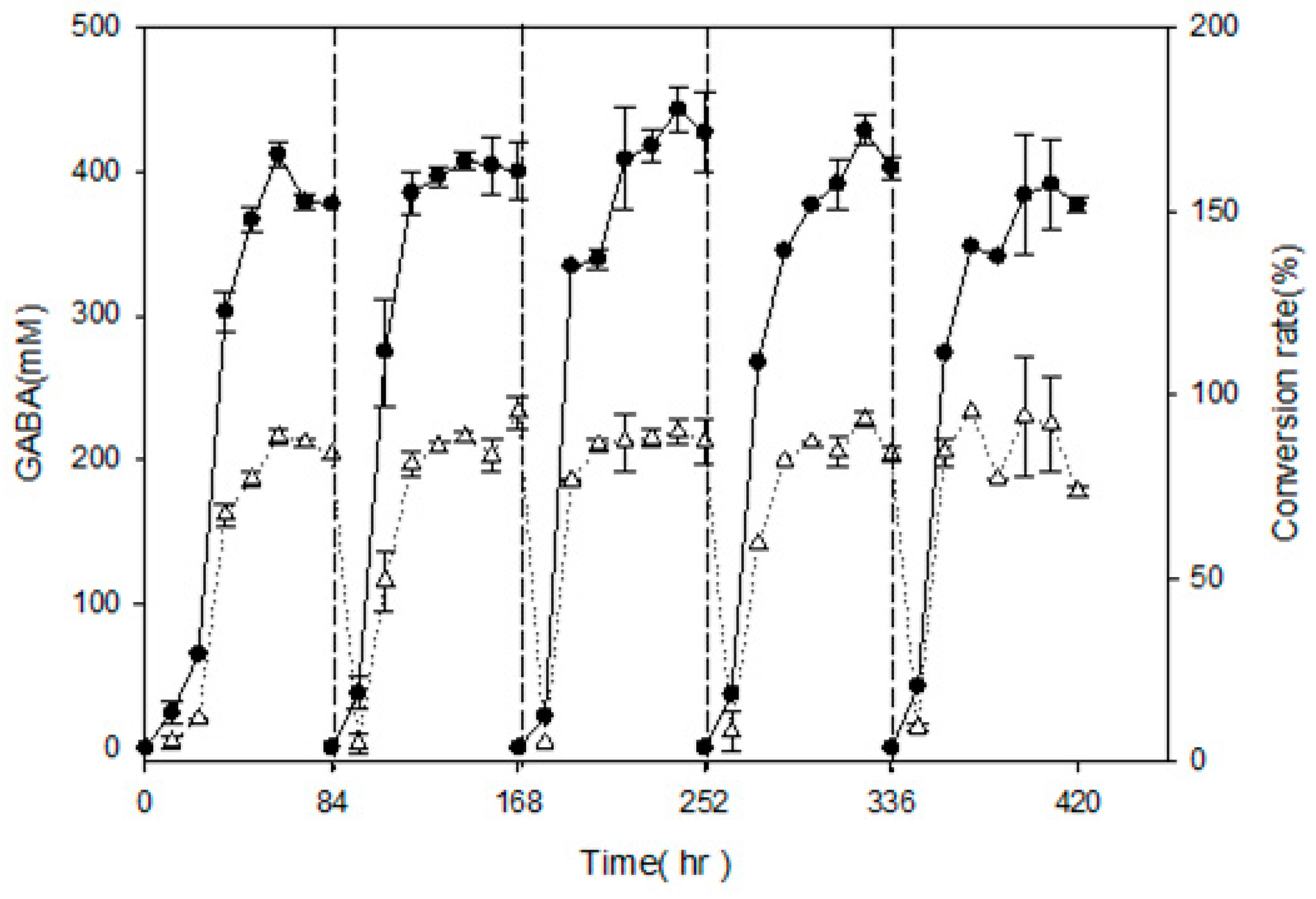
| No. | HEMA | PEGDA Mw: 200 | PEGDA Mw: 400 | PEGDA Mw: 700 | AA | Initiator | Tensile Strength (kg/cm2) | Water Content (%) |
|---|---|---|---|---|---|---|---|---|
| A | 95 | 3 | - | - | 1 | 1 | 9.31 | 31.41 |
| B | 93 | 3 | - | - | 3 | 1 | 7.26 | 31.56 |
| C | 91 | 3 | - | - | 5 | 1 | 6.61 | 32.45 |
| D | 95 | - | 3 | - | 1 | 1 | 7.52 | 32.10 |
| E | 93 | - | 3 | - | 3 | 1 | 6.76 | 32.94 |
| F | 91 | - | 3 | - | 5 | 1 | 5.96 | 34.63 |
| G | 95 | - | - | 3 | 1 | 1 | 7.10 | 34.75 |
| H | 93 | - | - | 3 | 3 | 1 | 5.20 | 34.91 |
| I | 91 | - | - | 3 | 5 | 1 | 3.95 | 34.95 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsueh, Y.-H.; Liaw, W.-C.; Kuo, J.-M.; Deng, C.-S.; Wu, C.-H. Hydrogel Film-Immobilized Lactobacillus brevis RK03 for γ-Aminobutyric Acid Production. Int. J. Mol. Sci. 2017, 18, 2324. https://doi.org/10.3390/ijms18112324
Hsueh Y-H, Liaw W-C, Kuo J-M, Deng C-S, Wu C-H. Hydrogel Film-Immobilized Lactobacillus brevis RK03 for γ-Aminobutyric Acid Production. International Journal of Molecular Sciences. 2017; 18(11):2324. https://doi.org/10.3390/ijms18112324
Chicago/Turabian StyleHsueh, Yi-Huang, Wen-Chang Liaw, Jen-Min Kuo, Chi-Shin Deng, and Chien-Hui Wu. 2017. "Hydrogel Film-Immobilized Lactobacillus brevis RK03 for γ-Aminobutyric Acid Production" International Journal of Molecular Sciences 18, no. 11: 2324. https://doi.org/10.3390/ijms18112324