Nitric Oxide Mediates Crosstalk between Interleukin 1β and WNT Signaling in Primary Human Chondrocytes by Reducing DKK1 and FRZB Expression
Abstract
:1. Introduction
2. Results
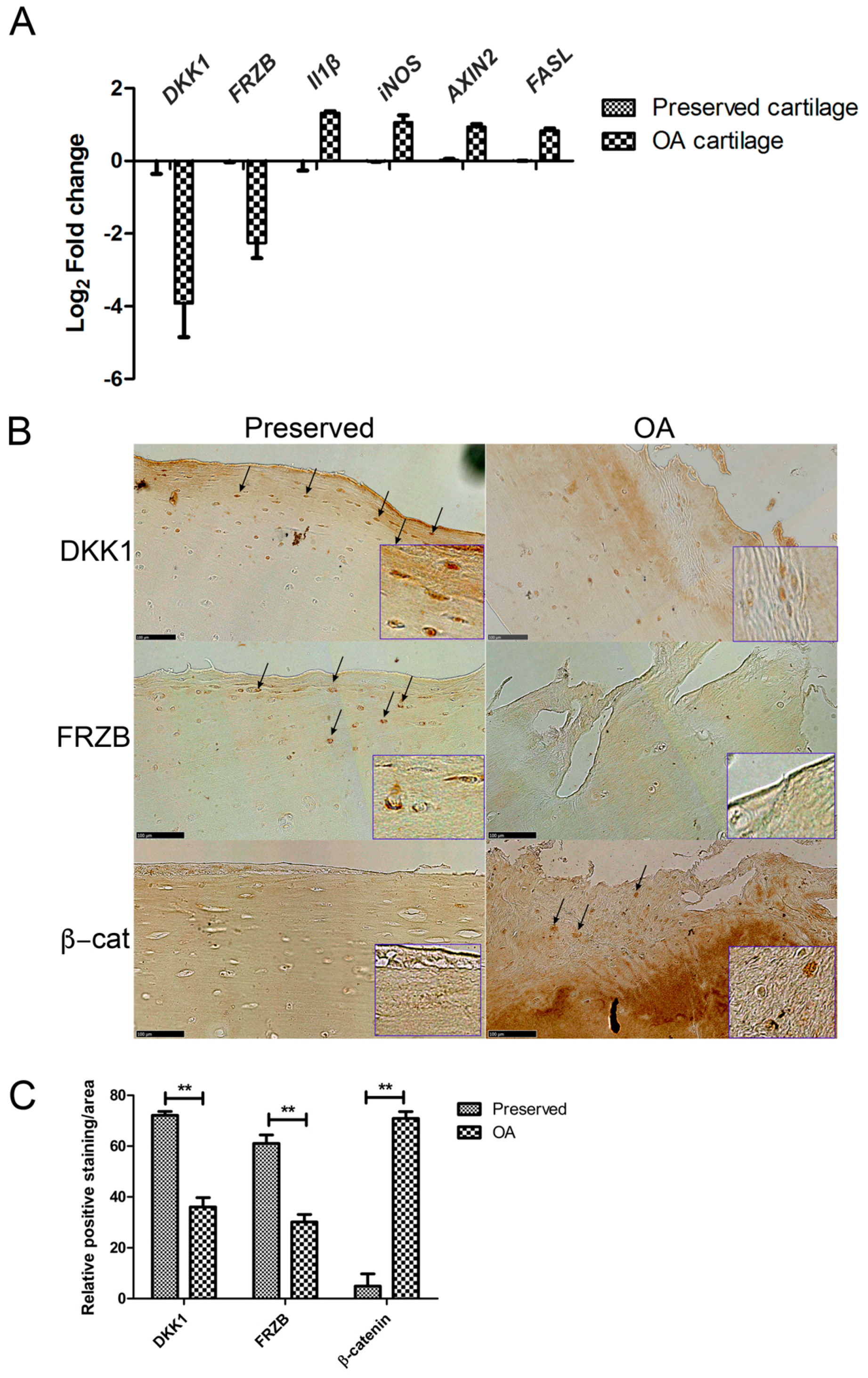
2.1. Expression of DKK1 and FRZB Is Decreased While IL1β, NOS2/iNOS, and AXIN2 Are Increased in Human OA
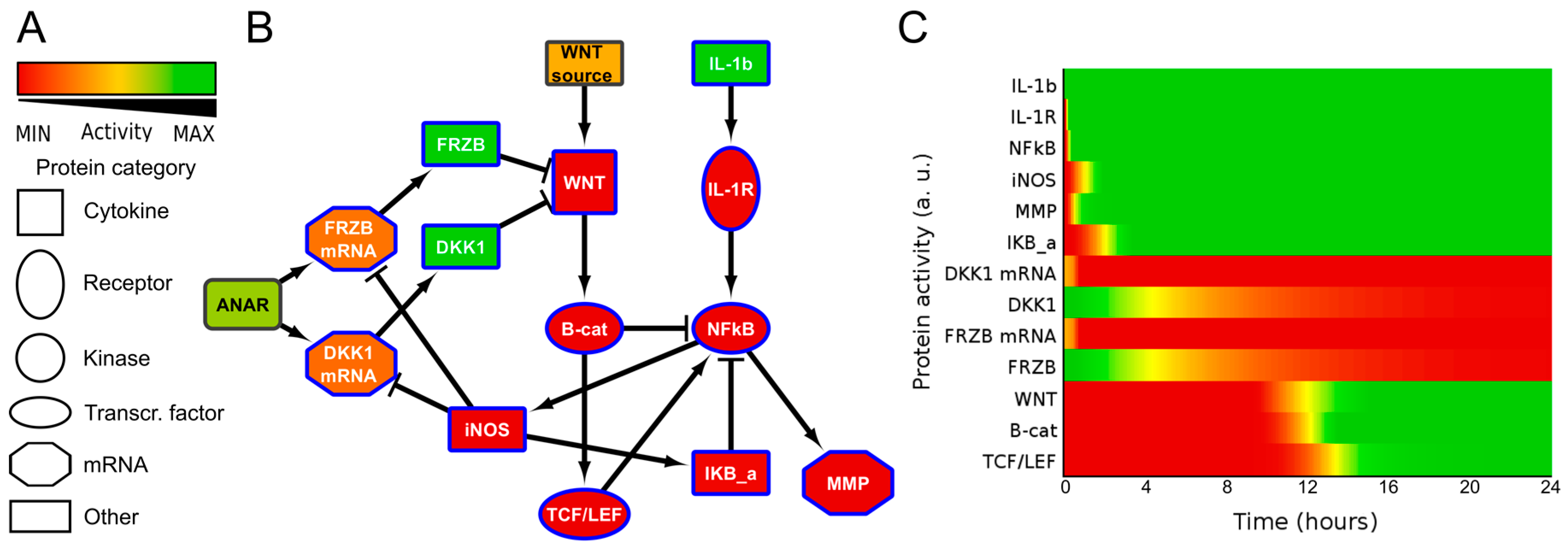
2.2. ANIMO Model Predicts That IL1β Upregulates WNT Signaling via iNOS/NO by Downregulating Expression of DKK1 and FRZB
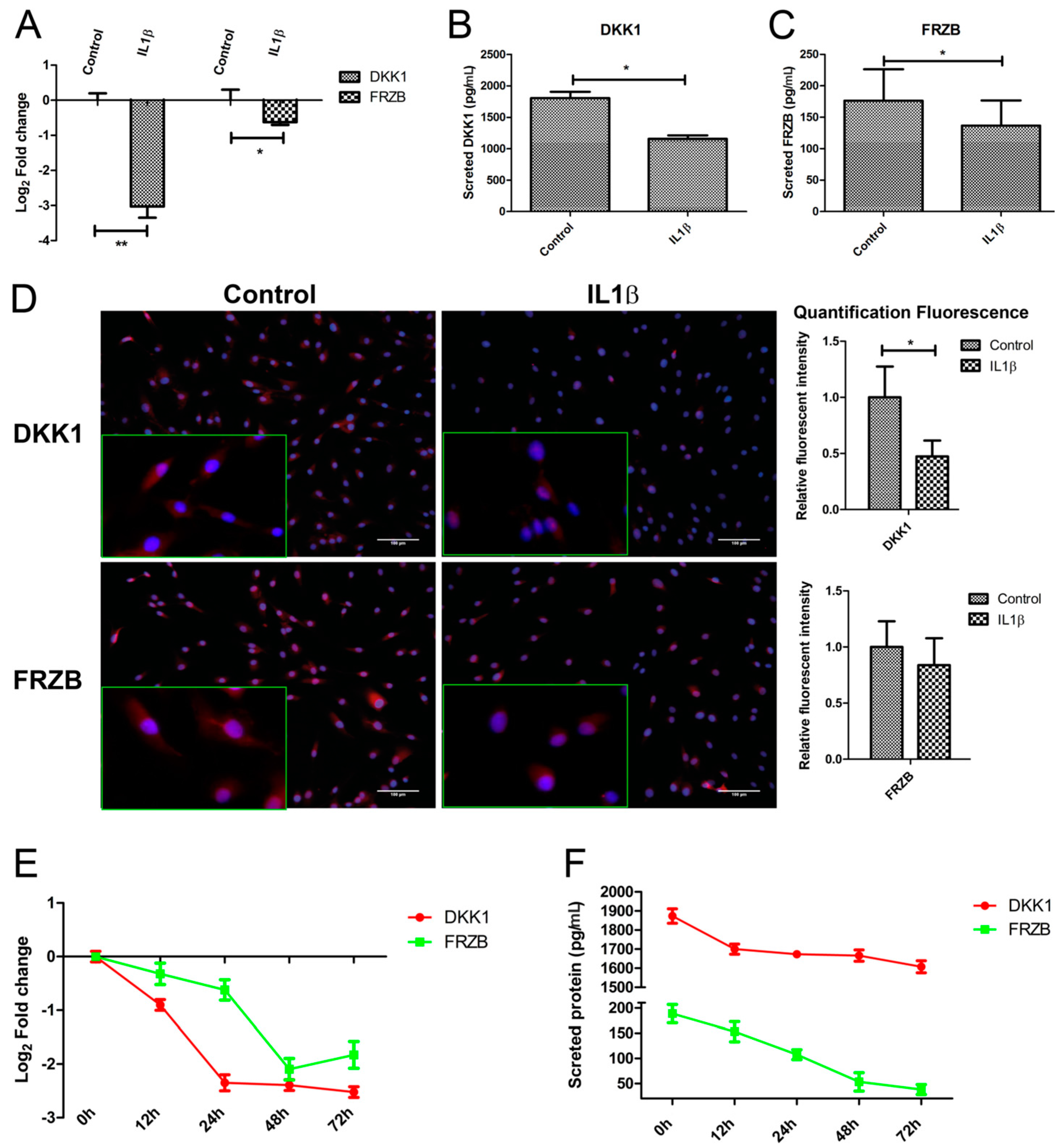
2.3. IL1β Decreased DKK1 and FRZB Expression in a Time- but Not Dose-Dependent Manner
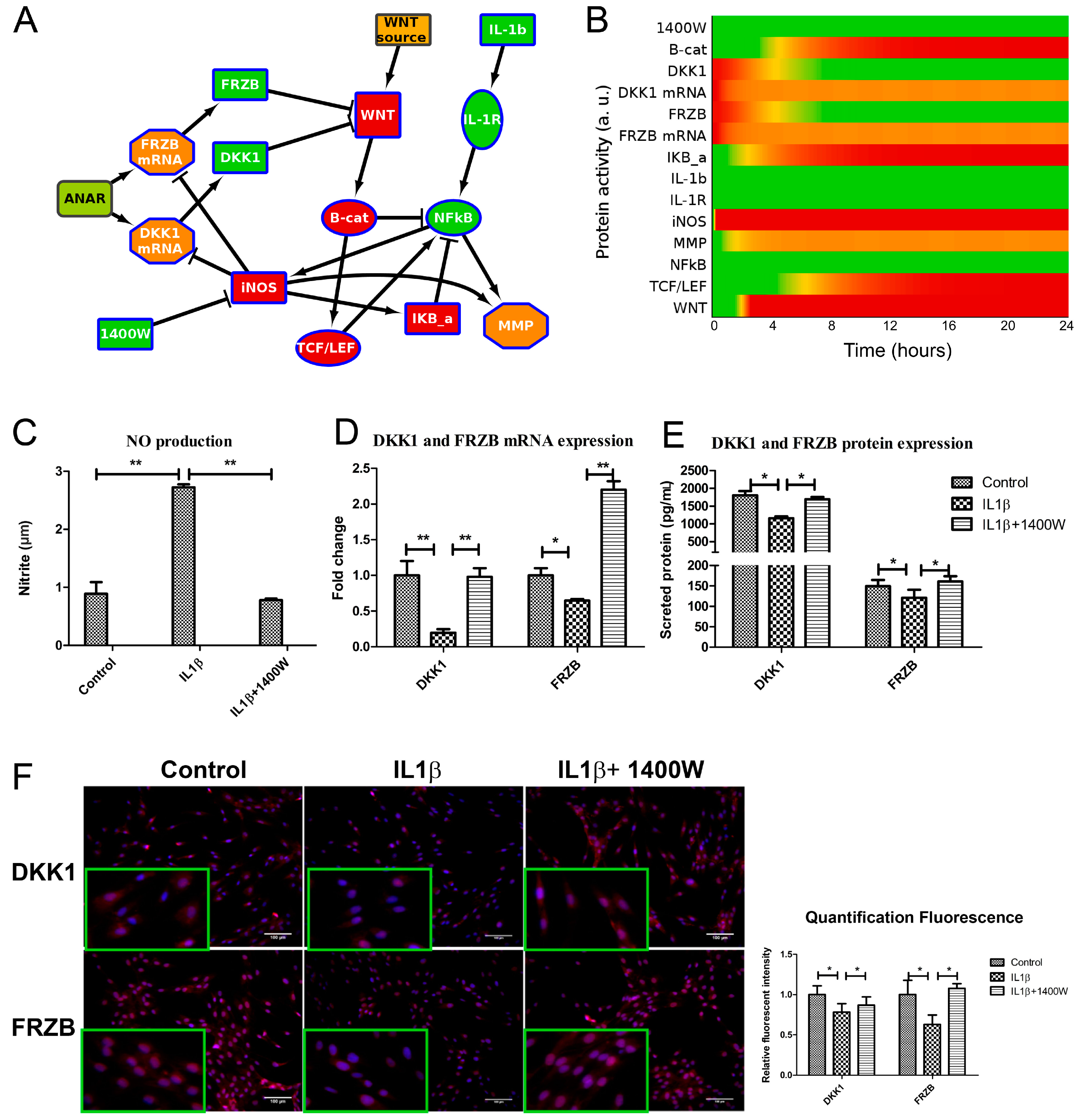
2.4. IL1β Decreased DKK1 and FRZB Expression through Upregulation of iNOS
2.5. Addition of 1400W Relieves the Break on DKK1 and FRZB Inhibition
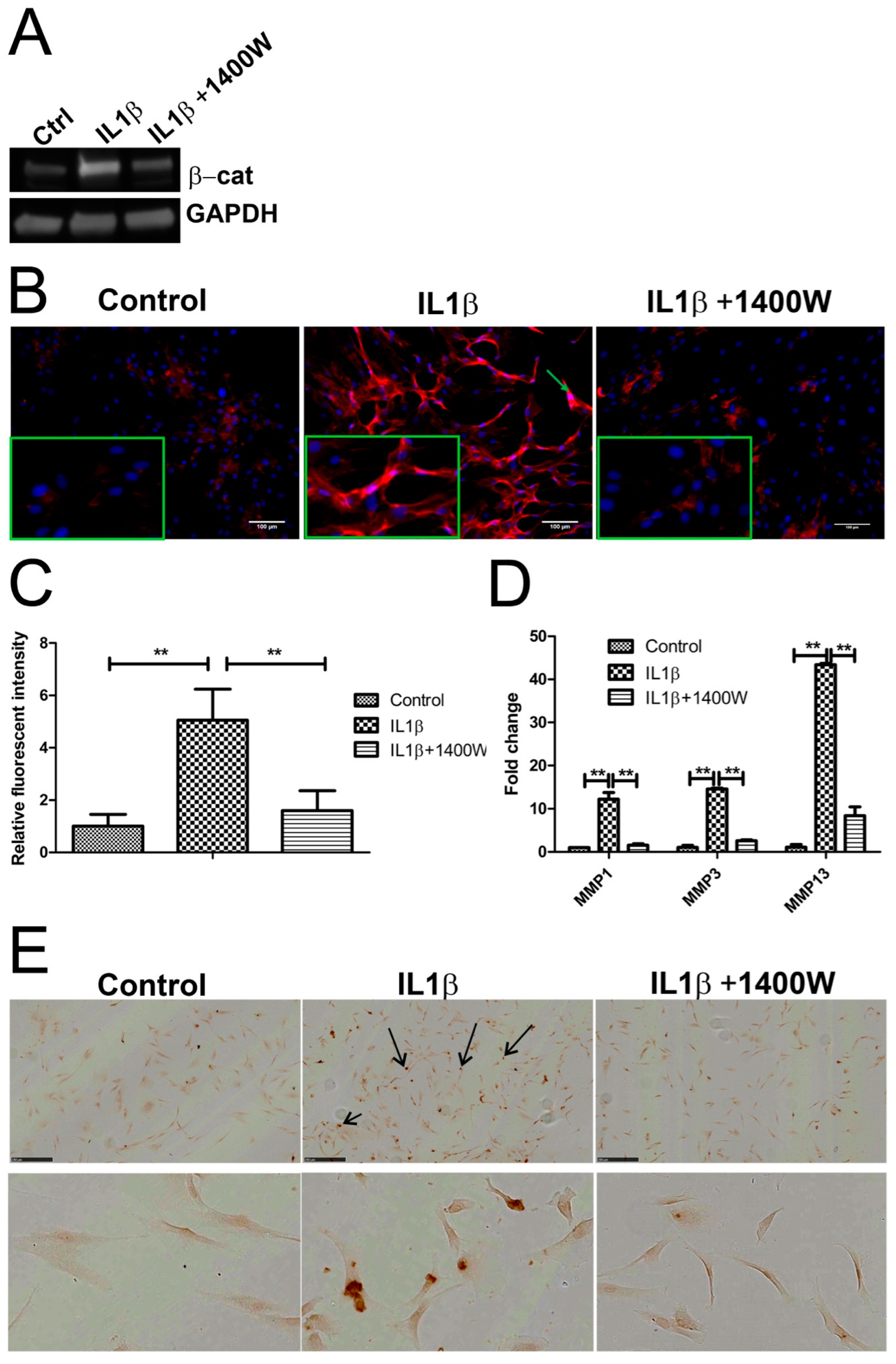
2.6. Blocking IL1β-Induced iNOS Decreased β-Catenin Expression
2.7. Computational Model Highlights Role of iNOS in Regulating MMP Expression
3. Discussion
4. Materials and Methods
4.1. ANIMO
4.2. Human Cartilage
4.3. RNA Isolation and qPCR Analysis
4.4. Immunohistochemistry (IHC) and Immunofluorescence
4.5. Human Primary Chondrocyte Isolation and Cell Culture
4.6. Recombinant Proteins and Reagents
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Western Blotting
4.9. NO Production Assay
4.10. Immunofluorescent Staining
4.11. Apoptosis Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACAN | Aggrecan |
| ANAR | ANAbolic regulator |
| ANIMO | Analysis of networks with interactive modeling |
| AXIN2 | Axis inhibition protein 2, mRNA |
| COL2A1 | Collagen 2 variant a1 |
| DKK1 | Dickkopf-1 |
| EGF | Epidermal growth factor |
| FASL | Fas ligand/CD95L-mRNA |
| FRZB | Frizzled related protein |
| FZD10 | Frizzled-10-mRNA |
| hCh | Human chondrocyte |
| IHC | Immunohistochemistry |
| IL | Interleukin |
| IL1R | IL1Receptor |
| iNOS/NOS2 | nitric oxide synthase |
| IκB | Inhibitor of κB |
| LEF | Lymphoid enhancer-binding factor |
| MMP3 | Matrix metalloproteinase 3- mRNA |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells/nuclear factor kappa B |
| NO | Nitric oxide |
| OA | Osteoarthritis |
| SNP | Single nucleotide polymorphisms |
| TCF4 | Transcription factor 4 |
| TNFa | Tumor necrosis factor α |
| WIF1 | Wnt inhibitory factor 1-mRNA |
| WNT | Wingless-type MMTV integration site family |
References
- Allen, K.D.; Golightly, Y.M. State of the evidence. Curr. Opin. Rheumatol. 2015, 27, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Issa, S.N.; Sharma, L. Epidemiology of osteoarthritis: An update. Curr. Rheumatol. Rep. 2006, 8, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, J.; Dowling, B.; Chapman, K.; Marcelline, L.; Mustafa, Z.; Southam, L.; Ferreira, A.; Ciesielski, C.; Carson, D.A.; Corr, M. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc. Natl. Acad. Sci. USA 2004, 101, 9757–9762. [Google Scholar] [CrossRef] [PubMed]
- Min, J.L.; Meulenbelt, I.; Riyazi, N.; Kloppenburg, M.; Houwing-Duistermaat, J.J.; Seymour, A.B.; Pols, H.A.; van Duijn, C.M.; Slagboom, P.E. Association of the frizzled-related protein gene with symptomatic osteoarthritis at multiple sites. Arthritis Rheum. 2005, 52, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.U.; Peeters, J.; Bakker, A.; Tylzanowski, P.; Derese, I.; Schrooten, J.; Thomas, J.T.; Luyten, F.P. Articular cartilage and biomechanical properties of the long bones inFrzb-knockout mice. Arthritis Rheum. 2007, 56, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Bougault, C.; Priam, S.; Houard, X.; Pigenet, A.; Sudre, L.; Lories, R.J.; Jacques, C.; Berenbaum, F. Protective role of frizzled-related protein B on matrix metalloproteinase induction in mouse chondrocytes. Arthritis Res. Ther. 2014, 16, R137. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E.; Nevitt, M.C.; Lui, L.-Y.; de Leon, P.; Corr, M. Wnt signaling antagonists are potential prognostic biomarkers for the progression of radiographic hip osteoarthritis in elderly Caucasian women. Arthritis Rheum. 2007, 56, 3319–3325. [Google Scholar] [CrossRef] [PubMed]
- Voorzanger-Rousselot, N.; Ben-Tabassi, N.C.; Garnero, P. Opposite relationships between circulating Dkk-1 and cartilage breakdown in patients with rheumatoid arthritis and knee osteoarthritis. Ann. Rheum. Dis. 2009, 68, 1513–1514. [Google Scholar] [CrossRef] [PubMed]
- Honsawek, S.; Tanavalee, A.; Yuktanandana, P.; Ngarmukos, S.; Saetan, N.; Tantavisut, S. Dickkopf-1 (Dkk-1) in plasma and synovial fluid is inversely correlated with radiographic severity of knee osteoarthritis patients. BMC Musculoskelet. Disord. 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.C.H.; Emons, J.; Sticht, C.; van Gool, S.; Decker, E.; Uitterlinden, A.; Rappold, G.; Hofman, A.; Rivadeneira, F.; Scherjon, S.; et al. Gremlin 1, Frizzled-related protein, and Dkk-1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012, 64, 3302–3312. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.C.; Bos, S.D.; Landman, E.B.; Georgi, N.; Jahr, H.; Meulenbelt, I.; Post, J.N.; van Blitterswijk, C.A.; Karperien, M. GREM1, FRZB and DKK1 mRNA levels correlate with osteoarthritis and are regulated by osteoarthritis-associated factors. Arthritis Res. Ther. 2013, 15, R126. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Huang, X.; Karperien, M.; Post, J. Correlation between Gene Expression and Osteoarthritis Progression in Human. Int. J. Mol. Sci. 2016, 17, 1126. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor α in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005, 52, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Heinegård, D.; Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2010, 7, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.-P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001, 44, 1237–1247. [Google Scholar] [CrossRef]
- Abramson, S.B.; Attur, M.; Yazici, Y. Prospects for disease modification in osteoarthritis. Nat. Clin. Pract. Rheumatol. 2006, 2, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R. The expression and regulation of nitric oxide synthase in human osteoarthritis-affected chondrocytes: Evidence for up-regulated neuronal nitric oxide synthase. J. Exp. Med. 1995, 182, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Carlson, C.S.; Carlo, M.D.; Cole, A. Detection of nitrotyrosine in aging and osteoarthritic cartilage: Correlation of oxidative damage with the presence of interleukin-1β and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum. 2002, 46, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Taskiran, D.; Stefanovicracic, M.; Georgescu, H.; Evans, C. Nitric-Oxide Mediates Suppression of Cartilage Proteoglycan Synthesis by Interleukin-1. Biochem. Biophys. Res. Commun. 1994, 200, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Ochs, R.L.; Schwarz, H.; Lotz, M. Chondrocyte apoptosis induced by nitric oxide. Am. J. Pathol. 1995, 146, 75–85. [Google Scholar] [PubMed]
- Abramson, S.B. Osteoarthritis and nitric oxide. Osteoarthr. Cartil. 2008, 16, S15–S20. [Google Scholar] [CrossRef]
- Ma, B.; van Blitterswijk, C.A.; Karperien, M. A Wnt/β-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheum. 2012, 64, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhong, L.; van Blitterswijk, C.A.; Post, J.N.; Karperien, M. T Cell Factor 4 Is a Pro-catabolic and Apoptotic Factor in Human Articular Chondrocytes by Potentiating Nuclear Factor κB Signaling. J. Biol. Chem. 2013, 288, 17552–17558. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhang, X.; Liu, Q.; Zhang, X.; Bartels, C.E.; Geller, D.A. Nitric Oxide Production Upregulates Wnt/-Catenin Signaling by Inhibiting Dickkopf-1. Cancer Res. 2013, 73, 6526–6537. [Google Scholar] [CrossRef] [PubMed]
- Lenas, P.; Moos, M.; Luyten, F.P. Developmental Engineering: A New Paradigm for the Design and Manufacturing of Cell-Based Products. Part II. From Genes to Networks: Tissue Engineering from the Viewpoint of Systems Biology and Network Science. Tissue Eng. Part B Rev. 2009, 15, 395–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef] [PubMed]
- Schivo, S.; Scholma, J.; Karperien, M.; Post, J.N.; van de Pol, J.; Langerak, R. Setting Parameters for Biological Models with ANIMO. Electron. Proc. Theor. Comput. Sci. 2014, 145, 35–47. [Google Scholar] [CrossRef]
- Schivo, S.; Scholma, J.; van der Vet, P.E.; Karperien, M.; Post, J.N.; van de Pol, J.; Langerak, R. Modelling with ANIMO: Between fuzzy logic and differential equations. BMC Syst. Biol. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Schivo, S.; Scholma, J.; Wanders, B.; Camacho, R.A.U.; van der Vet, P.E.; Karperien, M.; Langerak, R.; van de Pol, J.; Post, J.N. Modeling Biological Pathway Dynamics With Timed Automata. IEEE J. Biomed. Health Inf. 2014, 18, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Scholma, J.; Schivo, S.; Urquidi Camacho, R.A.; van de Pol, J.; Karperien, M.; Post, J.N. Biological networks 101: Computational modeling for molecular biologists. Gene 2014, 533, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, K.; Vuolteenaho, K.; Nieminen, R.; Moilanen, T.; Knowles, R.G.; Moilanen, E. Selective iNOS inhibitor 1400W enhances anti-catabolic IL-10 and reduces destructive MMP-10 in OA cartilage. Survey of the effects of 1400W on inflammatory mediators produced by OA cartilage as detected by protein antibody array. Clin. Exp. Rheumatol. 2008, 26, 275–282. [Google Scholar] [PubMed]
- Brendeford, E.M.; Andersson, K.B.; Gabrielsen, O.S. Nitric oxide (NO) disrupts specific DNA binding of the transcription factor c-Myb in vitro. FEBS Lett. 1998, 425, 52–56. [Google Scholar] [CrossRef]
- Garban, H.J.; Bonavida, B. Nitric Oxide Inhibits the Transcription Repressor Yin-Yang 1 Binding Activity at the Silencer Region of the Fas Promoter: A Pivotal Role for Nitric Oxide in the Up-Regulation of Fas Gene Expression in Human Tumor Cells. J. Immunol. 2001, 167, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Sparkman, L. Nitric oxide increases IL-8 gene transcription and mRNA stability to enhance IL-8 gene expression in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L764–L773. [Google Scholar] [CrossRef] [PubMed]
- Hartung, N.; Benary, U.; Wolf, J.; Kofahl, B. Paracrine and autocrine regulation of gene expression by wnt-inhibitor dickkopf in wild-type and mutant hepatocytes. BMC Syst. Biol. 2017, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Huang, X.; Rodrigues, E.D.; Leijten, J.C.; Verrips, T.; El Khattabi, M.; Karperien, M.; Post, J.N. Endogenous dkk1 and frzb regulate chondrogenesis and hypertrophy in three-dimensional cultures of human chondrocytes and human mesenchymal stem cells. Stem Cells Dev. 2016, 25, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Semënov, M.V.; Tamai, K.; Brott, B.K.; Kühl, M.; Sokol, S.; He, X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 2001, 11, 951–961. [Google Scholar] [CrossRef]
- Bafico, A.; Liu, G.; Yaniv, A.; Gazit, A.; Aaronson, S.A. Novel mechanism of wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 2001, 3, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Wang, S.; Julius, M.A.; Kitajewski, J.; Moos, M.; Luyten, F.P. The cysteine-rich frizzled domain of Frzb-1 is required and sufficient for modulation of Wnt signaling. Proc. Natl. Acad. Sci. USA 1997, 94, 11196–11200. [Google Scholar] [CrossRef] [PubMed]
- Leyns, L.; Bouwmeester, T.; Kim, S.H.; Piccolo, S.; De Robertis, E.M. Frzb-1 is a secreted antagonist of wnt signaling expressed in the spemann organizer. Cell 1997, 88, 747–756. [Google Scholar] [CrossRef]
- Bafico, A.; Gazit, A.; Pramila, T.; Finch, P.W.; Yaniv, A.; Aaronson, S.A. Interaction of Frizzled Related Protein (FRP) with Wnt Ligands and the Frizzled Receptor Suggests Alternative Mechanisms for FRP Inhibition of Wnt Signaling. J. Biol. Chem. 1999, 274, 16180–16187. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003, 116, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Gough, N.R. Understanding Wnt’s Role in Osteoarthritis. Sci. Signal. 2011, 4, ec134. [Google Scholar] [CrossRef]
- Blom, A.B.; van Lent, P.L.; van der Kraan, P.M.; van den Berg, W.B. To seek shelter from the wnt in osteoarthritis? Wnt-signaling as a target for osteoarthritis therapy. Curr. Drug Targets 2010, 11, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.K.; Otsuki, S.; Grogan, S.P.; Sah, R.; Terkeltaub, R.; D’Lima, D. Cartilage cell clusters. Arthritis Rheum. 2010, 62, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-G.; Yu, S.-S.; Ryu, J.-H.; Jeon, H.-B.; Yoo, Y.-J.; Eom, S.-H.; Chun, J.-S. Regulation of beta-catenin signaling and maintenance of chondrocyte differentiation by ubiquitin-independent proteasomal degradation of alpha-catenin. J. Biol. Chem. 2005, 280, 12758–12765. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.J.; Aigner, T. Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001, 3, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecil, D.L.; Johnson, K.; Rediske, J.; Lotz, M.; Schmidt, A.M.; Terkeltaub, R. Inflammation-Induced Chondrocyte Hypertrophy Is Driven by Receptor for Advanced Glycation End Products. J. Immunol. 2005, 175, 8296–8302. [Google Scholar] [CrossRef] [PubMed]
- Cecil, D.L.; Rose, D.M.; Terkeltaub, R.; Liu-Bryan, R. Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes. Arthritis Rheum. 2005, 52, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Cecil, D.L.; Appleton, C.T.G.; Polewski, M.D.; Mort, J.S.; Schmidt, A.M.; Bendele, A.; Beier, F.; Terkeltaub, R. The Pattern Recognition Receptor CD36 Is a Chondrocyte Hypertrophy Marker Associated with Suppression of Catabolic Responses and Promotion of Repair Responses to Inflammatory Stimuli. J. Immunol. 2009, 182, 5024–5031. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, E.; Borzi, R.M.; Vitellozzi, R.; Pagani, S.; Facchini, A.; Battistelli, M.; Penzo, M.; Li, X.; Flamigni, F.; Li, J.; et al. Differential requirements for ikkalpha and ikkbeta in the differentiation of primary human osteoarthritic chondrocytes. Arthritis Rheum. 2007, 58, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.; Lascau-Coman, V.; Jovanovic, D.; Fernandes, J.C.; Manning, P.; Connor, J.R.; Currie, M.G.; Martel-Pelletier, J. Selective inhibition of inducible nitric oxide synthase in experimental osteoarthritis is associated with reduction in tissue levels of catabolic factors. J. Rheumatol. 1999, 26, 2002–2014. [Google Scholar] [PubMed]
- Pelletier, J.P.; Jovanovic, D.V.; Lascau-Coman, V.; Fernandes, J.C.; Manning, P.T.; Connor, J.R.; Currie, M.G.; Martel-Pelletier, J. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: Possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 2000, 43, 1290–1299. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Schivo, S.; Scholma, J.; Wanders, B.; Camacho, R.A.U.; van der Vet, P.E.; Karperien, M.; Langerak, R.; van de Pol, J.; Post, J.N. Animo. Available online: http://fmt.cs.utwente.nl/tools/animo/ (accessed on 31 October 2017).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Park, K.S.; Guo, Z.; He, P.; Nagashima, M.; Shao, L.; Sahai, R.; Geller, D.A.; Hussain, S.P. Regulation of human nitric oxide synthase 2 expression by wnt beta-catenin signaling. Cancer Res. 2006, 66, 7024–7031. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, L.; Schivo, S.; Huang, X.; Leijten, J.; Karperien, M.; Post, J.N. Nitric Oxide Mediates Crosstalk between Interleukin 1β and WNT Signaling in Primary Human Chondrocytes by Reducing DKK1 and FRZB Expression. Int. J. Mol. Sci. 2017, 18, 2491. https://doi.org/10.3390/ijms18112491
Zhong L, Schivo S, Huang X, Leijten J, Karperien M, Post JN. Nitric Oxide Mediates Crosstalk between Interleukin 1β and WNT Signaling in Primary Human Chondrocytes by Reducing DKK1 and FRZB Expression. International Journal of Molecular Sciences. 2017; 18(11):2491. https://doi.org/10.3390/ijms18112491
Chicago/Turabian StyleZhong, Leilei, Stefano Schivo, Xiaobin Huang, Jeroen Leijten, Marcel Karperien, and Janine N. Post. 2017. "Nitric Oxide Mediates Crosstalk between Interleukin 1β and WNT Signaling in Primary Human Chondrocytes by Reducing DKK1 and FRZB Expression" International Journal of Molecular Sciences 18, no. 11: 2491. https://doi.org/10.3390/ijms18112491




